ABSTRACT
Objective
The analysis estimates projected population outcomes resulting from the introduction of a plant-derived influenza vaccine formulated as quadrivalent virus-like particles (QVLP) in Canada.
Methods
Using Monte Carlo simulations, the number of influenza cases, general practitioner visits, inpatient admissions, intensive care unit (ICU) admissions, and deaths due to influenza-associated illness were estimated under no vaccination, plant-derived QVLP vaccines only, or egg-derived vaccines only. The base case analysis examined the adult Canadian population in two subgroups: 18–64 years of age during the 2017/18 season and 65+ years of age during the 2018/19 season. Efficacy data were obtained from QVLP clinical trials. Vaccine effectiveness data for egg-derived vaccines were calculated from observational studies from the corresponding influenza seasons. Scenario analyses examined the impact of varying absolute vaccine effectiveness or vaccination coverage from base case inputs.
Results
In the base case analysis, plant-derived QVLP vaccines led to an additional reduction in the burden of influenza over egg-derived vaccines for both population subgroups. In the 18–64 subgroup, QVLP vaccines were associated with 2.63% (48,029; 95% credible interval [Crl]: 42,723–53,336) fewer influenza cases than egg-derived vaccines. In the 65+ subgroup, QVLP vaccines led to 4.82% (27,918; 95% Crl: 25,440–30,397) fewer influenza cases, and reductions in the number of inpatient admissions by 4.77% (1167; 95% CrI: 851–1483) and deaths by 4.75% (326; 95% CrI: 107–546) compared to egg-derived vaccines. Further reductions were observed in scenario analyses considering the potential increase in vaccine coverage.
Conclusion
Use of plant-derived QVLP influenza vaccines may contribute to greater reductions in influenza cases and influenza-related outcomes, including inpatient admissions and deaths, compared to egg-derived vaccines currently available in Canada.
Introduction
Each year, approximately 5–10% of adults in Canada are infected with influenza A and B viruses that circulate during seasonal epidemics.Citation1 The burden of seasonal influenza includes workplace absenteeism, emergency department (ED) visits, inpatient admissions, intensive care unit (ICU) admissions, and deaths.Citation1–4 Influenza is associated with an average of 12,200 inpatient admissionsCitation2 and 3500 deathsCitation4 annually in Canada. Vaccination is the primary strategy to control the spread of seasonal influenza and prevent influenza-associated illness and outcomes. In Canada, vaccination against influenza is recommended for individuals six months of age or older.Citation5
Vaccine-induced immunogenic response is specific to influenza A and B subtypes included in the annual vaccine formulation.Citation1,Citation6 Vaccine effectiveness (VE) against seasonal influenza viruses varies, and is impacted, at least in part, by the degree of antigenic match between circulating strains and the vaccine formulation.Citation6,Citation7 Vaccine formulations may be antigenically mismatched due to differences between circulating viruses or antigenic drift, resulting in lowered VE. While the predominant influenza strains vary each year, since 2013, the prevailing strains in Canada have been A(H1N1) and A(H3N2).Citation8 Lower VE against A(H3N2) viruses compared to A(H1N1) viruses have been documented in a number of influenza seasons,Citation6 including 2017/18Citation9 and 2018/19.Citation10 Reduction in VE, particularly against A(H3N2) viruses, may result from mutations acquired during egg-based vaccine manufacturing, and have been observed in previous influenza seasons.Citation7,Citation11,Citation12 Newer methods of influenza vaccine production, including recombinant and cell-culture based methods, have been developed to avoid occurrence of adaptive mutations and provide a better genetic match against circulating viral strains.Citation13
Plant-based production of influenza vaccines may address several limitations of currently licensed egg-derived vaccines. Transient expression of virus-like particles (VLP) in Nicotiana benthamiana plants enables production of quadrivalent influenza vaccines, QVLP, comprised of hemagglutinin proteins from two type A and two type B influenza strains.Citation14,Citation15 As with other recombinant and cell-culture based methods, plant-based production avoids the introduction of undesirable VE-reducing adaptive mutations that could be encountered in egg-based production.Citation14,Citation15 In addition, availability of plant-derived influenza vaccines may potentially increase vaccine uptake as a result of greater awareness of vaccine technologies and production processes by the segment of the population who prefer plant-derived products due to environmental or animal welfare concerns (e.g., strict or occasional vegetarians or vegans).
The safety and efficacy of a plant-derived QVLP influenza vaccine has been investigated in two global phase 3 studies.Citation16 The study populations were healthy adults aged 18–64 during the 2017/18 season and older adults aged 65+ in the 2018/19 season. Overall, the QVLP vaccine was well-tolerated and no major safety signals were identified. In the 18–64 study (n = 4818 QVLP; n = 4812 placebo) during a severe and prolonged influenza season, the absolute vaccine efficacy was 35.1% (95% CI: 17.9, 48.7) for the prevention of respiratory illness caused by vaccine-matched strains and 38.8% (95% CI: 27.8, 48.1) for the prevention of respiratory illness caused by any strain. In the 65+ study (n = 5996 QVLP; n = 6026 quadrivalent inactivated vaccine) during an even longer influenza season, the relative vaccine efficacy for the prevention of influenza-like illness caused by any virus strain was 8.8% (−16.7, 28.7) for the QVLP vaccine versus a quadrivalent inactivated vaccine (egg-based vaccine).
The objective of the current study is to simulate and compare the outcomes of seasonal influenza vaccination in Canada under settings of no influenza vaccination, only egg-derived vaccines (present-day scenario), and only plant-derived QVLP vaccines. The analysis estimates the number of additional influenza cases and influenza-related outcomes that could be prevented by the introduction of a QVLP influenza vaccine option in Canada.
Methods
Methodology
Monte Carlo simulations were used to model patient flow with multiple possible outcomes.Citation17 This approach was used to assess the impact of underlying uncertainty in input point estimates and to calculate the credible intervals around results. The conceptual framework of the model is depicted in Fig. S1. The simulations modeled three mutually exclusive settings where no influenza vaccines, only egg-derived vaccines, and only plant-derived QVLP vaccines were available.
The population was stratified into three groups: the susceptible, the immunized, and the infected. During each week of the modeled influenza season, the simulation determined the number of new cases considering the population not infected in the previous week and the vaccine coverage and attack rate for the current week. The susceptible population was renewed each week by removing those previously infected and immunized. The immunized population was derived from the proportion of the population vaccinated as well as the modeled VE. New cases led to increases in each of the outcomes evaluated: symptomatic and asymptomatic influenza-associated illness (referred to as influenza cases), symptomatic influenza-associated illness (referred to as symptomatic cases), general practitioner (GP) visits, emergency department (ED) visits, inpatient admissions, ICU admissions, and deaths. The model assumed that it was not possible to become infected twice during the same season. This assumption was made as rates for reinfection for the 2017/18 and 2018/19 influenza seasons were not reported in the literature. A graphic representation of the dynamic patient flow is available in the supplementary material Fig. S2.
The study population included two subgroups of Canadian adults, specifically, those aged 18–64 years and 65+ years. Canadian population estimates were obtained from Statistics Canada for those aged 18–64 in 2018 (n = 23,532,833), and for those aged 65+ in 2019 (n = 6,592,611).Citation18 Outcomes were further stratified by age into the following subgroups: 18–49, 50–64, 65–74, and 75+.
In the base case and each scenario analysis, patient flow was computed through the mean of 5000 simulations and 95% credible intervals were established around the point estimates for prevented cases and outcomes.
Model inputs in the base case analysis
All model inputs for the base case analysis are presented in Table S1.
Vaccine effectiveness
A frequentist indirect treatment comparisonCitation19 was conducted to compare the effectiveness of plant-derived QVLP and egg-derived vaccines using data obtained from QVLP randomized-controlled trials (RCTs) and real-world evidence (RWE) studies, respectively. Vaccine efficacy data obtained from an RCT was assumed to be equivalent to vaccine effectiveness (VE), where VE equaled the reduction in the risk of infection with the vaccine. In the absence of head-to-head VE data for each vaccine option versus placebo during the modeled influenza seasons, the analysis utilized RWE data for currently licensed egg-derived vaccines. RWE data were utilized from the same influenza season as the respective RCT to ensure that vaccine formulations and circulating strains had the greatest comparability. This approach was taken to account for the annual variation in circulating virus strains and differences in VE that would be consequently expected.
VE of the QVLP vaccine in the population aged 18–64 was sourced from a placebo-controlled, phase 3 RCT (CP-PRO-QVLP-012; NCT03301051) conducted during the 2017/18 season.Citation16 VE of egg-derived vaccines was obtained from a Centers for Disease Control & Prevention (CDC) RWE study, which examined the 2017/18 influenza season in the United States (US) using a test-negative design.Citation9 VE estimates for the US were used as a proxy for VE of egg-derived vaccines as only interim data was available for the 2017/18 season in Canada.Citation20 It was considered important to use end of season data due to the potential effects of intraseasonal waning immunity of egg-derived vaccines, which has been documented in previous seasons.Citation21
For the 65+ subgroup, VE of the QVLP vaccine was sourced from an active-comparator-controlled, phase 3 RCT (CP-PRO-QVLP-014; NCT03739112) conducted during the 2018/19 season.Citation16 VE of egg-derived vaccines was calculated using data from a CDC study that examined the 2018/19 influenza season in the US, and used a test-negative design.Citation10
Vaccine coverage
Vaccine coverage (VC) represented the total proportion of the Canadian population who were vaccinated during the course of the simulated influenza season. Base case VC was derived using data available from the PHAC for the 2017/18 influenza season, which was stratified for the 18–49 and 65+ subgroups.Citation1
Influenza case distribution and attack rates
The distribution of influenza cases in Canada was available from FluWatch for the population aged 18–64 in the 2017/18 season and for the population aged 65+ in the 2018/19 season, and correspond to the number of laboratory-confirmed influenza cases based on laboratory reporting.Citation22,Citation23 The baseline attack rates used in the analysis for the 18–49 and 50–64 subgroups were computed from the placebo arm of the CP-PRO-QVLP-012 RCT. The attack rate for the 65+ population was assumed to be equal to the attack rate of the 50–64 subgroup as CP-PRO-QVLP-014 was not a placebo-controlled trial.
Conditional probabilities of influenza-related outcomes
Conditional probabilities of GP visits, ED visits, inpatient admissions, and ICU admissions due to influenza were used to calculate model outcomes. These conditional probabilities were published by Molinari et al.,Citation24 with the exception of ICU admissions, which was sourced from Reed et al.Citation25
Model inputs in the scenario analyses
To understand how VC may be impacted by the introduction of the QVLP vaccine, an online survey was conducted to identify potential differences in willingness to be vaccinated with different influenza vaccine production technologies among the general adult population in the provinces of Alberta, British Columbia, Ontario, and Quebec (n = 802) in December 2019. This survey projected a 6.73% increase in VC with the introduction of plant-derived vaccines compared with currently available egg-derived vaccines with increased awareness of vaccine technologies and production [see supplementary material for survey questions). To model the impact of greater vaccine uptake, absolute VC was increased by 3%, 5%, or 6.73% over the base case inputs for the plant-derived QVLP vaccine only. In addition, scenarios considered the impact of varying absolute VE from base case inputs as well as using attack rates obtained from a RWE study by Molinari et al.Citation24
All scenarios are described in detail in .
Table 1. Description of scenario analyses
Results
Base case analysis
Results of the base case analysis are presented in for the Canadian population aged 18–64 and 65+. Full results stratified by age are presented in Tables S2–S4. In the base case analysis, outcomes with the egg-derived and plant-derived QVLP vaccines reflected differences in VE only, as all other variables were held constant (e.g., VC, attack rates). Plant-derived QVLP vaccines outperformed egg-derived vaccines in preventing influenza cases and influenza-related outcomes due to higher VE in both the 18–64 and 65+ subgroups.
Table 2. Number of influenza cases and influenza-related outcomes in the Canadian population aged 18–64 and 65+ with no vaccines, egg-derived vaccines only, and plant-derived QVLP vaccines only – base case results
In the 18–64 subgroup, the number of cases prevented with egg-derived vaccines versus no vaccination was 178,969 (95% CrI: 173,912–184,026), representing an 8.9% reduction ()). With plant-derived QVLP vaccines, an 11.3% reduction was observed, and 226,998 (95% CrI: 221,372–232,625) cases were prevented compared to no vaccination. Somewhat greater proportions of ED visits (9.6% vs 8.6%), inpatient admissions (9.6% vs 8.5%), and deaths (8.9% vs 8.4%) were prevented with plant-derived QVLP vaccines versus egg-derived vaccines.
Figure 1. Number of prevented influenza cases and influenza-related outcomes with plant-derived QVLP and egg-derived vaccines versus no vaccination in the base case analysis among the Canadian population (a) aged 18–64; and (b) aged 65+
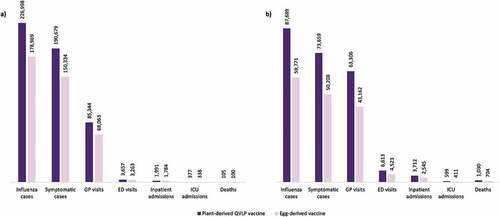
For the 65+ subgroup, the number of cases prevented with egg-derived vaccines versus no vaccination was 59,771 (Crl 95% 57,620–61,922), representing a 9.6% reduction ()). With plant-derived QVLP vaccines, a 13.6% reduction was observed, and 87,689 (Crl 95% 85,637–89,742) cases were prevented compared to no vaccination. Plant-derived QVLP vaccines were associated with substantially greater numbers of prevented ED visits (13.7% vs 9.3%), inpatient admissions (13.7% vs 9.4%), and deaths (13.7% vs 9.4%) compared to egg-derived vaccines.
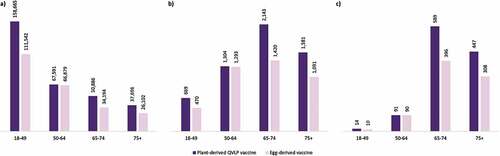
In the analysis stratified further by age, the majority of the cases prevented arose from the 18–49 subgroup ()). The greatest proportion of prevented inpatient admissions ()) and prevented deaths ()) occurred in the 65–74 subgroup. Overall, the 65+ subgroup represented approximately 65% of inpatient admissions and 91% of influenza deaths prevented with plant-derived QVLP vaccines compared to no vaccination.
Figure 2. Number of (a) prevented cases, (b) prevented inpatient admissions, and (c) prevented deaths with plant-derived QVLP and egg-derived vaccines versus no vaccination in the base case analysis among the adult Canadian population stratified by age
Scenario analyses
For each scenario, the number of influenza cases prevented with each vaccine option versus no vaccination was computed. These results are presented in for the 18–64 and 65+ subgroups, respectively. Results reported as positive values denote a higher number of prevented influenza cases compared to the base case analysis, while negative values denote fewer prevented cases. Complete results of scenario analyses are available in Tables S5–S7 and Tables S8–S10 for the 18–64 and 65+ subgroups, respectively.
Figure 3. Difference in the number of prevented influenza cases with plant-derived QVLP and egg-derived vaccines in scenario analyses relative to respective base case results among the Canadian population (a) aged 18–64a,b and (b) aged 65+.a–c
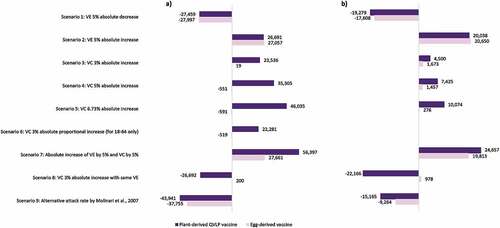
VE can vary with each influenza season based on factors such as the degree of match between the seasonal vaccine formulation and circulating influenza viruses.Citation6,Citation7 Scenario 1 examined the impact of a more severe influenza season with reduced vaccine protection by modeling a 5% absolute reduction in VE. In this scenario, the number of prevented cases with plant-derived QVLP vaccines were expectedly reduced from the base case by 12.10% (27,459; 95% CrI: 20,239–34,731) and 21.99% (19,279; 95% CrI: 16,362–22,196) for the 18–64 and 65+ subgroups, respectively. Scenario 2 modeled a 5% absolute increase in VE, which may be encountered in a season with greater degree of vaccine-virus matching. This translated to 11.76% (26,691; 95% Crl: 19,388–33,971) and 22.85% (20,038; 95% Crl: 17,161–22,914) fewer influenza cases with plant-derived QVLP vaccines over the base case for the 18–64 and 65+ subgroups, respectively.
An online survey among Canadian adults (n = 802; ≥18 years of age) identified a 6.73% absolute increase in willingness to get vaccinated with plant-derived vaccines among participants exposed to different influenza vaccine options potentially available (i.e., egg-derived, animal/mammalian cell-derived, insect cell-derived, and plant-derived), without detailed explanations about each (survey questionnaire and results are included in the supplementary material). The analysis explored more conservative increases in absolute VC of 3% (scenario 3) and 5% (scenario 4), in addition to 6.73% (scenario 5), with the plant-derived QVLP vaccine option in the two populations. For the 18–64 population, an additional scenario was tested where a 3% increase in VC was distributed proportionally for the age groups 18–49 and 50–64 based on the online survey findings (scenario 6). For scenarios 3–6, reductions in the number of influenza cases in the 18–64 subgroup ranged from 9.82% (22,281; 95% CrI: 15,087–29,414) with a 3% absolute increase in VC weighted by age to 20.28% (46,035; 95% Crl: 38,916–53,071) with a 6.73% absolute increase in VC. Scenarios 3–5 in the 65+ subgroup resulted in 5.13% (4500; 95% CrI: 1563–7437) to 11.49% (10,074; 95% CrI: 7129–13,020) fewer cases and 5.48% (56; 95% CrI: −73 to 186) to 11.68% (120; 95% Crl: −8 to 249) fewer deaths.
Overall, scenario analyses revealed that VE had a greater impact than VC on the number of influenza cases prevented with the plant-derived QVLP vaccine option for the population aged 65+. The number of prevented influenza cases was notably reduced from the base case in scenario 7 where absolute VE was equal for plant-derived QVLP and egg-derived vaccines, and absolute VC was increased by 3% for only plant-derived QVLP vaccines. Furthermore, a 5% absolute VE increase led to greater reduction in influenza cases (relative to no vaccination) when compared to a 5% absolute increase in VC for the 65–74 and 75+ subgroups (16.8% vs 14.4% and 16.8% vs 14.7%, respectively) (Figs. S3 and S4). In contrast, VC had somewhat greater impact than VE on the number of influenza cases prevented with plant-derived QVLP vaccines for those aged 18–64. An absolute VC increase of 5% translated to a higher number of prevented cases (relative to no vaccination) than an absolute VE increase of 5% in the 18–49 subgroup (15.6% vs 14.7%) and the 50–64 subgroup (9.9% vs 9.8%) (Figs. S3 and S4).
For both the 18–64 and 65+ subgroups, the most optimistic scenario for the plant-derived QVLP vaccine option was related to a simultaneous increase in absolute VC of 3% and absolute VE of 5% (scenario 8). In this scenario, 24.84% (56,397; 95% CrI: 49,169–63,596) and 28.12% (24,657; 95% CrI: 21,734–27,580) fewer influenza cases occurred for the 18–64 and 65+ subgroups, respectively. Stratified further by age, the greatest impacts were seen in the 65–74 and 75+ subgroups (Fig. S3); in each case, the number of influenza cases (relative to no vaccination) was reduced by 17.5%.
A less optimistic scenario resulted from modeling the impact of lower influenza attack rates reported in literature versus the base case rates observed in RCTs (scenario 9). The total number of cases was reduced from the base case by 19.36% (43,941; 95% CrI: 37,457–50,509) for the 18–64 subgroup and by 17.29% (15,165; 95% CrI: 12,504–17,825) for the 65+ subgroup.
Discussion
This study simulated the population benefits resulting from the introduction of a plant-derived QVLP influenza vaccine option in Canada for adults 18–64 and 65+ years of age. The model examined three mutually exclusive settings. Each vaccine option (egg-derived and plant-derived QVLP) was assumed to take up 100% of the total market share in their respective setting. In reality, the population outcomes resulting from the introduction of plant-derived QVLP vaccines would be related to the market share captured from egg-derived vaccine products available in Canada.
As expected, in the base case analysis, plant-derived QVLP vaccines outperformed egg-derived vaccines in preventing influenza cases and influenza-related outcomes due to greater VE. For the 18–64 subgroup, 2.63% more influenza cases were prevented with the plant-derived QVLP vaccines versus egg-derived vaccines over no vaccination. The majority of influenza-related outcomes prevented occurred in the 65+ age group. An estimated 4.82% more cases of influenza were prevented with the plant-derived QVLP vaccines over egg-derived vaccines in the base case analysis, leading to reductions in the number of inpatient admissions by approximately 1100 (4.77%) and deaths by 300 (4.75%). This result was expected as the elderly are more vulnerable to influenza-related morbidity and mortality.Citation1,Citation26 Overall findings suggest that plant-derived QVLP influenza vaccines may provide additional protection against seasonal influenza and influenza-associated outcomes compared to currently available egg-derived vaccines among the adult Canadian population. The introduction of plant-derived QVLP vaccines may therefore contribute to a further reduction in the overall burden of seasonal influenza in Canada.
The results of this analysis are consistent with a US mathematical model of the high-severity 2017/18 season, which projected substantial reductions in the number of influenza-related illnesses and hospitalizations with modest increases in VE or VC.Citation26 Among the adult population aged 18–49, a 5% absolute increase in VE was estimated to reduce the number of illnesses due to influenza by 173,000, compared to 213,000 with a 5% absolute increase in VC. Among adults aged 65+, a 5% absolute increase in VE was estimated to reduce the number of influenza-related hospitalizations by 19,000, compared to 5500 with a 5% absolute increase in VC. Similar findings were reached in the current analysis for the QVLP vaccine. Reductions in influenza-related outcomes were driven primarily by VE for the segment of the population aged 65+ and by VC in the population aged 18–64 (see Fig. S4). An absolute 5% increase in VE, as compared with an absolute 5% increase in VC, resulted in a greater number of prevented influenza cases (22.85% vs 8.47%) and inpatient admissions (22.92% vs 7.67%) for adults aged 65+. For the population aged 18–64, a 5% absolute increase in VE, as compared with a 5% absolute increase in VC, resulted in a lower number of prevented influenza cases (14.54% vs 15.40%) and inpatient admissions (8.23% vs 12.82%).
Improving VE is an important goal in preventing influenza-related outcomes. A(H3N2) viruses have been shown to be susceptible to antigenic change and undergo egg-adaptive mutations that reduce VE.Citation7,Citation11,Citation12 In a systematic review that covered influenza seasons between 2009 and 2016, pooled VE against A(H3N2) among those aged 65+ was estimated to be 43% in seasons with antigenic match between circulating and vaccine strains and 14% in seasons with antigenic mismatch.Citation27 Reduced VE against A(H3N2) viruses have been associated with increased number of influenza cases and outcomes among the elderly.Citation10 Plant-based influenza vaccine production entails transient expression of VLPs based on the genetic sequence of circulating viruses and avoids passaging in eggs. The likelihood of random egg-adaptive mutations is reduced with plant-derived QVLP vaccines as with other recombinant and cell-culture influenza vaccine production methods. As a result, QVLP vaccines and other recombinant or cell- based vaccines may offer greater protection in seasons where A(H3N2) viruses dominate.
Higher VC among the general population is another potential impact of QVLP influenza vaccine availability. Greater awareness of QVLP vaccine properties and advantages of plant-based vaccine production methods may potentially improve influenza vaccine uptake by a segment of the population. The analysis considered the impact of increased VC with the plant-derived QVLP vaccine on the proportion of prevented influenza-related outcomes. Previous analyses have identified the importance of increasing VC among younger adults for reducing the overall burden of influenza-associated illness and outcomes.Citation26,Citation28 Even with low VE, typical rates of vaccination among the general population have been projected to substantially reduce infections and influenza-related outcomes.Citation28 One analysis from the US perspective estimated that 21 million influenza infections could be prevented with a VE of 20% and VC of 43% compared to no vaccination.Citation28 The age-structured model showed greater sensitivity to VC than VE whereby increasing coverage among those aged 5–19 years and 30–39 years led to the greatest reduction in the burden of influenza when VE was low. In this analysis, a conservative absolute VC increase of 3% for plant-derived QVLP vaccines translated to 10.37% and 5.13% fewer influenza cases in the population aged 18–64 and 65+, respectively. Overall, results of this analysis agree with previous reports whereby an incremental increase in VE, supplemented by an increase in VC, led to a substantial reduction in the burden of seasonal influenza, especially among the population aged 65+.
Limitations
The current analysis has several limitations to note. In the absence of head-to-head VE data for each vaccine option versus placebo, the analysis utilized RWE data for currently licensed egg-derived vaccines. This limitation was addressed by sourcing RWE data from the same influenza season as the QVLP RCTs to ensure that the vaccine formulations and circulating strains had the greatest comparability, and therefore allowed for a more accurate comparison of plant-derived QVLP and egg-derived vaccines. However, model inputs for VE of egg-derived vaccines relied on RWE data from the US as end-of-season Canadian RWE data were not available; this introduced several biases.
The predominant circulating strains differed in the 2018/19 season between the two countries, where the predominant strain was A(H1N1) in Canada and A(H3N2) in the US. As egg-derived influenza vaccines are typically less effective against A(H3N2),Citation10 the analysis could have overestimated the prevented outcomes with plant-derived QVLP vaccines in the 65+ age group. The model also used US data to estimate conditional probabilities of influenza-related outcomes in the absence of Canadian data, which may have introduced bias due to differences in healthcare systems or coding of admissions.
Next, the utilization of egg-derived vaccine options differed in Canada and the US for the two influenza seasons considered. During the 2017/18 season, 97% of the vaccinated population under 65 received a quadrivalent vaccine in the US,Citation9 while in Canada an estimated 29% of administered vaccines were quadrivalent (based on data available for publicly-funded influenza vaccine doses purchased in provinces participating in the Canadian Sentinel Practitioner Surveillance Network).Citation29 This difference may bias VE and result in the overestimation of prevented outcomes with egg-derived vaccines in the 18–64 population. During the 2018/19 influenza season, an estimated 51% of vaccinated adults aged 65+ received the high dose inactivated trivalent vaccine (IIV3-HD) in the US.Citation10 In contrast, the active comparator in the RCT CP-PRO-QVLP-014 was the standard dose quadrivalent inactivated influenza vaccine (IIV4). The IIV3-HD vaccine has demonstrated greater efficacy and effectiveness against symptomatic influenza than the standard trivalent inactivated vaccine during the 2011/12 and the 2012/13 seasons,Citation30,Citation31 although no direct head-to head comparison has been carried out during the 2018/19 season or against the IIV4 vaccine during any influenza season. Considering these factors, the analysis could have overestimated prevented outcomes with egg-derived and plant-derived QVLP vaccines among the 65+ population. The analysis did not consider other recombinant or cell-culture based influenza vaccine options as these products were not available in Canada during the modeled influenza seasons of 2017/18 and 2018/19. However, a cell-based influenza vaccine option has since been approved by Health Canada.Citation32
In the analysis, the proportion of the population vaccinated each month was sourced from a survey;Citation1 this could have introduced bias if timing of vaccinations were misreported by participants. Influenza-related outcomes were modeled separately for the 18–64 and 65+ populations. As a result, the model did not consider the risk of influenza transmission between age subgroups or protection from seasonal influenza with vaccination through decreased exposure to infected individuals. Finally, Monte Carlo models do not account for indirect protection from herd immunity generated by vaccines. The incremental benefits of herd immunity increase with increasing vaccine-derived immunity, especially for an infectious disease such as influenza with a relatively low basic reproduction number.Citation33 This analysis may therefore underestimate the benefits of incremental increases in VC and VE.
Conclusion
In summary, this analysis estimated the number of prevented influenza cases and influenza-related outcomes that could result from the introduction of a plant-derived QVLP influenza vaccine option in Canada. In the base case analysis, plant-derived QVLP vaccines were associated with greater reductions in influenza cases and influenza-related outcomes compared to currently available egg-derived vaccines for the two modeled populations of adults aged 18–64 and 65+. In scenario analyses, reductions in influenza-related outcomes with QVLP vaccines were driven primarily by improvements in VC for the 18–64 subgroup and by improvements in VE for the 65+ subgroup. The findings suggest that the introduction of plant-derived QVLP vaccines could substantially reduce the burden of seasonal influenza in Canada through a combination of improved VE and increased vaccine uptake.
Author contributions
All authors contributed to the study design and data collection. L Ramjee, W Lemay, and G Tremblay developed the mathematical modeling and performed the analysis. The manuscript draft was written by N Vurgun and all authors reviewed, commented on, and approved the final manuscript.
Disclosure of potential conflicts of interest
L Ramjee, W Lemay, N Vurgun, and G Tremblay are employees of Purple Squirrel Economics, which received financial support from Medicago Inc. to conduct the analysis and for manuscript development. C Bauch, G Pullagura, and S Houle received honorariums at fair market value from Medicago Inc. as key opinion leaders. N Charland is an employee of Medicago Inc.
Ethical approval
The article does not involve any studies with human participants or animals.
Supplemental Material
Download MS Word (333.3 KB)Acknowledgments
Medicago wishes to acknowledge all the volunteers who participated in the QVLP clinical trials as well as the site investigators and their staff who conducted the studies with a high degree of professionalism.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1908797.
Additional information
Funding
References
- Public Health Agency of Canada. Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2017–2018. 2017 [accessed 2020 Apr 13]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2017-2018.html .
- Schanzer DL, McGeer A, Morris K. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada: influenza hospitalization multipliers. Influenza Other Respir Viruses. 2013;7:799–808. doi:10.1111/irv.12011 .
- Ng C, Ye L, Noorduyn SG, Hux M, Thommes E, Goeree R, Ambrose A, Andrew MK, Hatchette T, Boivin G, et al. Resource utilization and cost of influenza requiring hospitalization in Canadian adults: a study from the serious outcomes surveillance network of the Canadian Immunization Research Network. Influenza Other Respir Viruses. 2018;12:232–40. doi:10.1111/irv.12521 .
- Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T, Nishiura H. Estimating influenza deaths in Canada, 1992–2009. PLoS One. 2013;8:e80481. doi:10.1371/journal.pone.0080481 .
- National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2018–2019; 2018.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16:942–51. doi:10.1016/S1473-3099(16)00129-8.
- Lewnard J, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines. 2018;6:28. doi:10.3390/vaccines6020028.
- Public Health Agency of Canada. FluWatch annual report: 2018–19 influenza season. 2020 [accessed 2020 Apr 13]. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fluwatch/2018-2019/annual-report.html .
- Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. 2019;69:1845–53. doi:10.1093/cid/ciz075.
- Flannery B, Kondor RJG, Chung JR, Gaglani M, Reis M, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Mono AS, Martin ET, Belongia EA, McLean HQ, Kim SS, Blanton L, Kniss K, Budd AP, Brammer L, Stark TJ, Barnes JR, Wentworth DE, Fry AM, Patel M. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis Jiz. 2019;543. doi:10.1093/infdis/jiz543.
- Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, Fonseca K, Winter A-L, Gubbay JB, Krajden M, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9:e92153. doi:10.1371/journal.pone.0092153.
- Wu NC, Zost SJ, Thompson AJ, Oyen D, Nycholat CM, McBride R, Paulson JC, Hensley SE, Wilson IA. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLOS Pathog. 2017;13:e1006682. doi:10.1371/journal.ppat.1006682.
- Barr IG, Donis RO, Katz JM, McCauley JW, Odagiri T, Trusheim H, Tsai TF, Wentworth DE. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. Npj Vaccines. 2018;3:44. doi:10.1038/s41541-018-0079-z.
- D’Aoust M-A, Couture MM-J, Charland N, Trépanier S, Landry N, Ors F, Vézina L-P. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza: pandemic influenza vaccines from plants. Plant Biotechnol J. 2010;8:607–19. doi:10.1111/j.1467-7652.2009.00496.x.
- Le Mauff F, Mercier G, Chan P, Burel C, Vaudry D, Bardor M, Vézina L-P, Couture M, Lerouge P, Landry N, et al. Biochemical composition of haemagglutinin-based influenza virus-like particle vaccine produced by transient expression in tobacco plants. Plant Biotechnol J. 2015;13:717–25. doi:10.1111/pbi.12301.
- Ward BJ, Makarkov A, Séguin A, Pillet S, Trépanier S, Dhaliwall J, Libman MD, Vesikari T, Landry N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): two multicentre, randomised phase 3 trials. Lancet. 2020;396:1491–503. doi:10.1016/S0140-6736(20)32014-6.
- Jackson CH, Thompson SG, Sharples LD. Accounting for uncertainty in health economic decision models by using model averaging. J R Stat Soc Ser A Stat Soc. 2009;172:383–404. doi:10.1111/j.1467-985X.2008.00573.x .
- Statistics Canada. Table 17-10-0005-01 population estimates on July 1st, by age and sex. 2020 [accessed 2020 May 28]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 .
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi:10.1016/S0895-4356(97)00049-8 .
- Newall AT, Chen C, Wood JG, Stockwell MS. Within-season influenza vaccine waning suggests potential net benefits to delayed vaccination in older adults in the United States. Vaccine. 2018;36:5910–15. doi:10.1016/j.vaccine.2018.08.007 .
- Puig-Barberà J, Mira-Iglesias A, Tortajada-Girbés M, López-Labrador FX, Librero-López J, Díez-Domingo J, Carballido-Fernández M, Carratalá-Munuera C, Correcher-Medina P, Gil-Guillén V, et al. Waning protection of influenza vaccination during four influenza seasons, 2011/2012 to 2014/2015. Vaccine. 2017;35:5799–807. doi:10.1016/j.vaccine.2017.09.035 .
- PHAC. Influenza weekly reports 2017–18 season. 2018 [accessed 2020 July 13]. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance/weekly-reports-2017-2018-season.html .
- PHAC. Influenza weekly reports 2018–19 season. 2019 [accessed 2020 July 13]. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance/weekly-reports-2018-2019-season.html .
- Molinari NAM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi:10.1016/j.vaccine.2007.03.046 .
- Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, Butler L, Baumbach J, Hollick G, Bennett NM, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10:e0118369. doi:10.1371/journal.pone.0118369 .
- Hughes MM, Reed C, Flannery B, Garg S, Singleton JA, Fry AM, Rolfes MA. Projected population benefit of increased effectiveness and coverage of influenza vaccination on influenza burden in the United States. Clin Infect Dis Ciz. 2019;676. doi:10.1093/cid/ciz676 .
- Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect. 2017;75:381–94. doi:10.1016/j.jinf.2017.09.010 .
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018;115:5151–56. doi:10.1073/pnas.1802479115 .
- Skowronski DM, Chambers C, De Serres G, Sabaiduc S, Winter A-L, Dickinson JA, Gubbay JB, Drews SJ, Fonseca K, Charest H, et al. Vaccine effectiveness against lineage-matched and -mismatched influenza B viruses across 8 seasons in Canada, 2010–2011 to 2017–2018. Clin Infect Dis. 2019;68:1754–57. doi:10.1093/cid/ciy876 .
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45. doi:10.1056/NEJMoa1315727 .
- DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: a comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33:4988–93. doi:10.1016/j.vaccine.2015.07.006 .
- Sinilaite A, Gemmill I, Harrison R. Summary of the NACI supplemental statement on mammalian cell culture-based influenza vaccines. Can Commun Dis Rep. 2020;46:324–32. doi:10.14745/ccdr.v46i10a03 .
- Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis. 2011;52:911–16. doi:10.1093/cid/cir007 .
- CDC. “CDC Seasonal Flu Vaccine Effectiveness Studies”. 2020 [accessed 17 Jul 2020]. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm .
