ABSTRACT
The switch from using only trivalent oral polio vaccine (tOPV) to sequential schedules combining inactivated poliovirus vaccine (IPV) and bivalent oral polio vaccine (bOPV) for polio vaccination will cause changes to mucosal immunity against polio in infants, which plays an important role in preventing the poliovirus spread. Here, we analyzed mucosal immunity against poliovirus in the intestine during different sequential vaccination schedules. We conducted clinical trials in Guangxi Province, China on 1,200 2-month-old infants who were randomly assigned to one of three vaccination schedule groups: IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, with vaccine doses administered at 8, 12, and 16 weeks of age. Stool samples were collected from 10% of participants in each group before administration of the second vaccine doses and at 1, 2, and 4 weeks after the administrations of the second and third vaccine doses. Immunoglobulin A (IgA) in the stool samples was measured to analyze the mucosal immune response in the intestine. Because of the absence of poliovirus type 2 in bOPV, the vaccination schedule of IPV-IPV-bOPV did not sufficiently raise intestinal mucosal immunity against poliovirus type 2, although some cross-immunity was seen. The level of intestinal mucosal immunity was related to shedding status; shedders could produce intestinal mucosa IgA more quickly. The intestinal mucosal immunity level was not related to serum neutralizing antibody level. In the combined sequential vaccination schedule of IPV and bOPV, the risk of circulating vaccine-derived poliovirus type 2 (cVDPV2) may be increased owing to insufficient intestinal mucosal immunity against poliovirus type 2.
Introduction
Wild poliovirus (WPV) type 2 (WPV2) was declared eradicated by the Global Certification Commission on Polio Eradication (GCC) in September 2015, with WPV type 3 (WPV3) achieving a similar status in October 2019.Citation1 The oral poliovirus vaccine (OPV), a polio vaccine that was once widely used, greatly reduced the incidence of polio and has made a significant contribution to its prevention and control. OPV can induce high levels of intestinal mucosal immunity that prevent the spread of poliovirus along the fecal-oral route, which has been key for the rapid reduction in the incidence of polio infections, especially in developing countries.Citation2,Citation3 However, because OPV can cause both vaccine-associated paralytic polio (VAPP) cases and circulating vaccine-derived polioviruses (cVDPVs) and the number of poliomyelitis cases induced by WPV has gradually reduced, the World Health Organization (WHO)’s Strategic Advisory Group of Experts on Immunization (SAGE) proposed the gradual replacement of OPV with inactivated poliovirus vaccine (IPV). The first step in this strategy was to remove the polioviru type 2(PV2) components from OPV, thus producing bivalent OPV (bOPV, containing poliovirus types 1 [PV1] and 3 [PV3]).Citation4
Since May 2016, many countries, including China, have used an IPV and bOPV, combined sequential vaccination schedule in their major polio immunization program. However, although IPV does not directly cause virus shedding, because of its limited ability to cause specific intestinal immune responses to polio and the rapid inhibition of poliovirus replication by mucosal immunity, once individuals who were previously vaccinated with IPV are exposed to poliovirus or vaccine attenuated strains, virus replication and shedding will arise.Citation5–7 In fact, the proportion of shedders among IPV-vaccinated individuals who receive OPV is similar to that among previously non-vaccinated OPV recipients.Citation8 Unlike those vaccinated with the trivalent (t)OPV vaccination schedule, recipients of the new immunization strategy are not vaccinated with live attenuated PV2 vaccine, and this difference will cause some changes in the individual level of intestinal mucosal immunity as well as in the level of immunity against poliovirus within the population. During this transition phase of replacing OPV with IPV, there will be a long period in which IPV and bOPV must coexist, which brings uncertainty and complexity to the prevention and control of polio.
Data from clinical studies have shown that the immune strategy of vaccination with two doses of bOPV after one dose of IPV reduces serum neutralizing antibody levels against PV2 in the population.Citation9 As reported by Wright et al.,Citation10 more than half of the Latin American infants who received three doses of bOPV after receiving one dose of monovalent OPV type 2 (mOPV2) were found to be shedding, whereas only two infants were found to be shedding after being vaccinated with three doses of tOPV. Vaccination with OPV induces an effective specific mucosal antibody response, whereas IPV-induced mucosal immunity is limited. Finding ways to enhance mucosal immunity after the vaccine strategy switch has become an important issue.
Regarding the new polio vaccination schedules, detecting the status of intestinal mucosal immunity against poliovirus and analyzing the impact of different immune strategies on polio mucosal immunity are necessary for guiding the formulation of China’s polio immune prevention and control strategies and maintaining the country’s polio-free status. For this reason, based on previous randomized, blind, single-center, parallel-controlled clinical trials conducted in the China region, we further tested the efficiency of the polio-specific intestinal mucosal immune responses under variations of the new polio vaccination schedule strategy.
Materials and methods
Experimental design
During 2015–2016, randomized, parallel-controlled clinical trials were conducted in Guangxi Province, China; these trials were designed by the Institute of Medical Biology, Chinese Academy of Medical Sciences and the Guangxi Center for Disease Prevention and Control and were approved by the State Food and Drug Administration of China. The clinical trial protocol was verified and approved by the ethical committee of Guangxi Zhuang Autonomous Region (ClinicalTrials.gov ID: NCT03614702).
In total, 1,200 two-month-old infants, who had not yet received any vaccinations against polio, were recruited. The guardians of the included participants and their families confirmed that they were willing to voluntarily comply with the requirements of the clinical trial protocol; both the guardians of the participants and the research doctor signed an informed consent form prior to study enrollment. Participants were permitted to voluntarily withdraw at any time during the trial. An assignment table was used to randomly divided the participants into the following three groups: IPV-IPV-tOPV, IPV-IPV-bOPV and IPV-bOPV-bOPV. In all groups, the three immunizations were administered at 0, 28, and 56 days. Antibodies against poliovirus types 1, 2, and 3 were measured before vaccination and at 28 days after full vaccination. Stool collection was performed on the first 10% of subjects enrolled in each group. The participants’ guardians were requested to collect a stool sample before administration of the second vaccine dose and bring it to the vaccination site. Stool samples were also collected at 7, 14, and 28 days after the administrations of the second and third vaccine doses. Upon receipt, the stool samples were transferred into plastic bottles, labeled with the research number and collection time, and stored at −20°C until use in testing.
Measurement of stool IgA
Stool samples (1 g) were homogenized after adding 5 ml of phosphate buffered saline (PBS) (pH 7.4). The resulting supernatants were collected after the samples had been centrifuged for 20 min at 800–1,000 × g, and then used in enzyme-linked immunosorbent assays (ELISAs) to measure the level of anti-poliovirus IgA. Anti-human PV1, PV2, and PV3 IgA (PV1-IgA, PV2-IgA, and PV3-IgA, respectively) and ELISA kits for detecting PV1-IgA (Cat. No. KT37265), PV2-IgA (Cat. No. KT53156), and PV3-IgA (Cat. No. KT22871) were purchased from MSKBIO (Wuhan, China). Five concentration points (with dilution factors of 1:2, 1:4, 1:8, 1:16, and 1:32) and 10 wells were used for the standards, with parallel wells for each concentration. The initial concentrations of the PV1-IgA, PV2-IgA, and PV3-IgA standards were 24 U/L, 16 U/L, and 8 U/L, respectively; 50-µl of aliquots of each standard dilution were added, with an empty well used as a blank control. After the samples were loaded into the remaining wells, the plate was sealed with a plate membrane and incubated for 30 min at 37°C. After the wells were washed five times with wash solution (30-s incubation per wash). 50 µl of horseradish peroxidase (HRP)-conjugated reagent was added to each well (except the blank control), incubated, and washed as described above. Finally, 50 µl of Chromogen Solution A and B were added to each well, mixed by gentle shaking, and incubated at 37°C for 15 min. Reactions were terminated by adding 50 µl of stop solution to each well. The absorbances of the wells were read at an optical density (OD) of 450 nm using a Microtiter Plate Reader. The OD value of the blank control well was set as zero. Taking the concentration of the standards as the abscissa and the OD value as the ordinate, a standard curve was plotted, and the corresponding relative concentration was determined by the standard curve according to the OD value of the sample.
Serum neutralizing antibody testing
Venous blood samples collected before the first polio vaccine dose and at 28 days after the third polio vaccine dose were used by the National Institutes for Food and Drug Control to test the anti-poliovirus titer and seroconversion rate in accordance with the protocol recommended by the WHO. Seroconversion was defined as a neutralizing antibody titer of <1:8 before polio vaccination and of >1:8 against PV1, PV2, and PV3 after administration of three doses of polio vaccine. A neutralizing antibody titer of ≥1:8 before polio vaccination was considered to indicate the presence of maternally transferred antibodies; for such cases, seroconversion was defined as a four-fold increase in the polio-specific antibody response after three doses of polio vaccine. The changes in anti-poliovirus antibody titer after vaccination were analyzed. Both the maximum dilution and the maximum reported titer were 16,384; in cases where the actual titer was greater than 16,384, the value used in calculations was 16,384.
Statistical analyses
All statistical tests used were two-sided, and values of p < .05 were considered to indicate a statistically significant difference. An analysis of variance (ANOVA) was used to detect differences in the relative concentration of IgA between the vaccination schedule groups. The t-test was used for the comparison of means between two groups. The correlation between the relative concentration of mucosal IgA and log2 serum neutralization titers was measured by Pearson χ2 test. Statistical analysis was conducted using the software SPSS.16.0.
Results
Intestinal mucosal immune responses induced by different sequential vaccination schedules
A total of 1,200 healthy 2-month-old infants were enrolled in the clinical trials conducted in Guangxi Province, China and randomly assigned to one of the following three vaccination schedules: IPV-bOPV-bOPV, IPV-IPV-tOPV, or IPV-IPV-bOPV. The first, second, and third vaccine doses were administered at 8, 12, and 16 weeks of age, respectively (). Fecal samples were collected from 10% of the participants to detect poliovirus-specific IgA antibodies in the stool for analysis of the intestinal mucosal immune response (). Regardless of the immunization program group, IPV was used for the first vaccine dose, so stool samples were collected and analyzed starting just prior to administration of the second vaccine dose. Fecal samples were collected at seven timepoints: before administration of the second vaccine dose and at 1, 2, and 4 weeks after the administrations of the second and third vaccine doses (). A total of 797 fecal samples were collected; the numbers of samples collected per group at each timepoint are shown in and Supplementary Table 1.
Figure 1. Timepoints of sample collection. The three vaccine doses were administered at the ages of 8, 12, and 16 weeks old, respectively. Stool samples were collected seven times from a subset of the participants (before administration of the second vaccine dose and at 1, 2, and 4 weeks after the administrations of the second and third vaccine doses)
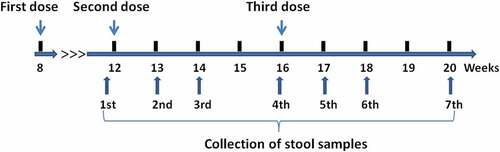
Figure 2. Trial enrollment and outcome profile. Stool samples were collected before administration of the second vaccine dose and at 1, 2, and 4 weeks after the administrations of the second and third vaccine doses, yielding a total of 797 samples (owing to some participants failing to provide samples at all timepoints, not all participants have data for each sampling timepoint). bOPV, bivalent (poliovirus types 1 and 3) oral polio vaccine; IPV, inactivated polio vaccine; tOPV, trivalent oral polio vaccine
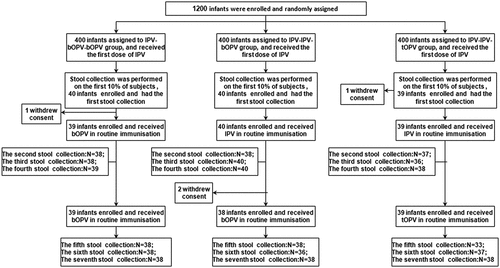
The results of IgA detection in the stool samples showed that after administration of the second vaccine dose, there was a significant difference in the relative concentrations of IgA between those vaccinated with IPV and those vaccinated with bOPV (). Because bOPV is a live attenuated oral vaccines containing PV1 and PV3, the mucosal immune responses to PV1 and PV3 in the infants vaccinated with bOPV was significantly higher than those of infants vaccinated with IPV after administration of the second vaccine dose ( and Supplementary Table 2; mean of PV1-IgA at 13 weeks: 0.22, −0.13, and −0.12 for IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, respectively, p < .05; mean of PV3-IgA at 14 weeks: 0.25, 0.04, and 0.03 for IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, respectively, p < .01 by ANOVA). Some PV2-IgA could also be detected in participants who received a single dose of bOPV.
Table 1. Poliovirus-specific intenstinal mucosal IgA immunized with IPV-bOPV-bOPV, IPV-IPV-tOPV, IPV-IPV-tOPV
Although all three vaccination schedule groups received an OPV (bOPV or tOPV) as the final vaccine dose, we found varying degrees of mucosal immunity were elicited after administration of the third vaccine dose.
For PV2-IgA, similar relative concentrations were detected in the IPV-bOPV-bOPV and IPV-IPV-tOPV groups after administration of the third vaccine dose, both of which were significantly higher than that in the IPV-IPV-bOPV group ( and Supplementary Table 2; mean of PV2-IgA at 18 weeks of age: 0.49, 0.21, and 0.40 for IPV-IPV-tOPV, IPV-IPV-bOPV, and IPV-bOPV-bOPV, respectively; IPV-IPV-tOPV vs IPV-IPV-bOPV, p < .01 by t-test; IPV-IPV-bOPV vs IPV-bOPV-bOPV, p < .05 by t-test). Four weeks after the third dose of vaccine, both PV1-IgA and PV3-IgA could be detected in all groups, but PV2-IgA was undetectable in the IPV-IPV-bOPV group.
For PV1-IgA, the administration of bOPV (as the second vaccine dose) followed by a second administration of bOPV stimulated mucosal immunity more rapidly compared with the other tested vaccination schedules. One week after administration of the third vaccine dose (17 weeks of age), the relative concentration of PV1-IgA in the IPV-bOPV-bOPV group was higher than those in the IPV-IPV-bOPV and IPV-IPV-tOPV groups ( and Supplementary Table 2; mean of PV1-IgA at 17 weeks of age: 0.65, 0.23, and 0.34 for IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, respectively; IPV-bOPV-bOPV vs IPV-IPV-tOPV, p < .01 by t-test; IPV-bOPV-bOPV vs IPV-IPV-bOPV, p < .05 by t-test). However, the level of PV1-IgA increased in the IPV-IPV-tOPV group at the second week after administration of the third vaccine dose and was significantly higher than those in the other two groups ( and Supplementary Table 2; mean of PV1-IgA at 18 weeks of age: 0.56, 0.77, and 0.44 for IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, respectively; IPV-bOPV-bOPV vs IPV-IPV-tOPV, p < .05 by t-test; IPV-bOPV-bOPV vs IPV-IPV-bOPV, p < .001 by t-test).
For PV3-IgA, the relative concentration in the IPV-bOPV-bOPV group trended slightly higher than those in the other two groups, but this difference was not statistically significant. Two weeks after administration of the third vaccine dose (at 18 weeks of age), the relative concentrations of PV3-IgA in the IPV-bOPV-bOPV and IPV-IPV-tOPV groups were similar and significantly higher than in the IPV-IPV-bOPV group ( and Supplementary Table 2; mean of PV3-IgA at 18 weeks of age: 0.32, 0.32, and 0.18 for IPV-bOPV-bOPV, IPV-IPV-tOPV, and IPV-IPV-bOPV, respectively; IPV-bOPV-bOPV vs IPV-IPV-bOPV, p < .01 by t-test; IPV-IPV-tOPV vs IPV-IPV-bOPV, p < .01 by t-test). In terms of poliovirus type, the overall IgA levels after administration of the third vaccine dose were: PV1-IgA > PV2-IgA > PV3-IgA. In addition, we observed that even in the absence of OPV2 (i.e., in the IPV-bOPV-bOPV group), the level of PV2-IgA was slightly higher than that of PV3-IgA after the third dose of polio vaccine. This may be the result of differences in the mucosal immunity induced by different types of polio vaccine.
Effect of viral shedding on the mucosal immune response
We classified the participants as shedders or non-shedders according to shedding status after administration of the third polio vaccine dose and analyzed their respective intestinal mucosal response to each poliovirus type. The polio-specific intestinal mucosal IgA growth curves of shedders and non-shedders had different characteristics. Compared with non-shedders, shedders showed a rapid stimulation of mucosal immune responses (). Regardless of the vaccination schedule, for most of groups, at one week (17 weeks of age) after receiving a dose of OPV, the level of mucosal IgA produced by the shedders was significantly increased from previous levels, unlike that produced by the non-shedders, which did not show much change over the same period. At two weeks (17–18 weeks of age) after receiving a dose of OPV, the growth rate of mucosal IgA in the shedders began to decline, whereas the opposite trend was seen for the non-shedders during this period. At three to four weeks (18–20 weeks of age) after receiving a dose of OPV, the mucosal IgA level of non-shedders was maintained at a relatively stable level (), whereas the relative concentrations of mucosal IgA in the shedders began to decline significantly during this period ().
Figure 3. The relative concentration of intestinal mucosal IgA specific to each type of poliovirus between shedders and non-shedders in each sequential vaccination schedule. The third vaccine dose was administered at 16 weeks of age. Participants were classified as shedders or non-shedders according to their viral shedding status after administration of the third vaccine dose. (a–c) The relative concentration of PV1-IgA between shedders (n = 11) and non-shedders (n = 28) in IPV-bOPV-bOPV (a), shedders (n = 21) and non-shedders (n = 18) in IPV-IPV-tOPV (b), and shedders (n = 18) and non-shedders (n = 20) in IPV-IPV-bOPV (c). (d–f) The relative concentration of PV3-IgA between shedders (n = 8) and non-shedders (n = 31) in IPV-bOPV-bOPV (d), shedders (n = 19) and non-shedders (n = 20) in IPV-IPV-tOPV (e), and shedders (n = 24) and non-shedders (n = 14) in IPV-IPV-bOPV (f). (g) The relative concentration of PV2-IgA between shedders (n = 24) and non-shedders (n = 15) in IPV-IPV-tOPV. A t-test was used for comparison of the means of intestinal mucosal IgA between the shedders and non-shedders at the same timepoints. *p < .05, **p < .01, ***p < .001
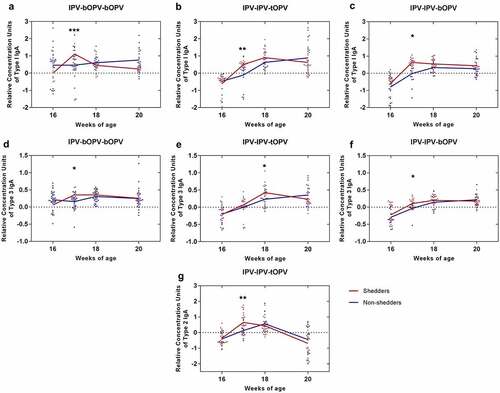
We found that the levels of PV1- IgA and PV3-IgA in infants who were classified as non-shedders after administration of the last dose of bOPV in the IPV-bOPV-bOPV group were higher than those of shedders at the time of last bOPV inoculation (16 weeks of age) (). This finding suggests that the shedding rate was reduced by the mucosal immunity induced by the previous dose of bOPV.
Relationship between intestinal mucosal immunity and serum neutralizing antibody
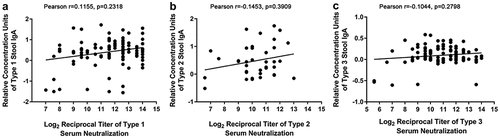
Previous trials have not found any significant differences in the levels of PV1- and PV3-specific serum neutralizing antibodies of infants vaccinated with IPV-IPV-bOPV, IPV-IPV-tOPV, or IPV-bOPV-bOPV, which all reached nearly 100%. Here, we measured whether there is a correlation between the relative concentration of poliovirus-specific intestinal IgA and serum neutralizing antibodies. Because only the infants in the IPV-IPV-tOPV group were vaccinated with OPV2 and the PV2-specific serum neutralizing antibody level can reach close to 100%, we used the data from the IPV-IPV-tOPV group when analyzing the correlation between PV2-IgA and anti-PV2 serum neutralizing antibodies. The analysis revealed that the relative concentrations of intestinal IgA specific for each poliovirus type were not related to the levels of serum poliovirus neutralizing antibodies (, Pearson: r = 0.1115, p = .2318 for PV1-IgA and PV1 serum neutralization titer; r = 0.1453, p = .3909 for PV2-IgA and PV2 serum neutralization titer; r = 0.1044, p = .2798 for PV3-IgA and PV3 serum neutralization titer). We further analyzed the relationship between serum neutralizing antibodies and IgA in each immunization program. The results show that there was no significant correlation between the levels of serum neutralizing antibodies and IgA, regardless of which immunization program was performed, although the relative concentration of IgA induced by different immunization programs varied (Supplementary Figure 1).
Figure 4. Relationship between intestinal mucosal immunity and serum neutralizing antibody . (a) PV1-IgA and PV1-specific serum neutralizing antibody at one week after administration of the last vaccine dose (17 weeks of age; n = 109). (b) PV2-IgA and PV2-specific serum neutralizing antibody at 17 weeks of age (n = 38); data from the IPV-IPV-tOPV group only. (c) PV3-IgA and PV3-specific serum neutralizing antibody at 17 weeks of age (n = 109). The x-coordinate of each point corresponds to the log2 reciprocal titer of serum neutralization; the y-coordinate of each point corresponds to the relative concentration units of IgA
Discussion
Immunization with live attenuated polio vaccine mimics natural viral infection because the virus replicates at the natural site of infection, i.e., in the intestine, where it induces a local secretory IgA response, thereby reducing the intestinal shedding of poliovirus.Citation11 To reduce the probability of generating cVDPVs in polio vaccine recipients, enhancing the polio-specific mucosal immunity generated by vaccination is critical.Citation12 We found that the participants in the IPV-bOPV-bOPV group who were classified as non-shedders after receiving the second dose of bOPV had a slightly higher level of IgA than those classified as shedders. The IgA triggered by the first dose of bOPV appears to reduce shedding by infants when they are re-exposed to poliovirus.
Because bOPV does not contain the OPV2 component, the IPV-IPV-bOPV and IPV-bOPV-bOPV vaccination schedules do not cause sufficiently high serum neutralizing antibody levels in the population.Citation9 Furthermore, the intestinal mucosal PV2-IgA response is also insufficient owing to the limited ability of IPV to induce IgA. However, we detected enteric PV2-IgA in some of the infants in the IPV-IPV-bOPV or IPV-bOPV-bOPV vaccination schedule groups after the administration of bOPV. In this study, the level of PV2-IgA detected in the IPV-bOPV-bOPV group, which received two doses of bOPV, was similar to that detected in the IPV-IPV-tOPV group, which received one dose of tOPV. This may be the result of cross-immunity induced by bOPV against PV2 or may indicate that bOPV can enhance the mucosal response induced by IPV, even to PV2.Citation13 Additionally, some participants may have been exposed to PV2 during this study.
We observed that, compared with the non-shedders, relatively high levels of IgA were detected earlier after OPV inoculation in the shedders, but the IgA levels in the shedders then decreased faster than those of the non-shedders. It may be that the attenuated poliovirus strains replicate in a short period of time in the intestinal tracts of the shedders, consequently making their intestinal tracts more receptive to a stronger mucosal immune response, such that the amount of IgA secreted by the shedders is higher than that secreted by the non-shedders in the early stage. With the produced poliovirus-specific IgA then neutralizing the poliovirus replicating within the intestinal tract of shedders, the IgA is consumed rapidly.
The complete eradication of polio includes not only the elimination of WPV but also the elimination of cVDPV.Citation14 In situations where sanitation is poor and fecal-oral transmission is dominant, OPV is a more effective vaccine than IPV for blocking polio transmission. However, using OPV carries with it the risk of VAPP and cVDPV, which is a negative factor for achieving and sustaining complete polio eradication. In the context of global WPV2 eradication, stopping the use of tOPV is necessary to eliminate cVDPV type 2 (cVDPV2);Citation15 however, doing so also has some potential risks.Citation16 With the global cessation of vaccination with OPV2, the population’s mucosal immunity to PV2 is reduced, which may lead to the spread and rapid prevalence of cVDPV2.Citation17 In fact, there have been multiple outbreaks of cVDPV2 after the switch to using bOPV for polio vaccination,Citation18 and cVDPVs have been designated a Public Health Emergency of International Concern. Notably, mOPV2 can quickly stimulate the body’s mucosal immunity, so it has become the vaccine of the choice for rapidly suppressing the cVDPV2 crisis in these regions.Citation19 However, reusing OPV2 brings with it the risk of developing new strains of cVDPV2. To minimize this risk, a consortium has developed a novel OPV2 (nOPV2), with the aim of stockpiling such vaccine for emergency purposes, i.e., if needed in response to an outbreak of cVDPV2. The nOPV2 was engineered to be more genetically stable with a lower likelihood of reversion to neurovirulence, while retaining the benefits of Sabin OPV.Citation20 Two nOPV2 candidates have completed phase 2 clinical trials in adults with encouraging results.Citation21
The WHO announced the eradication of WPV3 in 2019,Citation1 but WPV1 has recently experienced the worst epidemic crisis since 2014.Citation18 As of October 2019, 73 cases of WPV1 have occurred globally, compared with 15 WPV1 cases during the same period in 2018.Citation18 Currently, in Pakistan and Afghanistan, the number of WPV1 cases is increasing. Furthermore, virus sequencing has indicated new mutations in the strains isolated in Pakistan and Afghanistan, and the WPV1 crisis in these regions continues to increase.Citation22 Based on the existing immune strategy, strengthening the level of immunity against poliovirus, especially mucosal immunity, should quickly suppress the virus’s replication and transmission – this is very important in these areas. Reserves of nOPV and its usage in the times of crisis to strengthen immunity against polio is currently an effective method for quickly stimulating the population’s polio immunity.Citation23
In countries where WPV has already been eradicated, VAPP and cVDPV have become the most important polio-related issues. In these developed countries, sufficient public health facilities and resources are available to perform vaccination with IPV. So a full-scale IPV strategy is applied to vaccinating infants. Because of high vaccination coverage and good child health conditions in these regions, after receiving the three vaccine doses in a standard polio vaccination course, more than 95% of vaccinees are positive for antibodies specific to the three types of poliovirus and exhibit long-term immunity. However, potential transmission may exist even in IPV-only countries; for example, after the continuous use of IPV alone for many years (2005–2013), Israel detected WPV1 in sewage samples (February 2013–March 2014), and non-symptomatic polio cases were found.Citation24 This should act as a wake-up call for complacency in the complete eradication of poliovirus. In less developed countries, owing to factors such as the poor nutrition and health of children, the relatively low immunogenicity of polio vaccine in children, the lack of public health facilities and resources, and the relatively low vaccination coverage, the level of immunity against poliovirus in infants is relatively low.Citation25 Under these circumstances, once polio cases due to cVDPV or WPV occur, they can easily spread among the population. At present, these underdeveloped countries are in the transition period from using OPV for polio vaccination to using IPV. Because of the problem of IPV resources, polio immunity in some areas is insufficient.Citation26 Also, the use of OPV2 has ceased. As children in these underdeveloped countries cannot obtain sufficient PV2-specific immunity from a dose of IPV, this adds uncertain factors to the success of polio prevention and control during this transition period.
Once WPV is finally eradicated, IPV may become the only vaccine used in the final stage of polio eradication, and countries around the world may eventually terminate the use of OPV. However, the OPV strains and WPV strains in the environment have always existed, so in the current transition stage and the future IPV maintenance stage, one of keys to preventing further polio epidemics will still be to stimulate the mucosal immunity of the population against poliovirus replication. At present, teams have begun to focus on the use of special types of adjuvants to strengthen the mucosal immunity induced by IPV.Citation27 This brings new ideas for how to achieve the complete eradication of poliovirus in the future all-IPV vaccination phase. Currently, developing effective strategies that can adapt to the prevalence of polio in various regions and continuous monitoring of polio cases are necessary.
Disclosure of potential conflicts of interest
All authors have completed the Unified Competing Interest form and declare that they have no competing interests.
Supplemental Tables
Download MS Word (19.4 KB)Supplemental Figure 1
Download TIFF Image (347.6 KB)Acknowledgments
We thank the research staff of the Guangxi Province Center for Disease Control and Prevention, Liujiang City Center for Disease Control and Prevention, and all clinical investigators for their contributions to the clinical trial and sample collection.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- World Health Organization. Two out of three wild poliovirus strains eradicated. 2019 Oct 24 [accessed 2019 Oct 25]. https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated.
- Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163(1):1–6. doi:10.1093/infdis/163.1.1.
- Laassri M, Lottenbach K, Belshe R, Wolff M, Rennels M, Plotkin S, Chumakov K. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis. 2005;192(12):2092–98. doi:10.1086/498172.
- World Health Organization. Global eradication of wild poliovirus type 2 declared. 2015 Sep 20 [accessed 2015 Oct 6] http://www.polioeradication.org/mediaroom/newsstories/Global-eradication-of-wild-poliovirus-type-2-declared/tabid/526/news/1289/Default.aspx.
- Herremans MM, Van Loon AM, Reimerink JH, Rumke HC, Van Der Avoort HG, Kimman TG, Koopmans MP. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin Diagn Lab Immunol. 1997;4(5):499–503. doi:10.1128/CDLI.4.5.499-503.1997.
- Henry JL, Jaikaran ES, Davies JR, Tomlinson AJ, Mason PJ, Barnes JM, Beale AJ. A study of polio vaccination in infancy: excretion following challenge with live virus by children given killed or living poliovaccine. J Hyg (Lond). 1966;64(1):105–20. doi:10.1017/S0022172400040389.
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, et al. Expert review on poliovirus immunity and transmission. Risk Anal. 2013;33(4):544–605. doi:10.1111/j.1539-6924.2012.01864.x.
- Hird TR, Grassly NC, Andino R. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4):e1002599. doi:10.1371/journal.ppat.1002599.
- O’Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, Oberste MS, Self S, Borate BR, Asturias EJ, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis. 2015;15(11):1273–82. doi:10.1016/S1473-3099(15)00219-4.
- Wright PF, Connor RI, Wieland-Alter WF, Hoen AG, Boesch AW, Ackerman ME, Oberste MS, Gast C, Brickley EB, Asturias EJ, et al. Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect Dis. 2016;16(12):1377–84. doi:10.1016/S1473-3099(16)30169-4.
- Valtanen S, Roivainen M, Piirainen L, Stenvik M, Hovi T. Poliovirus-specific intestinal antibody responses coincide with decline of poliovirus excretion. J Infect Dis. 2000;182(1):1–5. doi:10.1086/315684.
- Wright PF, Wieland-Alter W, Ilyushina NA, Hoen AG, Arita M, Boesch AW, Ackerman ME, Van Der Avoort H, Steven Oberste M, Pallansch MA, et al. Intestinal immunity is a determinant of clearance of poliovirus after oral vaccination. J Infect Dis. 2014;209(10):1628–34. doi:10.1093/infdis/jit671.
- Resik S, Tejeda A, Mach O, Fonseca M, Diaz M, Alemany N, Heng Hung L, Aleman Y, Mesa I, Garcia G, et al. Does simultaneous administration of bivalent (types 1 and 3) oral poliovirus vaccine and inactivated poliovirus vaccine induce mucosal cross-immunity to poliovirus type 2? Clin Infect Dis. 2018;67(suppl_1):S51–S6. doi:10.1093/cid/ciy604.
- Sutter RW, Platt L, Mach O, Jafari H, Aylward RB. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–8. doi:10.1093/infdis/jiu222.
- Thompson KM, Duintjer Tebbens RJ. Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes. BMC Infect Dis. 2015;15(1):374. doi:10.1186/s12879-015-1113-7.
- World Health Organization. Statement of the twenty-sixth polio IHR emergency committee. 2020 Oct 22 [accessed 2020 Oct 23] https://www.who.int/news/item/22-10-2020-statement-of-the-twenty-sixth-polio-ihr-emergency-committee.
- Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, Patel M. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. 2016;15(6):693–708. doi:10.1586/14760584.2016.1140041.
- World Health Organization. Statement of the twenty-second IHR emergency committee regarding the international spread of poliovirus. 2019 [accessed 2019 Oct 3]. https://www.who.int/news-room/detail/03-10-2019-statement-of-the-twenty-second-ihr-emergency-committee-regarding-the-international-spread-of-poliovirus.
- Nicoletta Previsani RHT, Graham T, Jafari HS. Guidelines for containment of poliovirus following type-specific polio eradication—worldwide. Morbidity and Mortality Weekly Report. 2015.
- Van Damme P, Coster I, Bandyopadhyay AS, Suykens L, Rudelsheim P, Neels P, Oberste MS, Weldon WC, Clemens R, Revets H, et al. Poliopolis: pushing boundaries of scientific innovations for disease eradication. Future Microbiol. 2019;14(15):1321–30. doi:10.2217/fmb-2019-0196.
- De Coster I, Leroux-Roels I, Bandyopadhyay AS, Gast C, Withanage K, Steenackers K, De Smedt P, Aerssens A, Leroux-Roels G, Oberste MS, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials. Lancet. 2021;397(10268):39–50. doi:10.1016/S0140-6736(20)32541-1.
- Duintjer Tebbens RJ, Thompson KM. Evaluation of proactive and reactive strategies for polio eradication activities in Pakistan and Afghanistan. Risk Anal. 2019;39(2):389–401. doi:10.1111/risa.13194.
- Thompson KM, Duintjer Tebbens RJ. Lessons from globally coordinated cessation of serotype 2 oral poliovirus vaccine for the remaining serotypes. J Infect Dis. 2017;216(suppl_1):S168–S75. doi:10.1093/infdis/jix128.
- Kopel E, Kaliner E, Grotto I. Lessons from a public health Emergency — importation of wild poliovirus to Israel. N Engl J Med. 2014;371(11):981–83. doi:10.1056/NEJMp1406250.
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13(5):926–39. doi:10.1093/clinids/13.5.926.
- Sutter RW, Cochi SL. Inactivated poliovirus vaccine supply shortage: is there light at the end of the tunnel? J Infect Dis. 2019;220(10):1545–46. doi:10.1093/infdis/jiy739.
- Norton EB, Bauer DL, Weldon WC, Oberste MS, Lawson LB, Clements JD. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine. 2015;33(16):1909–15. doi:10.1016/j.vaccine.2015.02.069.
