ABSTRACT
Cervical cancer is ranked as the fourth most common cancer in women worldwide. Monoclonal antibody has created a new dimension in the immunotherapy of many diseases, including cervical cancer. The antibody’s ability to target various aspects of cervical cancer (oncoviruses, oncoproteins, and signaling pathways) delivers a promising future for efficient immunotherapy. Besides, technologies such as hybridoma and phage display provide a fundamental platform for monoclonal antibody generation and create the opportunity to generate novel antibody classes including, T cell receptor (TCR)-like antibody. In this review, the current immunotherapy strategies for cervical cancer are presented. We have also proposed a novel concept of T cell receptor (TCR)-like antibody and its potential applications for enhancing cervical cancer therapeutics. Finally, the possible challenges in TCR-like antibody application for cervical cancer therapeutics have been addressed, and strategies to overcome the challenges have been highlighted to maximize the therapeutic benefits.
1. Introduction
Cervical cancer is the abnormal growth of the cervix cells located at the lower end of the womb with possible invasion of different parts of the body (metastasis).Citation1 Ranked as the fourth most common cancer of women worldwide, the World Health Organization (WHO) reported approximately 570000 new cases in 2018.Citation2 Based on histological typing, cervical cancer is characterized by squamous cell carcinoma (80%) and adenocarcinomas (20%), but at times a combination of both types (adenosquamous carcinoma) is present.Citation3 Symptoms include vaginal bleeding (intermenstrual, post-menopausal and postcoital bleeding), irregular vaginal discharge, and pain in the lower abdomen (pelvic) as well as during sexual intercourse.Citation4 The primary cause of cervical cancer is associated with human papillomavirus (HPV) infection.Citation5 In general, cervical cancer is preventable due to its delayed development and easily detectable via cytological approach along with the availability of efficient treatments, including antibody-based immunotherapy.Citation6 In addition, the disease is preventable with proper HPV screening and vaccinations.Citation7
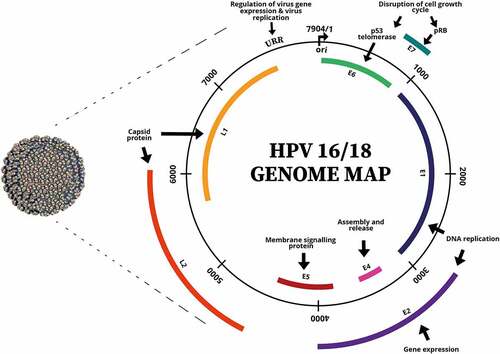
Human papillomavirus (HPV) ()Citation8 derives from the Papillomaviridae family; it is a non-enveloped virus with double-stranded circular DNA.Citation9 HPV subtypes can account for about 100 viral strains with HPV 16 and 18 strains being the main causative strains for approximately 70% of cervical cancer cases worldwide.Citation10 HPV 18 is strongly associated with adenocarcinoma of the cervix and HPV 16 is usually detected in squamous cell carcinoma.Citation11 HPV’s oncogenicity is largely contributed by E6 and E7 oncoproteins whereby E6 oncoprotein promotes cell proliferation by repressing the function of p53 tumor-suppressor protein which prevents apoptosis and cell cycle arrest.Citation12 On the other hand, E7 oncoprotein inactivates the retinoblastoma (Rb) protein (tumor-suppressor), which results in cell immortalization.Citation13
Figure 1. The virion and genome map of human papillomavirus (HPV). (Adapted from (Robboy et al.).Citation8
Despite having preventive measures, cervical cancer incidence rates remain high worldwide, thus further treatments are required. Treatments for cervical cancer are given based on the stages and severity of the cancer, which may include a combination of surgery, radiation, chemotherapy, targeted therapy (drugs or monoclonal antibodies), and immunotherapy.Citation14,Citation15 In this review, the current immunotherapy strategies for cervical cancer are presented. Besides, the fundamental concept of monoclonal antibody therapy and its significance in targeting cervical cancer have been discussed. We have also proposed a novel concept of T cell receptor (TCR)-like antibody and its potential applications for enhancing cervical cancer therapeutics. Finally,possible challenges in TCR-like antibody application for cervical cancer therapeutics have been addressed, and strategies to overcome these challenges have been highlighted to maximize the therapeutic benefits.
2. Current treatment strategies for cervical cancer
The need for effective cancer immunotherapy does not exclude cervical cancer. As such, researchers are continually investigating different approaches to combat this disease.
2.1. Monoclonal antibody therapy in cervical cancer
Monoclonal antibody therapy is a part of the targeted therapy and serves as one of the treatment options available for cervical cancer patients. Administrated through intravenous (IV) infusion, the monoclonal antibodies are expected to target/inhibit receptors that activate signaling pathways responsible for the growth, survival, and metastasis of cancer, deactivating immune response suppressor generated by the tumor cells, and targeting effector immune cells.Citation16
Recently, several monoclonal antibodies were approved by the U.S. Food and Drug Administration (FDA) to be incorporated into cervical cancer treatment. Bevacizumab, a vascular endothelial growth factor (VEGF)-A inhibitor is one of the approved monoclonal antibodies used in cervical cancer treatment to block VEGF-associated pathways that ultimately delay tumor progression and improve prognosis.Citation17,Citation18 In addition, combination therapy of bevacizumab and chemotherapy has proven to increase the survival rate of advanced cervical cancer patients.Citation19 In another strategy to target cervical cancer, monoclonal antibodies were utilized as immune checkpoint inhibitors. The inhibitors can evoke immune responses against tumor cells by interrupting/blocking a specific checkpoint protein that hampers the function of immune cells.Citation20 Pembrolizumab is a U.S. FDA-approved antibody for advanced and recurrent cervical cancer treatment that inhibits the immune checkpoint, programmed cell death 1 (PD-1) expression to improve T and dendritic cell responses in cervical cancer.Citation21–23 Recently, the U.S. FDA granted a Fast Track designation to another PD-1 inhibitor monoclonal antibody, balstilimab, for metastatic cervical cancer treatment after the antibody reported favorable objective response rates both as monotherapy and as a combination with zalifrelimab (CTLA-4 inhibitor) with no adverse side effects.Citation24
The many prospects of monoclonal antibody applications for cervical cancer diagnostics and, more importantly, therapeutics have further broadened the research scope. This is evident through the drastic increase in the number of antibodies under clinical trial investigation summarized in . Besides being a targeted approach, monoclonal antibody therapy encounters several hiccups in the aspects of antibody resistance as well as poor penetration and distribution of antibody into the tumor during treatment that impacts the treatment efficacy.Citation25
Table 1. Monoclonal antibodies under clinical trial investigation for cervical cancer therapeutics
2.2. Targeted gene delivery therapy
The current cervical cancer immunotherapy approaches are mostly based on targeting HPV E6 and E7 oncogenes, contributing mainly to cervical carcinogenesis. The success of genome sequencing has led researchers to manipulate the technology to generate several genome engineering tools specific to HPV E6 and E7. In this approach, the gene expression of E6 and E7 oncogenes are impeded by utilizing elements such as small interfering RNA (siRNA), antisense DNA or RNA molecules, RNA and DNA enzymes known as ribozymes and DNAzymes, respectively, to ultimately hinder the roles of E6 and E7 oncogenes.Citation26 Advancement in this technology has also enabled the silencing of E6 and E7 expression via transcription activator-like effertor nucleases (TALENs), zinc finger nucleases (ZFN), and clustered regularly interspaced short palindromic repeat-associated nucleases (CRISPR/Cas9) RNA-guided endonuclease, which contributes significantly in treating advanced stages of cancer.Citation27 This therapeutic approach is highly favorable in treating cervical carcinoma, primarily contributed by engineering the oncogene expressions and functions. However, the implementation of this strategy requires advanced technology, and high cost, which may not be feasible for low-socioeconomic populations.Citation26 Besides, further validation is needed as the technology is still under evaluation with minimal ongoing clinical trials to date.Citation28
2.3. Therapeutic vaccine
Therapeutic vaccine is a broad strategy based on T cell responses which can be subcategorized to live bacterial/viral vector vaccines (recombinant E6 and E7 vectors introduced to the host cells to elicit T cell responses), subunit peptide/protein vaccines (HPV-antigenic peptide/protein introduced and presented by antigen-presenting cells to stimulate T cell responses), nucleic acid, DNA/RNA-based vaccines (naked HPV DNA/RNA vectors administered to trigger T cell responses via MHC I presentation) and cell-based vaccines (isolation and manipulation ex vivo of patient’s cells (dendritic, T lymphocytes or tumor cells) before transferring to patients).Citation29 Despite being a holistic approach in treating cervical cancer, therapeutic vaccines may face several setbacks in terms of toxicity, particularly in immunocompromised individuals, the neutralizing antibodies generated in the initial immunization stage may reduce vaccine efficacy in the subsequent immunization rounds, HLA-restriction which prevents the implementation of a generalized vaccination, a requirement for additional booster components to overcome low-grade immunogenicity, and patient-specific (cell-based vaccines) making the vaccine inapplicable for the overall population.Citation30 Besides, all the vaccines are still under evaluation, and there are no therapeutic vaccines approved for cervical cancer to date.Citation31
2.4. Adoptive T cell transfer therapy
Adoptive T cell transfer therapy is another promising strategy for cervical cancer therapeutics which comprises tumor-infiltrating lymphocytes, TILs (administration of autologous T cell with selected tumor specificity that has been cultured ex vivo into the cancer patients), and genetically engineered T cells (transfer of autologous genetically modified T cell receptors with selected tumor antigen specificity into the patients) which can be further divided to T cell receptor (TCR) and chimeric antigen receptor (CAR).Citation32 T cell response is evoked in TCR T cell therapy when the autologous T cells with desired antigen specificity detect antigen presented on MHC complex of the tumor cells.Citation33 On the other hand, CARs offer a similar effector mechanism as TCR, exceptional to being MHC/HLA-independent.Citation32 Single-chain variable fragment (scFv) of the CAR T cells contributes mainly to the antigen-binding features and is found to demonstrate stronger affinity compared to the receptors of a typical T cell.Citation34 In general, both therapies have indicated promising outcomes in the aspects of strong cytotoxicity and cervical tumor regression, and most importantly, serve as the primary treatment plan for advanced cervical cancer patients.Citation35 highlights the adoptive T cell transfer therapies under investigation for cervical cancer therapeutics. One of the significant challenges with the adoptive T cell approach is toxicity (lymphodepleting preparative regimen, cytokine or immune-related toxicity). Therefore, there is a critical need for extensive evaluation and evidence to ensure treatment efficacy and, most crucially, safety. The therapy is also patient-specific and not applicable as a standard treatment option for all cervical cancer patients.
Table 2. Current adoptive cell therapy (ACT) under investigation for cervical cancer treatment
Recently, the first-in-human clinical study utilizing genetically engineered T cells to target HPV-associated epithelial cancers was presented by Dorab et al.Citation36 In this approach, patients with metastatic HPV 16-positive cancer pre-treated with platinum-based therapy were administered with genetically engineered T cells, known as E6 T-cell receptor T cells, with a targeted specificity to HPV 16 E6. The outcome of the study revealed that E6 T-cell receptor T cells implicated tumor regression with a moderate response rate that may be contributed by resistance to T cell-mediated recognition. In terms of toxicity, neither adverse autoimmune incidents nor off-target toxicities were observed. However, the researchers have addressed the challenges associated with the overall study and hiccups associated with resistance to T cell-mediated recognition. Nevertheless, the study outcomes serve as useful guidelines for future investigations on genetically engineered T cells in cervical cancer therapeutics.
The therapeutic vaccines and adoptive T cell transfer therapy applications are based mainly on T cell responses. Despite proving to be promising platforms for cancer immunotherapy, the expected therapeutic effect could not be achieved. Although the underlying causes remain unclear, several studies have demonstrated evidence of dysfunction/exhaustion of T cells in the tumor microenvironment.Citation37 The T cell exhaustion state is a consequence of continuous exposure to similar antigen by pre-exposed T cells that result in the decline of T cell’s effector mechanisms (reviewed by Zhang et al.).Citation38 Unfortunately, T cells’ dysfunctionality has been evident in TILCitation39 and CAR T cellsCitation40 studies. Hence, researchers are focusing on strategies to tackle this hurdle as well as to develop alternative approaches such as T cell receptor (TCR)-like antibody that surpasses the bottleneck effects of T cell exhaustion to achieve effective cervical cancer therapeutics.
3. T cell receptor-like antibody
The applications and benefits of monoclonal antibodies, either as a solo treatment or in combination with other therapeutic options such as chemotherapy in many cancers, including cervical cancer, have led to the continuous development and improvement of monoclonal antibodies.
T cell receptor (TCR)-like antibody/TCR-mimic antibody is a novel group of antibodies that is atypical and difficult to be generated until recent developments in technologies such as genetic engineering and phage display technology made it possible.Citation41 The idea behind TCR-like antibody is based on the unique roles of the T (T cell receptor) and B cells (antibody). T cell (T cell receptor) recognizes the antigenic peptide presented on the MHC molecules of all nucleated cells. On the other hand, B cell (antibodies) distinguishes three-dimensional antigen form whether soluble or membrane-bound and has a more excellent capability to eliminate diseases due to its broader effector mechanisms.Citation42
The highly polymorphic MHC class I and II molecules, also known as Human Leukocyte Antigen (HLA) in humans, perform different antigen presentation in which MHC class I displays antigenic peptides derived from infected/mutated cells to the cytotoxic T cell (CD8), resulting in the elimination of the infected cells (internal immunosurveillance) while MHC class II presents antigenic peptide from antigen-presenting cells (APCs) to the helper T cell (CD4) resulting in intracellular signaling transmission for B cell initiation for antibody production and secretion, macrophages for phagocytosis and cytotoxic T cell.Citation43,Citation44 As such, the selection of a specific MHC molecule type for the TCR-like antibody generation is subjected to the target disease and the desirable downstream applications.
The dual functionality of TCR-like antibody in sandwiching the best of the humoral (antibody) and cell-mediated immunity in a single approach has gained many researchers’ attention. As the name indicates, the antibody mimics the function of a T cell receptor by distinguishing antigenic peptide presented on major histocompatible complex (MHC) molecule and establishes fundamental antibody defense mechanisms, such as antibody-dependent cell cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and recruitment of immune cells for phagocytosis (antibody-dependent cellular phagocytosis (ADCP)).Citation45 In addition to performing the functions of a basic antibody, TCR-like antibody can be further explored for other applications, including antibody-based immunotoxins, antibody-toxin fusion molecule along with combination treatment with other chemotherapeutic agents, making it an ideal therapeutic candidate for many diseases, such as infectious disease, viral infections, autoimmune diseases, and cancer.Citation46–50 The list of TCR-like antibodies against various diseases, including cancer, has been summarized by He and colleagues, and Høydahl and colleagues, which include additional information such as antigen type, epitope sequence, HLA allele, target disease, antibody function, format, and generation method.Citation51,Citation52
A TCR-like antibody can be generated through either hybridoma technology or phage display technology where phage display proved to be more beneficial in the aspects of antibody generation with high peptide specificity, straightforward antibody production with high yield, stability, and purity via bacterial expression system and possible conjugation of the antibody fragments with transport proteins.Citation46 In phage display technology, TCR-like antibody is selected through the panning process, which involves (1) binding of MHC complex that displays the antigenic target peptide (target) to a solid surface (microtiter plate, beads, etc.), (2) incubation of high-density antibody phage library to target potential binders (panning), (3) elimination of nonspecific binders through washing, (4) target binder recovery, and (5) amplification of target antibody binders using a bacterial host which will be used for further panning rounds.Citation53,Citation54 The panning process can also be conducted via an automated system, commonly preferred for high throughput generation of antibodies.Citation55
4. The applications of T cell receptor (TCR)-like antibody in cancer immunotherapy
TCR-like antibody has drawn the attention of many researchers recently, especially for the potential applications in cancer immunotherapy. TCR-like antibody’s ability to identify tumor-specific antigens/tumor-associated antigens (most intracellular) presented on MHC molecules serves as a strong platform for effective cancer immunotherapy.Citation56 These antigens are derived from tumor-generated proteins, cancer-causing viral proteins, or neoantigens that undergo degradation by the cell proteasome to produce a shorter antigenic peptide sequence, which is then migrated into the endoplasmic reticulum (ER) for MHC class I binding before the peptide-MHC complex is transferred via Golgi apparatus to be presented on the cell surface.Citation57–59 Upon tumor antigen-MHC recognition, the TCR-like antibody activates a broad spectrum of mechanisms including the essential antibody functions like ADCC, CDC, phagocytosis, recruitment of immune cells along with other potential applications, such as antibody-based immunotoxin, antibody-toxin fusion molecules, and combinational therapy with chemotherapy, which increases the efficacy of cancer treatment as a whole.Citation46
In order for TCR-like antibody to perform basic antibody effector mechanisms, the incorporation of the Fc region into the TCR-like antibody fragments is essential as the Fc mediates most of the antibody functions mentioned above.Citation60 Several researchers have demonstrated the successful generation and functionality of complete TCR-like IgGs capable of inducing apoptosis in selected breast cancer cells (TCR-like antibody: RL4B, RL6A, and RL1B), complement cascade activation for complement-dependent cytotoxicity in vitro (TCR-like antibody: RL4B, RL6A, and 8FA (acute myeloid leukemia)) and ADCC (TCR-like antibody: RL4A, RL6A and 8FA).Citation61–63 ESK1 is another TCR-like antibody that has demonstrated ADCC in an in vivo approach against Wilms Tumor (WT1) oncoprotein responsible for many cancers, including leukemia.Citation64
The incorporation/conjugation of TCR-like antibody with elements such as toxin or drugs (TCR-like antibody-immunotoxin) serves as an alternate approach in cancer immunotherapy with the focal point of tumor elimination with minimal side effects. In the aspect of the immunotoxin, the TCR-like antibody is chemically conjugated with a recombinant toxin derived from bacteria (Diphtheria toxin (DT) and Pseudomonas exotoxin) or plant (A chain of ricin (RTC), gelonin, and dodecahedron) with bacterial toxin being preferred due to effective cytotoxicity with minimal side effects in human.Citation65 The primary strategy of immunotoxin is to prevent protein synthesis in the tumor cells, which ultimately leads to cell death.Citation66 Upon recognition of the antigen presented on the MHC molecules of the tumor cell, the immunotoxin binds and enters the cell via endocytosis in which the toxin undergoes a specific translocation mechanism (depending on the toxin used) and catalyzes adenine diphosphate (ADP)-ribosylation of elongation factor 2 (EF2)’s diphthamide residue that stops protein synthesis and consequently cell death via apoptosis.Citation65 At present, several studies have successfully provided the proof of concept for TCR-like antibody-immunotoxin, including Klechevsky and colleagues who demonstrated the capability of Fab-immunotoxin (Pseudomonas exotoxin) to detect melanoma antigenic peptide, MART-1-HLA-A2*01 molecule and subsequently induce cell death in human melanoma cells.Citation67 The potential of TCR-like antibody-immunotoxin was further validated by the discovery of another Fab-immunotoxin, specific to a breast and prostate cancer antigenic protein known as TCR gamma alternative reading frame protein (TARP), which successfully demonstrated cell cytotoxicity.Citation68 Adding to the antibody list are two other derivatives of TCR-mimic antibodies conjugated with cytotoxic agent monomethyl auristatin E (MMAE), ESK-MMAE, and Q2L-MMAE, which was generated against Wilms tumor 1 (WT1) oncoprotein.Citation69 The TCR-like antibody-drug-conjugates are expected to eliminate cancer cells by transferring the toxin to the tumor cells upon antigen peptide MHC (pMHC) recognition by the antibody. However, the moderate level of cytotoxicity observed in the initial stage of the antibodies led to the development of a bispecific (Bi)-TCR-like-ADC with improved cytotoxicity deduced from both in vitro and in vivo study.Citation69 In another study by Kurosawa and colleagues, the antibody clone #21-3 against tumor-associated antigen survivin-2B was successfully generated with dual functionality of a TCR-like antibody (intracellular tumor peptide recognition) as well as T cell engager (activation of T cell mechanism), resulting in enhanced cytotoxicity against the tumor cells in vitro.Citation70 Despite these favorable findings, further evaluation and more substantial evidence are necessary for fruitful and effective cancer immunotherapy since toxin is involved.
TCR-like antibody fusion/conjugated molecule is another promising strategy for cancer as well as other immunotherapies. The fusion molecule can be derived from cytokines (interferon) that have the ability to perform immunomodulatory roles at the tumor site.Citation46 The TCR-like antibody can also be conjugated with scFv dimers, known as diabodies or tandem scFvs, which have proven to enhance affinity by doubling the interaction between the tumor and effector immune cells, resulting in increased cytotoxicity to establish more effective immunotherapy.Citation52 Another promising approach for targeting cancer is the engineering of chimeric antigen receptor (CAR) with TCR-like antibody to induce specific tumor cell elimination by cytotoxic T cells. TCR-like antibody CAR, which is engineered with the intracellular domain of CD3 molecule required for T cell activation, can detect/recognize the pMHC molecules presented on the cancer cells, resulting in the elimination of the tumor cells via T cell activation.Citation71 The generation of TCR-like antibody CAR in the single-chain variable fragment (scFv) format delivers added therapeutic advantages in terms of affinity and specificity as it maintains a typical antibody’s features and demonstrates a stronger affinity compared to the receptors of a typical T cell.Citation34 The success garnered from several FDA-approved CAR-based therapies in effectively treating cancers such as B-cell lymphomas, chronic lymphocytic leukemia, and melanoma gives great hope and potential for beneficial cancer treatment with TCR-like antibody CAR therapy.Citation51,Citation72
5. TCR-like antibody immunotherapy in cervical cancer
TCR-like antibody acquires the potentials to shed some light on effective cervical cancer therapy as well. Ideally, TCR-like antibody will be able to detect intracellular cervical cancer antigenic peptides presented on the MHC class I molecules of the tumor and establish a wide range of defense mechanisms including ADCC, CDC, phagocytosis, activating other immune components as well as further applications such as immunotoxin (), TCR-like antibody fusion molecule and combinational therapy with chemotherapy as discussed earlier to achieve an effective treatment for cervical cancer.Citation46 Most importantly, the effector mechanisms of TCR-like antibody are mostly shielded from T cell exhaustion, which overcomes the hiccups faced in T cell-based immunotherapy and adds more therapeutic value in targeting cervical cancer.
Figure 2. Overview of T cell receptor (TCR)-like antibody applications in cervical cancer immunotherapy. (a) TCR-like antibody-dependent cell cytotoxicity (ADCC); (b) Complement-dependent cytotoxicity (CDC) mediated by TCR-like antibody; (c) TCR-like antibody-dependent cellular phagocytosis (ADCP); (d) Induction of tumor cell apoptosis by TCR-like antibody; (e) TCR-like antibody-based CAR for tumor cell lysis by T cells; (f) TCR-like antibody conjugated with drug/toxin (Immunotoxin)
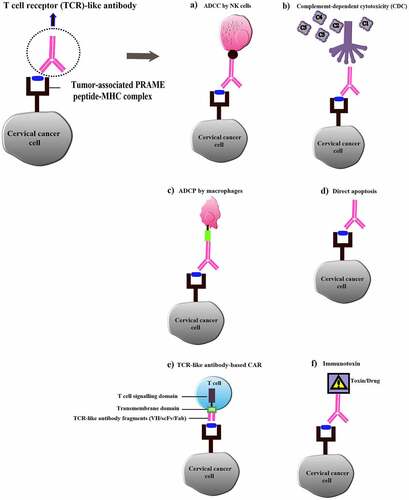
Recently, various cervical cancer biomarkers have been identified with great potentials and provide strong platforms for the diagnostics and therapeutics of cervical cancer.Citation73 Biomarkers deliver several essential purposes that include preliminary cancer diagnosis, enhance histopathology screening accuracy, evaluate a patient’s risk factor for cervical cancer, and determine suitable treatment plans based on evaluating and monitoring high-risk and post-treatment individuals.Citation74 In targeting cervical cancer, several biomarkers serve as prominent antigenic targets for TCR-like antibody generation. Peptides derived from the HPV oncoprotein E6 and E7 serve as ideal candidates since these proteins are responsible for most of the cervical carcinogenesis.Citation13 The cancer-testis (CT) antigens are unique antigens that are limitedly expressed in the testis germ cells of a healthy individual but found to be upregulated in several cancer malignancies such as ovarian, breast, renal, colorectal, and, most importantly, cervical.Citation75 The difference in these antigens’ expression levels helps distinguish between healthy and cancer patients (biomarkers) and also plays vital roles in cancer therapeutics by serving as a target antigen for TCR-like antibody generation. Some of the CT antigens identified in cervical cancer with such potential include sperm-associated antigen 9 (SPAG), BAP31, MAGE (A3, A4, A6, and A12), GAGE-3/6, PRAME, and LAGE-1.Citation76–78 Using a CT antigen PRAME peptide ALY, a TCR-like human IgG1 antibody (Pr20) was successfully generated in a recent study with the ability to perform ADCC against leukemia cells in vivo, proving the efficacy of CT antigen as a target for TCR-like antibody generation, as well as adding therapeutic values for cancer immunotherapy including cervical cancer.Citation79 Besides, there is evidence of the successful generation of several other TCR-like antibodies () against different tumor antigenic markers similarly expressed in cervical cancer, emphasizing the prospects of generating TCR-like antibodies for cervical cancer therapeutics utilizing the same antigenic target.
Table 3. List of generated TCR-like antibodies developed for therapeutic purpose
Antigen presentation on MHC molecules is the core, and crucial element for TCR-like antibody generation as the antibody recognizes the antigen presented, which distinguishes a TCR-like antibody from a typical antibody. The causative agent for cervical cancer, HPV is known to employ several escape mechanisms to overcome the immune defenses, including downregulation of Toll-like receptor 9 (TR9) expressions which inhibits inflammatory signaling and interleukin (IL)-1-β generation, suppression of NF-ĸB pathway, upregulation of P13-K pathway in Langerhans cells, inhibition of HPV infected cell apoptosis, and unfortunately the downregulation of antigen processing and MHC presentation.Citation80–83 Some of the possible mechanisms responsible for MHC downregulation by the HPV virus include the (1) inhibition of transporter associated with antigen processing (TAP) required for MHC assembly, (2) disruption of the ER-associated protein degradation (ERAD) resulting in MHC class I heavy chain disintegration, (3) blocking MHC I molecule migration to the plasma membrane and subsequently prevent MHC antigen presentation, and (5) MHC endocytosis.Citation84 Since peptide-MHC presentation is crucial for TCR-like antibody generation, the HPV escape mechanisms mentioned above would be a significant hurdle for generating TCR-like antibodies for cervical cancer therapeutics.
Fortunately, several favorable findings recently from the analysis of cervical cancer patient samples proved otherwise. It has been found that the expression of MHC class I in HPV positive cancer cells was similar to the negative control cells (HPV negative tumor and non-cancerous cell), which hypothesize that HPV may not be the contributing factor for MHC downregulation.Citation85 A similar finding was obtained from the analysis of more than 800 human cervical cancer data retrieved from The Cancer Genome Atlas (TCGA), which provided further evidence that denies the role of HPV in MHC repression of cervical cancer.Citation86 Most importantly, there is evidence that supports the existence of MHC class I molecules in low- and high-grade squamous intraepithelial lesions and is not completely lost despite the effect of HPV on the transporter associated with antigen processing (TAP), which provides some hope and opportunities for the application of TCR-like antibody in cervical cancer immunotherapy.Citation87 Under this circumstance, it is understandable that antigen presentation on the cervical tumor cells may be at low density.
Three strategies can be employed to improve antigen-MHC presentation (target density) and subsequent recognition by TCR-like antibody. The first strategy is to utilize several cytokines like IFNγ, which have been proven to enhance MHC presentation by upregulating the expression of proteasome activator LMP2, LMP7, and MECL-1, TAP1/TAP2 and MHC heavy chains, while TNFα improved the stability and functionality of MHC molecule.Citation88 The second strategy involves administering a mild dosage of chemotherapeutic agents and ionizing radiation, topotecan (TPT). This strategy successfully induced the MHC class I expression and presentation in breast cancer cells.Citation89 The administration of inhibitors is the third promising strategy to enhance the antigen-MHC presentation. This is evident in a study where the expression of MHC class I molecules was increased in esophageal and gastric cancer upon administration of inhibitors, such as MEK inhibitor, that suppress mitogen-activated protein kinase (MAPK) pathway and Erlotinib inhibitor, which represses EGFR pathway.Citation90
Besides employing strategies to improve the peptide-MHC presentation, TCR-like antibody fusion molecules can also improve the overall treatment efficacy in a low peptide-MHC presented environment. ESK1-BiTE, a TCR-like antibody conjugated with a bispecific T-cell engager (BiTE) specific to WT1 oncoprotein successfully eliminated leukemia and solid tumor cells in an animal model with low antigen presentation by MHC class I HLA-A*02 molecules.Citation91 Although WT1 is not the prognostic marker for cervical cancer, the approach of conjugating BiTE to TCR-like antibody targeting cervical cancer can still be adapted to enhance the antigenic peptide-MHC presentation.Citation92 In another study, a TCR-like antibody conjugated with the drug illustrated favorable cytotoxic effects against breast and colon cancer cells under low target MCH density which further validated TCR-like antibody’s efficacy under low MHC presentation.Citation93
Multimerization of single-chain T cell receptor (scTCR) is another promising approach to improve tumor antigen-MHC recognition in cervical cancer therapeutics. Using this approach, scTCR multimers were successfully generated recently with the ability to demonstrate enhanced recognition of p53 oncopeptide on HLA-A2.1.Citation94 The method enables excellent visualization and quantification of peptide-MHCs, specifically on cervical cancer tumor cells presenting low MHC complexes. Besides, multimeric TCRs acquire the potential to differentiate single amino acid differences, which will be very useful in evaluating cross-reactivity.Citation95 Overall, the strategies discussed above provide relief to overcome a significant hurdle in cervical cancer treatment (low antigen MHC density) and open the door for a promising combinational therapy with TCR-like antibody to deliver maximum immunotherapy benefits to overcome cervical cancer.
It is undeniable that TCR-like antibodies can deliver huge prospects for the future of cervical cancer immunotherapy. However, several hurdles need to be addressed to ensure the effectiveness of these antibodies in cervical cancer treatment. HLA-restriction of epitope serves as the main challenge of TCR-like antibody due to the specificity of TCR-like antibody to a particular HLA type.Citation96 In other words, the TCR-like antibody will only be beneficial for individuals of a specific HLA type. Besides, some evidence links HLA prevalence to factors, such as geographic location and disease susceptibility. The HLA distribution pattern of the HLA-A2 gene is predominantly found in the world population; meanwhile, HLA-A11 and HLA-A24 genes were found to be concentrated in the Asian population.Citation97,Citation98 On the other hand, specific HLA genes such as HLA-DQB*:0602 and HLA-DRB1*1501 have been associated with the susceptibility to HPV infection.Citation99 As such, these factors can be considered during HLA selection for TCR-like antibody generation to gain maximum benefit. In previous research, all three HLAs were considered for TCR-like antibody evaluation to benefit a wide range of populations.Citation47,Citation100 The second major challenge associated with TCR-like antibody, especially in targeting cancer, is the suppressing antigen presentation on HLA molecule by the tumor.Citation101 Strategies to overcome this hurdle and optimize the application of TCR-like antibodies have been elaborated in detail above. Besides that, it is crucial for the recognition of TCR-like antibody to be highly specific to the target-HLA and not to other similar peptides presented on other cells (TCR-like antibody distinguishes only several amino acids from a peptide sequence) to eliminate cross-reactivity, which could be potentially fatal.Citation56 In short, these hurdles are merely hiccups in TCR-like antibody application, which can be overcome and improvised.
Overall, the ability of TCR-like antibody to perform various defense mechanisms, such as ADCC, CDC, antibody-based immunotoxin, and antibody fusion molecules along with combinational therapy with chemotherapy and radiation, highlights TCR-like antibody as the golden goose in cancer immunotherapy. As such, TCR-like antibody serves as a novel group of antibodies with a wide range of therapeutic potentials, which can be ideally channeled to effective cervical cancer immunotherapy.
6. Conclusion
Despite the availability of several preventive measures, the mortality rate and reported cases of cervical cancer remain high. As such, there is a desperate need to develop different strategies to improvise the effectiveness of cervical cancer therapy and ensure maximum therapeutic benefits are obtained. The bright prospect of monoclonal antibodies for both efficient and beneficial cervical cancer immunotherapy is undeniably evident through a wide range of target strategies (HPV oncoproteins and various signaling pathways), increased FDA-approved monoclonal antibodies for immunotherapy as well as the drastic rise in the number of monoclonal antibodies under clinical trial investigations. Besides, the positive outcomes from the combinational therapy approach using monoclonal antibodies and chemotherapy/radiotherapy have shown to benefit many patients of different severity, further validating the efficiency of monoclonal antibody. With the rapid advancement in science and technology, a promising platform is available to successfully incorporate monoclonal antibodies and future novel antibodies like TCR-like antibodies for the broader and effective cervical cancer immunotherapy.
Author contributions
Sylvia Annabel Dass: Conceptualization, Writing Original Draft and Writing Review and Editing. Rehasri Selva Rajan: Writing Review and Editing. Gee Jun Tye: Conceptualization and Writing Review and Editing. Venugopal Balakrishnan: Conceptualization, Writing Review and Editing and Supervision
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Funding
References
- InformedHealth.org. Cervical cancer: overview. 2006; 2017 Dec 14 [accessed 2019 Nov 10]. https://www.ncbi.nlm.nih.gov/books/NBK279259/.
- WHO. Cervical cancer. 2019 [accessed 2019 Nov 30]. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/.
- Denny L, Herrero R, Levin C, Kim JJ. Cervical Cancer In: Gelband H, Jha P, Sankaranarayan R, Horton S, eds. Cancer: Disease Control Priorities. Washington (DC): The International Bank for Reconstruction and Development / The World Bank, 2015:69–84
- Mwaka AD, Orach CG, Were EM, Lyratzopoulos G, Wabinga H, Roland M. Awareness of cervical cancer risk factors and symptoms: cross-sectional community survey in post-conflict northern Uganda. Health Expectations. 2016;19(4):854–67. doi:10.1111/hex.12382.
- Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164:1017–25.
- Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4(12):1795–811. doi:10.15419/bmrat.v4i12.386.
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–82. doi:10.1016/S0140-6736(18)32470-X.
- Robboy SJ, Anderson MC, Russell P. The etiology of cervical cancer. In: Fu SY, editor. Pathology of the female reproductive tract. London: Churchill Livingstone, Elsevier Science; 2000. p. 146–64
- Luria L, Cardoza-Favarato G. Human papillomavirus. StatPearls [Internet]: 2019. [accessed 2020 Jan 20]. https://www.ncbi.nlm.nih.gov/books/NBK448132/.
- Braaten KP, Laufer MR. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev Obstet Gynecol. 2008;1:2–10.
- Woodman CBJ, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. doi:10.1038/nrc2050.
- Wang -C-CJ, Palefsky JM. Human papillomavirus (HPV) infections and the importance of HPV vaccination. Curr Epidemiol Rep. 2015;2(2):101–09. doi:10.1007/s40471-015-0039-3.
- Yim E-K, Park J-S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37(6):319–24. doi:10.4143/crt.2005.37.6.319.
- PATE Board. Cervical cancer treatment (PDQ®): patient version. PDQ Cancer Information Summaries 2019; 2020 Jan 10 [accessed 2002 Nov 8]. https://www.ncbi.nlm.nih.gov/books/NBK65985/.
- Grace PCY, Paul de S, Levon MK. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets. 2013;13(2):205–20. doi:10.2174/1568009611313020009.
- Bellati F, et al. Monoclonal antibodies in gynecological cancer: a critical point of view. J Immunol Res. 2011;2011(Article ID 890758):16.
- Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Tamaya T. Clinical implication of expression of vascular endothelial growth factor-C in metastatic lymph nodes of uterine cervical cancers. Br J Cancer. 2004;91(3):466–69. doi:10.1038/sj.bjc.6601963.
- Eskander RN, Tewari KS. Development of bevacizumab in advanced cervical cancer: pharmacodynamic modeling, survival impact and toxicology. Future Oncol (London, England). 2015;11(6):909–22. doi:10.2217/fon.14.276.
- Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240). Lancet. 2017;390(10103):1654–63. doi:10.1016/S0140-6736(17)31607-0.
- Heinzerling L, De Toni EN, Schett G, Hundorfean G, Zimmer L. Checkpoint inhibitors. Dtsch Arztebl Int. 2019;116(8):119–26. doi:10.3238/arztebl.2019.0119.
- Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJM, Van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–47. doi:10.1158/1078-0432.CCR-09-1652.
- Liu Y, Wu L, Tong R, Yang F, Yin L, Li M, You L, Xue J, Lu Y. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol. 2019;10:65–65. doi:10.3389/fphar.2019.00065.
- AACR. Pembrolizumab OK’d for cervical cancer. Cancer Discov. 2018;8(8):904.
- Tucker N. FDA grants fast track designation to balstilimab in metastatic cervical cancer. Targeted Oncology; 2020 Apr 8 [accessed 30 May 2020]. https://www.targetedonc.com/view/fda-grants-fast-track-designation-to-balstilimab-in-metastatic-cervical-cancer.
- Chen W, Li T, Wang J, Liang L, Huang D, Yan G, Tian Y, Zhang X, Zhang W. Clinical study of nimotuzumab combined with concurrent radiochemotherapy for treatment of locally advanced cervical cancer. Cancer Manag Res. 2019;11:8157–65. doi:10.2147/CMAR.S191134.
- Cao Y, Deng L, Lian S, Jiang Y. Research on the efficacy of cisplatin and nimotuzumab combined with concurrent chemoradiotherapy on locally advanced cervical cancer. J buon. 2019;24:2013–19.
- Santin AD, Sill MW, McMeekin DS, Leitao MM, Brown J, Sutton GP, Van le L, Griffin P, Boardman CH. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a gynecologic oncology group study. Gynecol Oncol. 2011;122(3):495–500. doi:10.1016/j.ygyno.2011.05.040.
- Hong DS, Concin N, Vergote I, de Bono JS, Slomovitz BM, Drew Y, et al. Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer. Clinical Cancer Research 2020; 26:1220–8
- Lheureux S, Butler MO, Clarke B, Cristea MC, Martin LP, Tonkin K, Fleming GF, Tinker AV, Hirte HW, Tsoref D, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4(7):e173776. doi:10.1001/jamaoncol.2017.3776.
- Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J, López-Picazo JM, Machiels J-P, Delord J-P, Evans TRJ, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkmate 358 trial. J Clin Oncol. 2019;37(31):2825–34. doi:10.1200/JCO.19.00739.
- Rischin D, Gil-Martin M, González-Martin A, Brana I, Hou JY, Cho D, Falchook G, Formenti S, Jabbour S, Moore K, et al. 75P-cemiplimab, a human PD-1 monoclonal antibody, in patients (pts) with recurrent or metastatic cervical cancer: interim data from phase I cohorts. Ann Oncol. 2018;29:x27–x28. doi:10.1093/annonc/mdy487.006.
- Papadopoulos KP, Johnson ML, Lockhart AC, Moore K, Falchook GS, Formenti SC, Naing A, Carvajal RD, Rosen LS, Weiss GJ, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 2020;26(5):1025. doi:10.1158/1078-0432.CCR-19-2609.
- Tse K-Y. Avelumab with axitinib in persistent or recurrent cervical cancer after platinum-based chemotherapy (ALARICE); 2019 Feb 18 [accessed 2020 Mar 31]. https://clinicaltrials.gov/ct2/show/NCT03826589#contacts.
- Mayadev J, Nunes AT, Li M, Marcovitz M, Lanasa MC, Monk BJ. CALLA: efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer. 2020;30(7):1065–70. doi:10.1136/ijgc-2019-001135.
- Roche H-L A phase II, safety, and efficacy study of tiragolumab plus atezolizumab and atezolizumab monotherapy in patients with metastatic and/or recurrent PD-L1−positive cervical cancer (NCT04300647); ClinicalTrials.gov, 2020 [ accessed 2020 July 17]. https://clinicaltrials.gov/ct2/show/NCT04300647#contacts.
- Friedman CF, Snyder Charen A, Zhou Q, Carducci MA, Buckley De Meritens A, Corr BR, et al. Phase II study of atezolizumab in combination with bevacizumab in patients with advanced cervical cancer. Journal for immunotherapy of cancer 2020; 8:e001126.
- Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer. 2020;30(1):139–43. doi:10.1136/ijgc-2019-000880.
- Cruz E, Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics. 2019;13:33–51.
- Bharti AC, Singh T, Bhat A, Pande D, Jadli M. Therapeutic strategies for human papillomavirus infection and associated cancers. Front Biosci (Elite Ed). 2018;10(1):15–73. doi:10.2741/e808.
- Pal A, Kundu R. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 2020;10:3116–3116. doi:10.3389/fmicb.2019.03116.
- Áyen Á, Jiménez Martínez Y, Boulaiz H. Targeted gene delivery therapies for cervical cancer. Cancers. 2020;12(5):1301. doi:10.3390/cancers12051301.
- Kumar S, Biswas M, Jose T. HPV vaccine: current status and future directions. Med J Armed Forces India. 2015;71(2):171–77. doi:10.1016/j.mjafi.2015.02.006.
- Nayereh KG, Khadem G. Preventive and therapeutic vaccines against human papillomaviruses associated cervical cancers. Iran J Basic Med Sci. 2012;15:585–601.
- Barra F, Della Corte L, Noberasco G, Foreste V, Riemma G, Di Filippo C, Bifulco G, Orsi A, Icardi G, Ferrero S, et al. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J Obstetrics Gynaecol Res. 2020;46(7):989–1006. doi:10.1111/jog.14276.
- Rohaan MW, Wilgenhof S, Haanen J. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474(4):449–61. doi:10.1007/s00428-018-2484-0.
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi:10.1038/nrc2355.
- Stoiber S, Cadilha BL, Benmebarek M-R, Lesch S, Endres S, Kobold S. Limitations in the design of chimeric antigen receptors for cancer therapy. Cells. 2019;8(5):472. doi:10.3390/cells8050472.
- Orbegoso C, Murali K, Banerjee S. The current status of immunotherapy for cervical cancer. Rep Pract Oncol Radiother. 2018;23(6):580–88. doi:10.1016/j.rpor.2018.05.001.
- Piersma SJ, Jordanova ES, Van Poelgeest MIE, Kwappenberg KMC, Van der Hulst JM, Drijfhout JW, Melief CJM, Kenter GG, Fleuren GJ, Offringa R, et al. High number of intraepithelial CD8+tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67(1):354–61. doi:10.1158/0008-5472.CAN-06-3388.
- Jazaeri A, Gontcharova V, Blaskovich M, Kunkalla K, Masteller E, Fardis M, Chartier C. 873P in vivo persistence of iovance tumour-infiltrating lymphocytes LN-145 in cervical cancer patients. Ann Oncol. 2020;31:S642. doi:10.1016/j.annonc.2020.08.1012.
- Jazaeri AA, Edwards RP, Wenham RM, Matsuo K, Fleming GF, O’Malley DM, Slomovitz BM, Monk BJ, Brown RJ, Suzuki S, et al. A phase 2, multicenter study to evaluate the efficacy and safety using autologous tumor infiltrating lymphocytes (LN-145) in patients with recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2018;36(15_suppl): TPS5604–TPS5604. doi:10.1200/JCO.2018.36.15_suppl.TPS5604.
- Doran SL, Stevanović S, Adhikary S, Gartner JJ, Jia L, Kwong ML, Faquin WC, Hewitt SM, Sherry RM, Yang JC, Rosenberg SA. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37(30):2759–68. doi:10.1200/JCO.18.02424.
- Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33(4):547–62. doi:10.1016/j.ccell.2018.03.012.
- Zhang Z, Liu S, Zhang B, Qiao L, Zhang Y, Zhang Y. T cell dysfunction and exhaustion in cancer. Front Cell Dev Biol. 2020;8:17–17. doi:10.3389/fcell.2020.00017.
- Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6(6):e1792–e1792. doi:10.1038/cddis.2015.162.
- Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y, Zhang T, Liu C, Zhou J. Understanding the mechanisms of resistance to CAR T-cell therapy in malignancies. Front Oncol. 2019;9:1237–1237. doi:10.3389/fonc.2019.01237.
- Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc Natl Acad Sci U S A. 1996;93(5):1820–24. doi:10.1073/pnas.93.5.1820.
- Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol. 2013;4:302–302. doi:10.3389/fimmu.2013.00302.
- Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31(1):443–73. doi:10.1146/annurev-immunol-032712-095910.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Helper T cells and lymphocyte activation. In: Molecular biology of the cell. New York: Garland Science; 2002. https://www.ncbi.nlm.nih.gov/books/NBK26827/
- Dubrovsky L, Dao T, Gejman RS, Brea EJ, Chang AY, Oh CY, Casey E, Pankov D, Scheinberg DA. T cell receptor mimic antibodies for cancer therapy. OncoImmunology. 2016;5(1):e1049803.
- Cohen M, Reiter Y. T-cell receptor-like antibodies: targeting the intracellular proteome therapeutic potential and clinical applications. Antibodies. 2013;2(3):517–34. doi:10.3390/antib2030517.
- Dass SA, Norazmi MN, Dominguez AA, Miguel MESGS, Tye GJ. Generation of a T cell receptor (TCR)-like single domain antibody (sDAb) against a mycobacterium tuberculosis (Mtb) heat shock protein (HSP) 16kDa antigen presented by Human Leukocyte Antigen (HLA)-A*02. Mol Immunol. 2018;101:189–96. doi:10.1016/j.molimm.2018.07.001.
- Lai J, Choo JAL, Tan WJ, Too CT, Oo MZ, Suter MA, Mustafa FB, Srinivasan N, Chan CEZ, Lim AGX, et al. TCR–like antibodies mediate complement and antibody-dependent cellular cytotoxicity against Epstein-Barr virus–transformed B lymphoblastoid cells expressing different HLA-A*02 microvariants. Sci Rep. 2017;7(1):9923. doi:10.1038/s41598-017-10265-6.
- Weidanz JA, Piazza P, Hickman-Miller H, Woodburn D, Nguyen T, Wahl A, Neethling F, Chiriva-Internati M, Rinaldo CR, Hildebrand WH, et al. Development and implementation of a direct detection, quantitation and validation system for class I MHC self-peptide epitopes. J Immunol Methods. 2007;318(1):47–58. doi:10.1016/j.jim.2006.09.019.
- Dahan R, Tabul M, Chou YK, Meza-Romero R, Andrew S, Ferro AJ, Burrows GG, Offner H, Vandenbark AA, Reiter Y, et al. TCR-like antibodies distinguish conformational and functional differences in two vs. four-domain auto-reactive MHC II-peptide complexes. Eur J Immunol. 2011;41(5):1465–79. doi:10.1002/eji.201041241.
- He Q, Liu Z, Liu Z, Lai Y, Zhou X, Weng J. TCR-like antibodies in cancer immunotherapy. J Hematol Oncol. 2019;12(1):99. doi:10.1186/s13045-019-0788-4.
- Høydahl LS, Frick R, Sandlie I, Løset GÅ. Targeting the MHC ligandome by use of TCR-like antibodies. Antibodies (Basel, Switzerland). 2019;8(2):32.
- Hammers CM, Stanley JR. Antibody phage display: technique and applications. J Invest Dermatol. 2014;134(2):e17–e17. doi:10.1038/jid.2013.521.
- Kabir ME, Krishnaswamy S, Miyamoto M, Furuichi Y, Komiyama T. An improved phage-display panning method to produce an HM-1 killer toxin anti-idiotypic antibody. BMC Biotechnol. 2009;9(1):99. doi:10.1186/1472-6750-9-99.
- Turunen L, Takkinen K, Söderlund H, Pulli T. Automated panning and screening procedure on microplates for antibody generation from phage display libraries. J Biomol Screen. 2009;14(3):282–93. doi:10.1177/1087057108330113.
- Trenevska I, Li D, Banham AH. Therapeutic antibodies against intracellular tumor antigens. Front Immunol. 2017;8(1001). doi:10.3389/fimmu.2017.01001.
- Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12(1):93. doi:10.1186/s13045-019-0787-5.
- Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501–948501. doi:10.1155/2015/948501.
- Wieczorek M, Abualrous ET, Sticht J, Álvaro-Benito M, Stolzenberg S, Noé F, Freund C. Major Histocompatibility Complex (MHC) Class I and MHC class II proteins: conformational plasticity in antigen presentation. Front Immunol. 2017;8(292). doi:10.3389/fimmu.2017.00292.
- van Erp EA, Luytjes W, Ferwerda G, Van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10(548). doi:10.3389/fimmu.2019.00548.
- Sergeeva A, He H, Ruisaard K, St John L, Alatrash G, Clise-Dwyer K, Li D, Patenia R, Hong R, Sukhumalchandra P, et al. Activity of 8F4, a T cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia. 2016;30. doi:10.1038/leu.2016.57
- Wittman VP, Woodburn D, Nguyen T, Neethling FA, Wright S, Weidanz JA. Antibody targeting to a class I MHC-peptide epitope promotes tumor cell death. J Immunol. 2006;177(6):4187. doi:10.4049/jimmunol.177.6.4187.
- Dubrovsky L, Dao T, Gejman RS, Brea EJ, Chang AY, Oh CY, Casey E, Pankov D, Scheinberg DA. T cell receptor mimic antibodies for cancer therapy. Oncoimmunology. 2015;5(1):e1049803–e1049803. doi:10.1080/2162402X.2015.1049803.
- Ataie N, Xiang J, Cheng N, Brea EJ, Lu W, Scheinberg DA, Liu C, Ng HL. Structure of a TCR-mimic antibody with target predicts pharmacogenetics. J Mol Biol. 2016;428(1):194–205. doi:10.1016/j.jmb.2015.12.002.
- Aruna G. Immunotoxins: a review of their use in cancer treatment. J Stem Cells Regen Med. 2006;1:31–36.
- Kreitman RJ. Immunotoxins for targeted cancer therapy. Aaps J. 2006;8:E532–E551.
- Klechevsky E, Gallegos M, Denkberg G, Palucka K, Banchereau J, Cohen C, Reiter Y. Antitumor activity of immunotoxins with T-cell receptor-like specificity against human melanoma xenografts. Cancer Res. 2008;68(15):6360–67. doi:10.1158/0008-5472.CAN-08-0928.
- Epel M, Carmi I, Soueid‐Baumgarten S, Oh S, Bera T, Pastan I, Berzofsky J, Reiter Y. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies. Eur J Immunol. 2008;38(6):1706–20. doi:10.1002/eji.200737524.
- Shen Y, Li Y-M, Zhou -J-J, Zhou Z, Xu Y-C, Zhao W-B, Chen S-Q. The antitumor activity of TCR-mimic antibody-drug conjugates (TCRm-ADCs) targeting the intracellular Wilms tumor 1 (WT1) oncoprotein. Int J Mol Sci. 2019;20(16):3912. doi:10.3390/ijms20163912.
- Kurosawa N, Wakata Y, Ida K, Midorikawa A, Isobe M. High throughput development of TCR-mimic antibody that targets survivin-2B80-88/HLA-A*A24 and its application in a bispecific T-cell engager. Sci Rep. 2019;9(1):9827. doi:10.1038/s41598-019-46198-5.
- Akatsuka Y. TCR-like CAR-T cells targeting MHC-bound minor histocompatibility antigens. Front Immunol. 2020;11(257). doi:10.3389/fimmu.2020.00257.
- Zhang G, Wang L, Cui H, Wang X, Zhang G, Ma J, Han H, He W, Wang W, Zhao Y, et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci Rep. 2014;4:3571. doi:10.1038/srep03571.
- Yim E-K, Park J-S. Biomarkers in cervical cancer. Biomark Insights. 2007;1:215–25.
- Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007;23(4):315–30. doi:10.1155/2007/678793.
- Suri A, Saini S, Sinha A, Agarwal S, Verma A, Parashar D, Singh S, Gupta N, Jagadish N. Cancer testis antigens: a new paradigm for cancer therapy. Oncoimmunology. 2012;1(7):1194–96. doi:10.4161/onci.20686.
- Dang E, Yang S, Song C, Jiang D, Li Z, Fan W, Sun Y, Tao L, Wang J, Liu T, et al. BAP31, a newly defined cancer/testis antigen, regulates proliferation, migration, and invasion to promote cervical cancer progression. Cell Death Dis. 2018;9(8):791. doi:10.1038/s41419-018-0824-2.
- Garg M, Kanojia D, Salhan S, Suri S, Gupta A, Lohiya NK, Suri A. Sperm-associated antigen 9 is a biomarker for early cervical carcinoma. Cancer. 2009;115(12):2671–83. doi:10.1002/cncr.24293.
- Sarcevic B, Spagnoli GC, Terracciano L, Schultz-Thater E, Heberer M, Gamulin M, Krajina Z, Oresic T, Separovic R, Juretic A, et al. Expression of cancer/testis tumor associated antigens in cervical squamous cell carcinoma. Oncology. 2003;64(4):443–49. doi:10.1159/000070305.
- Chang AY, Dao T, Gejman RS, Jarvis CA, Scott A, Dubrovsky L, Mathias MD, Korontsvit T, Zakhaleva V, Curcio M, Hendrickson RC. A therapeutic T cell receptor mimic antibody targets tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest. 2017;127(7):2705–18. doi:10.1172/JCI92335.
- Spaans VM, Trietsch MD, Peters AAW, Osse M, Ter Haar N, Fleuren GJ, Jordanova ES. Precise classification of cervical carcinomas combined with somatic mutation profiling contributes to predicting disease outcome. Plos One. 2015;10(7):e0133670. doi:10.1371/journal.pone.0133670.
- Jiang W, Xiang L, Pei X, He T, Shen X, Wu X, Yang H. Mutational analysis of KRAS and its clinical implications in cervical cancer patients. J Gynecol Oncol. 2018;29(1):e4. doi:10.3802/jgo.2018.29.e4.
- Shen Y, Wei X, Jin S, Wu Y, Zhao W, Xu Y, Pan L, Zhou Z, Chen S. TCR-mimic antibody-drug conjugates targeting intracellular tumor-specific mutant antigen KRAS G12V mutation. Asian J Pharm Sci. 2020;15(6):777–85. doi:10.1016/j.ajps.2020.01.002.
- Skora AD, Douglass J, Hwang MS, Tam AJ, Blosser RL, Gabelli SB, Cao J, Diaz LA, Papadopoulos N, Kinzler KW. Generation of MANAbodies specific to HLA-restricted epitopes encoded by somatically mutated genes. Proc Natl Acad Sci USA. 2015;112(32):9967–72. doi:10.1073/pnas.1511996112.
- Nakamura H, Taguchi A, Kawana K, Baba S, Kawata A, Yoshida M, Fujimoto A, Ogishima J, Sato M, Inoue T, et al. Therapeutic significance of targeting survivin in cervical cancer and possibility of combination therapy with TRAIL. Oncotarget. 2018;9(17):13451–61. doi:10.18632/oncotarget.24413.
- Vranic S, Cyprian FS, Akhtar S, Al Moustafa A-E. The role of Epstein–Barr virus in cervical cancer: a brief update. Front Oncol. 2018;8(113). doi:10.3389/fonc.2018.00113.
- Ahmed M, Lopez-Albaitero A, Pankov D, Santich BH, Liu H, Yan S, Xiang J, Wang P, Hasan AN, Selvakumar A, O’Reilly RJ. TCR-mimic bispecific antibodies targeting LMP2A show potent activity against EBV malignancies. JCI Insight. 2018;3(4):e97805. doi:10.1172/jci.insight.97805.
- Napoletano C, Bellati F, Tarquini E, Tomao F, Taurino F, Spagnoli G, Rughetti A, Muzii L, Nuti M, Panici PB. MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic factors and effects of chemotherapy. Am J Obstet Gynecol. 2008;198(1):99.e1–7. doi:10.1016/j.ajog.2007.05.019.
- Holland CJ, Crean RM, Pentier JM, de Wet B, Lloyd A, Srikannathasan V, Lissin N, Lloyd KA, Blicher TH, Conroy PJ, Hock M. Specificity of bispecific T cell receptors and antibodies targeting peptide-HLA. J Clin Invest. 2020;130(5):2673–88. doi:10.1172/JCI130562.
- Cai S, Han K. Research on expression and importance of p53, p16 and VEGF-C in cervical cancer. J Gynecol Obstet Biol Reprod (Paris). 2015;44(7):639–45. doi:10.1016/j.jgyn.2014.07.012.
- Low L, Goh A, Koh J, Lim S, Wang CI. Targeting mutant p53-expressing tumours with a T cell receptor-like antibody specific for a wild-type antigen. Nat Commun. 2019;10(1):5382–5382. doi:10.1038/s41467-019-13305-z.
- Saeed M, Schooten E, Van Brakel M, Cole D, Ten Hagen TLM, Debets R. T cells expressing a TCR-like antibody selected against the heteroclitic variant of a shared MAGE-A epitope do not recognise the cognate epitope. Cancers. 2020;12(5):1255. doi:10.3390/cancers12051255.
- Kwek ME-J, Kwek J, Lim YH, Lim SL, Wong WL. Metastatic squamous cell carcinoma of the cervix secreting ectopic serum beta human chorionic gonadotropin. 2018.
- Neethling FA, Ramakrishna V, Keler T, Buchli R, Woodburn T, Weidanz JA. Assessing vaccine potency using TCRmimic antibodies. Vaccine. 2008;26(25):3092–102. doi:10.1016/j.vaccine.2008.02.025.
- Luo Q, Zhang S, Wei H, Pang X, Zhang H. Roles of Foxp3 in the occurrence and development of cervical cancer. Int J Clin Exp Pathol. 2015;8:8717–30.
- Dao T, Mun SS, Scott AC, Jarvis CA, Korontsvit T, Yang Z, Liu L, Klatt MG, Guerreiro M, Selvakumar A, et al. Depleting T regulatory cells by targeting intracellular Foxp3 with a TCR mimic antibody. OncoImmunology. 2019;8(7):e1570778. doi:10.1080/2162402X.2019.1570778.
- Mathias MD, Sockolosky JT, Chang AY, Tan KS, Liu C, Garcia KC, Scheinberg DA. CD47 blockade enhances therapeutic activity of TCR mimic antibodies to ultra-low density cancer epitopes. Leukemia. 2017;31(10):2254–57. doi:10.1038/leu.2017.223.
- Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol. 2019;9:682–682. doi:10.3389/fonc.2019.00682.
- Fausch SC, Fahey LM, Da Silva DM, Kast WM. Human papillomavirus can escape immune recognition through Langerhans cell phosphoinositide 3-kinase activation. J Immunol. 2005;174(11):7172–78. doi:10.4049/jimmunol.174.11.7172.
- Shreya K, Laura MF, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7(1):79–89. doi:10.2174/156800907780006869.
- Gonçalves MAG, Donadi EA. Immune cellular response to HPV: current concepts. Braz J Infect Dis. 2004;8:1–9.
- Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110(2):163–69. doi:10.1046/j.1365-2567.2003.01738.x.
- Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195(3):346–55. doi:10.1002/jcp.10290.
- Gameiro SF, Zhang A, Ghasemi F, Barrett J, Nichols A, Mymryk J. Analysis of class I major histocompatibility complex gene transcription in human tumors caused by human papillomavirus infection. Viruses. 2017;9(9):252. doi:10.3390/v9090252.
- Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011;4(3):361–75. doi:10.1007/s12307-011-0066-7.
- Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98(4):1108–15. doi:10.1182/blood.V98.4.1108.
- Wan S, Pestka S, Jubin RG, Lyu YL, Tsai Y-C, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. Plos One. 2012;7(3):e32542. doi:10.1371/journal.pone.0032542.
- Yang X, Xie S, Yang X, Cueva JC, Hou X, Tang Z, Yao H, Mo F, Yin S, Liu A, et al. Opportunities and challenges for antibodies against intracellular antigens. Theranostics. 2019;9(25):7792–806. doi:10.7150/thno.35486.
- Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, De Morais Guerreiro MD, Veomett N, et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol. 2015;33(10):1079–86. doi:10.1038/nbt.3349.
- Lu J, Gu Y, Li Q, Zhong H, Wang X, Zheng Z, Hu W, Wen L. Wilms’ tumor 1 (WT1) as a prognosis factor in gynecological cancers: a meta-analysis. Medicine. 2018;97(28):e11485–e11485. doi:10.1097/MD.0000000000011485.
- Lowe DB, Bivens CK, Mobley AS, Herrera CE, McCormick AL, Wichner T, Sabnani MK, Wood LM, Weidanz JA. TCR-like antibody drug conjugates mediate killing of tumor cells with low peptide/HLA targets. mAbs. 2017;9(4):603–14. doi:10.1080/19420862.2017.1302630.
- Zhu X, Belmont HJ, Price-Schiavi S, Liu B, Lee H-I, Fernandez M, Wong RL, Builes J, Rhode PR, Wong HC, et al. Visualization of p53 264–272/HLA-A*0201 complexes naturally presented on tumor cell surface by a multimeric soluble single-chain T cell receptor. J Immunol. 2006;176(5):3223–32. doi:10.4049/jimmunol.176.5.3223.
- He Q, Jiang X, Zhou X, Weng J. Targeting cancers through TCR-peptide/MHC interactions. J Hematol Oncol. 2019;12(1):139. doi:10.1186/s13045-019-0812-8.
- Chang AY, Gejman RS, Brea EJ, Oh CY, Mathias MD, Pankov D, Casey E, Dao T, Scheinberg DA. Opportunities and challenges for TCR mimic antibodies in cancer therapy. Expert Opin Biol Ther. 2016;16(8):979–87. doi:10.1080/14712598.2016.1176138.
- Krausa P, Iii MB, Savage D, Hui KM, Bunce M, Ngai JLF, Teo DLT, Ong YW, Barouch D, Allsop CEM, et al. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45(4):223–31. doi:10.1111/j.1399-0039.1995.tb02444.x.
- Li D, Toji S, Watanabe K, Torigoe T, Tsukahara T. Identification of novel human leukocyte antigen-A*11:01-restricted cytotoxic T-lymphocyte epitopes derived from osteosarcoma antigen papillomavirus binding factor. Cancer Sci. 2019;110(4):1156–68. doi:10.1111/cas.13973.
- Maciag PC, Schlecht NF, Souza PS, Franco EL, Villa LL, Petzl-Erler ML. Major histocompatibility complex class II polymorphisms and risk of cervical cancer and human papillomavirus infection in Brazilian women. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1183.
- Dass SA, Norazmi MN, Acosta A, Sarmiento ME, Tye GJ. TCR-like domain antibody against mycobacterium tuberculosis (Mtb) heat shock protein antigen presented by HLA-A*11 and HLA-A*24. Int J Biol Macromol. 2020;155:305–14. doi:10.1016/j.ijbiomac.2020.03.229.
- Garrido F. MHC/HLA class I loss in cancer cells. Adv Exp Med Biol. 2019;1151:15–78.
