ABSTRACT
HPV vaccination is recommended for U.S. adolescents at ages 11–12 and requires two versus three doses if the series is started before age 15. We evaluated how talking about recommended age or fewer doses motivates on-time HPV vaccination. Our national, online experiment randomized 1,263 parents of adolescents to view one of three messages about HPV vaccination recommendations or no message. Messages framed guidelines as recommending: vaccination at age 11–12; fewer doses for those who start vaccination at age 11–12; or, fewer doses for those who start vaccination before age 15. We then assessed parents’ preferred age for HPV vaccination, categorizing preferences of ≤12 years as on-time. Parents who viewed “at age 11–12” versus no message more often preferred on-time HPV vaccination (63% vs. 43%, p < .05) and did not differ from those viewing “fewer doses at age 11–12” (63% vs. 64%, p > .05). Parents who viewed “fewer doses before age 15” less often preferred on-time HPV vaccination (39%, p < .05). Recommending HPV vaccination at age 11–12 encouraged on-time vaccination, while offering fewer doses had little impact. Providers should avoid framing HPV vaccination guidelines in reference to age 15 because doing so may discourage on-time vaccination by introducing confusion about the recommended age.
Widespread human papillomavirus (HPV) vaccination could prevent nearly all cervical cancer, as well as many cases of five other cancers and genital warts.Citation1,Citation2 National recommendations call for routine HPV vaccination by age 12,Citation3 but relatively few U.S. adolescents meet this goal.Citation4 Improving HPV vaccination timeliness is critical for protecting adolescents prior to HPV exposure. Younger adolescents also have a better immunologic response to the vaccine, which may translate into improved effectiveness.Citation5
Differences in HPV vaccine dosing schedules by age may provide an incentive for on-time HPV vaccination. In 2016, the U.S. Advisory Committee on Immunization Practices (ACIP) revised recommendations to reduce the number of required doses from three to two for adolescents initiating the series before age 15, with three doses still recommended for adolescents initiating at age 15 or older.Citation3 Offering fewer doses to be up-to-date could incentivize vaccination timeliness by reducing the cost and inconvenience associated with traveling to primary care office visits, where most HPV vaccine doses are delivered in the U.S. However, the updated recommendations could also disincentive vaccination timeliness if introducing information about initiating the series before age 15 resulted in confusion about the definition of on-time HPV vaccination (i.e., by age 12). Supporting providers in effectively and efficiently framing national recommendations is crucial, given that a provider’s recommendation is the strongest and most consistent predictor of HPV vaccination.Citation6,Citation7 To understand how providers can best communicate national recommendations, we assessed the impact of framing messages to include information about fewer doses (two versus three) and recommended age (at age 11–12) on parents’ preference for on-time HPV vaccination.
We conducted a national online survey of U.S. parents of adolescents in 2017–2018. We have described the methods previously and review them briefly here.Citation8 Study participants were members of a probability-based, national panel maintained by a survey company. Eligible respondents were parents of a 9- to 17-year-old child who was not yet fully vaccinated against HPV, defined as having had <2 doses. Parents with more than one eligible child completed the survey about the youngest child.
Of 2,857 parents contacted to participate in the survey, a total of 1,834 parents responded by visiting the survey website, completing a screener to confirm eligibility, and providing informed consent. Of these parents, 1,313 (72%) met eligibility criteria and provided informed consent. After we excluded 50 panelists who did not complete at least two-thirds of the survey, our analytic sample consisted of 1,263 parents. The response rate was 61%, using the American Association for Public Research Response Rate 4 calculation.Citation9 The Institutional Review Board at the University of North Carolina at Chapel Hill approved the study protocol.
To examine the impact of information about recommended age and fewer HPV vaccine doses, the survey randomly assigned parents to one of four message conditions:
No message;
“National recommendations are for children to get the HPV vaccine at age 11 or 12” (at age 11–12);
“According to national recommendations, children who start the HPV vaccine at age 11 or 12 only need two doses, instead of three” (fewer doses at age 11–12); or
“According to national recommendations, children who start the HPV vaccine before age 15 only need two doses, instead of three” (fewer doses before age 15).
Messages appeared in plain text.
After receiving the assigned message condition, the survey measured parents’ preference for the age of HPV vaccination with one item: “At what age do you think children should get the first dose of the HPV vaccine?” To capture the full range of parents’ preferences, response options were “8 years or younger”, “9–10”, “11–12”, “13–14”, “15–16”, “17–18”, “19 or older”, or “never.” Using these responses, we categorized parents into those who preferred “on-time HPV vaccination” (age ≤12) versus otherwise (age ≥13 or never). The survey company provided data on parents’ age, education, race/ethnicity, and annual household income.
We used unweighted logistic regression to assess the odds of preference for on-time HPV vaccination (yes/no), comparing the at age 11–12 message condition as the reference to the remaining conditions. We selected at age 11–12 as the reference because it is the recommended age for HPV vaccination. Our model did not include covariates because our checks of randomization identified no differences among experimental conditions with respect to demographic characteristics.
We conducted sensitivity analyses to probe the robustness of our findings in the absence of parents who may have more extreme views on HPV vaccine timing. First, we reran our analysis after removing parents who reported “8 years or younger” as the age at which children should get the first dose of HPV vaccine (n = 51) as that age range is earlier than national recommendations. Second, we reran our analysis after removing parents who reported “never” as the age at which children should get the first dose of HPV vaccine (n = 195). For both models, the significance and direction of the findings remained the same; therefore, we do not report further on these analyses. We used SAS 9.4 to conduct all analysis. Statistical tests were two-tailed with a critical alpha of 0.05.
Most parents were non-Hispanic white (70%), Hispanic (14%), or non-Hispanic Black (10%, ). Over one-fourth of parents (28%) had a high school or less education, and nearly one-fifth (19%) had a household income of less than 35,000. USD
Table 1. Sample characteristics, United States, 2017 (n = 1,263)
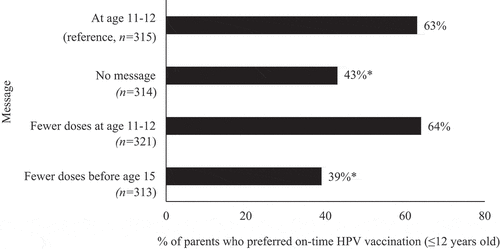
Preference for on-time HPV vaccination was lower among parents who received no message compared to those who received the reference message of at age 11–12 (43% versus 63%; odds ratio [OR]: 0.45; 95% CI: 0.32:0.61) (). We did not find evidence to suggest that the fewer doses at age 11–12 message improved preference for on-time HPV vaccination over the at age 11–12 message (64% versus 63%; OR:1.06; 95% CI:0.77:1.46). The fewer doses before age 15 message elicited lower preference for on-time HPV vaccination compared to the at age 11–12 message (39% versus 63%; OR: 0.37; 95% CI:0.27:0.51).
Figure 1. Parents’ preference for on-time HPV vaccination by national recommendation message. *p < .05
The findings of this brief report suggest that framing national recommendations to emphasize routine administration of HPV vaccine at age 11–12 increases parents’ preference for on-time vaccination, while offering information on fewer doses may have little additional benefit. Furthermore, framing recommendations as involving fewer doses before age 15 may actually discourage on-time HPV vaccination. Our study did not directly probe why this message was discouraging, but we speculate that the mention of age 15 may subtly anchor parents’ preferences by incorrectly suggesting that routine administration extends to this age.Citation10 Such a message may be particularly problematic given that many providers report that they feel less urgency to recommend on-time HPV vaccination, compared to other routinely administered vaccines.Citation6,Citation11–14 In this way, ambiguous information about the recommended age for HPV vaccination could join other cues from providers that unintentionally discourage on-time vaccination.
In terms of implications for clinical practice, our study may offer opportunities for simplifying messaging about complex recommendations for routine HPV vaccination. By leading with the recommended ages of 11–12 and avoiding mention of age 15, providers have the best chance of clearly communicating the goal. Providers may wish to reserve further discussion about the recommended number of doses for only those parents who express subsequent hesitation to vaccinate and need additional information. Prior studies suggest that framing HPV vaccination as cancer prevention and discussing the improved effectiveness of HPV vaccination at younger ages may also be important in supporting parents in their decision making about when to vaccinate.Citation6,Citation13,Citation15,Citation16
Strengths of this study include use of a randomized controlled design and data from a large, national sample of parents. Our findings should also be interpreted in light of several limitations. Most notably, our experiment tested HPV vaccine messages in an online survey; parents’ interpretation of such messages may differ when delivered by providers in more naturalistic clinical settings. Although diverse in terms of race/ethnicity and socio-economic status, our sample was limited to parents of adolescents who were not fully vaccinated against HPV because they represent a high-priority population for public health intervention; our findings may be less generalizable to parents of fully vaccinated adolescents. Finally, our study relied on self-reported measures, including those of adolescent’s vaccination status.
In conclusion, our randomized controlled study found evidence to suggest that recommended age is more important than number of doses for encouraging on-time HPV vaccination. By demonstrating that message framing can encourage parents’ preferences, these findings can support health care providers in effectively and efficiently delivering HPV vaccination recommendations to parents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial disclosure
Brewer has served on paid advisory boards and/or received research grants from Merck, Pfizer and GSK.
Additional information
Funding
References
- Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi:10.1056/NEJMoa1405044.
- Arbyn M, Bryant A, Beutels P, Martin-Hirsch PP, Paraskevaidis E, Van Hoof E, Steben M, Qiao Y, Zhao F-H, Schneider A. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database System Rev. 2011;2011(4):4. doi:10.1002/14651858.CD009069.
- Meites E. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016; 65.
- Bednarczyk RA, Ellingson MK, Omer SB. Human Papillomavirus vaccination before 13 and 15 years of age: analysis of National immunization survey teen data. J Infect Dis. 2019;220(5):730–34. doi:10.1093/infdis/jiy682.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM. Immunogenicity of the 9-Valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Jama. 2016;316(22):2411–21. doi:10.1001/jama.2016.17615.
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine. 2016;34(9):1187–92. doi:10.1016/j.vaccine.2016.01.023.
- Donahue KL, Hendrix KS, Sturm LA, Zimet GD. Human Papillomavirus vaccine initiation among 9–13-year-olds in the United States. Prev Med Rep. 2015;2:892–98. doi:10.1016/j.pmedr.2015.10.003.
- Margolis MA, Brewer NT, Shah PD, Calo WA, Gilkey MB. Stories about HPV vaccine in social media, traditional media, and conversations. Prev Med. 2019;118:251–56. doi:10.1016/j.ypmed.2018.11.005.
- American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys. 9th ed. Oakbrook Terrace, IL: AAPOR; 2016.
- Tversky A, Kahneman D. Judgment under uncertainty: heuristics and Biases. Science. 1974;185(4157):1124–31. doi:10.1126/science.185.4157.1124.
- Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1673–79. doi:10.1158/1055-9965.EPI-15-0326.
- Henrikson NB, Tuzzio L, Gilkey MB, McRee AL. “You’re never really off time”: healthcare providers’ interpretations of optimal timing for HPV vaccination. Prev Med Rep. 2016;4:94–97. doi:10.1016/j.pmedr.2016.05.002.
- Perkins RB, Clark JA, Apte G, Vercruysse JL, Sumner JJ, Wall-Haas CL, Rosenquist AW, Pierre-Joseph N. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics. 2014;134(3):e666–e674. doi:10.1542/peds.2014-0442.
- McRee AL, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. J Pediatr Health Care. 2014;28(6):541–49. doi:10.1016/j.pedhc.2014.05.003.
- Shah PD, Calo WA, Gilkey MB, Boynton MH, Alton Dailey S, Todd KG, Robichaud MO, Margolis MA, Brewer NT. Questions and Concerns About HPV Vaccine: a Communication Experiment. Pediatrics. 2019;143(2):2. doi:10.1542/peds.2018-1872.
- Gilkey MB, Zhou M, McRee AL, Kornides ML, Bridges JFP. Parents’ views on the best and worst reasons for guideline-consistent HPV vaccination. Cancer Epidemiol Biomarkers Prev. 2018;27(7):762–67. doi:10.1158/1055-9965.EPI-17-1067.
