ABSTRACT
Aims: An observational study of a retrospective cohort was performed to assess the impact of influenza vaccination (IV) on the risk of SARS-CoV-2 infection in a population of middle-aged people for 8 weeks after IV and compared with an unvaccinated group.
Patients and methods: Data from 1098 middle-aged patients (53.7 ± 4.7 years) after IV and 1205 unvaccinated patients (50.1 ± 6.8 years) were analyzed based on medical documentation. The inclusion criteria were age between 40 − 60 years and IV in the period from 1−30 September 2020. The incidence of infection with SARS-CoV-2 was confirmed by PCR and the classification of ICD-10 (U07.1).
Results and conclusions: After IV, patients had significantly fewer SARS-CoV–2 infections than the unvaccinated patients (P = .017). The hazard ratio was 0.74 (95% CI: 0.54−0.89). IV may partially reduce the risk of SARS-CoV-2 infection.
Introduction
COVID-19 is an infectious disease caused by a newly discovered coronavirus, SARS-CoV-2. The course of SARS-CoV-2 infections is diverse and ranges from asymptomatic, through mild respiratory disease (similar to a cold), to severe pneumonia with acute respiratory distress syndrome and/or multiple organ failure. Approximately 80% of people with SARS-CoV-2 infection do not require treatment, and the disease clears up on its own. However, one in six people had a more severe disease course and breathing problems requiring treatment.Citation1,Citation2
Currently, some new vaccines against COVID-19 are commercially available, and the process of vaccination has started in many counties.Citation3 However, it will be probably take a long time to complete vaccinations in the whole population, and the longitudinal effectiveness of the available vaccines it is still an open question. Regardless, there have been many observations made with various known drugs regarding whether they protect or moderate the course of such an infection. An indirect form of protection, recommended by the WHO, is the influenza vaccination.Citation3 There are also different activities that can protect people during the COVID-19 pandemic: for example, the WHO recommended influenza vaccination (IV). According to this recommendation, is unclear if simultaneous infection with both influenza and SARS-CoV-2 viruses results in a more severe disease.Citation4 However, such a procedure protects against the overlap of both infections and facilitates easier diagnosis of SARS-CoV-2 infection in patients with fever, cough and other symptoms.
Despite the significant difference between influenza and COVID-19, doubts could be raised about whether the use of the IV may partially protects against SARS-CoV-2 infection. Is the the importance of the nonspecific immune response described by other authors may be significant in partial protection against COVID-19 ?
The aim of this study was to assess the impact of IV on the risk of SARS-CoV-2 infection and to assess the number of patients hospitalized due to SARS-CoV-2 in a population of middle-aged people in the standard statistical model of age, sex and comorbidities for the Polish population in 2020 and to compare the group receiving IV to a similar middle-aged population that did not receive IV.
Patients and methods
Study design
This study was a multicentre, observational study of a retrospective, cohort performed between September and November 2020 in Poland with the participation of 10 outpatient clinics (in the southwest area). Patient data were made available with the signed consent of these centers, and the patients signed consent forms to be accepted when publishing the data. The study was based on an 8-week observation period following influenza vaccination.
Patients
The influenza-vaccinated group and unvaccinated patients were randomly selected based on the use of computer software (Allocation, Microsoft, Poland) as two-thirds of the analyzed total from the starting groups, namely, 1679 patients for the vaccinated group and 2197 patients for the unvaccinated group. The randomization procedure allows verification and adjustment of the analyzed groups for the statistical model of a Polish middle-aged inhabitant (age, sex, comorbidities) based on data from a statistical year.Citation5 Subsequently, the data of 1108 vaccinated patients and 1450 unvaccinated patients were analyzed based on medical documentation. The inclusion criteria for the influenza vaccinated group were as follows: age between 40 and 60 years and influenza vaccination in the period from 1−30 September 2020. The exclusion criteria were as follows: previous confirmed infection with SARS-CoV-2 (positive PCR and ICD confirmation for SARS-CoV-2) until the time of influenza vaccination and the need for any quarantine due to COVID-19.
The unvaccinated group consisted of similar participants who had decided not to receive the flu vaccine. The inclusion criteria are described as follows: age between 40 and 60 years and no influenza vaccination for the 2020/2021 flu season. The exclusion criteria were the same as those in the vaccinated group. The characteristics of the groups are presented in .
Table 1. Characteristics of the study patients
Data of all variables were obtained based on medical documentation (ICD codes, treatment). Vaccinated patients received an IV with commercially available products during September 2020 for the 2020/2021 influenza season using the following: inactivated INFLUVAC® TETRA quadrivalent influenza vaccine (BGP Pharma ULC, Ontario) or inactivated VAXIGRIP® TETRA quadrivalent influenza vaccine 2020 (Sanofi Aventis, New Zealand). The vaccines were administered through intramuscular or subcutaneous injection after examination by a doctor and according to the restrictions indicated by the manufacturers. Patients with immunodeficiencies or who had any adverse events after IV in the past were excluded.
Outcomes
The incidence of SARS-CoV-2 infection, hospitalization due to COVID-19 disease and the requirement of mechanical ventilation were monitored.
The incidence of SARS-CoV-2 infection was confirmed simultaneously by positive PCR results and the presence of ICD-10 as U07.1 diagnosis (‘COVID-19 virus identified’ is assigned to a disease diagnosis of COVID-19 confirmed by laboratory testing) during the 8 weeks after IV. The confirmed hospitalizations for SARS-CoV-2 with the classification as U 07.1 ICD-10 code were analyzed based on documentation.
All patients without positive PCR results and with a U07.2 diagnosis (‘COVID-19 virus not identified’ is assigned to a clinical or epidemiological diagnosis of COVID-19 where laboratory confirmation is inconclusive or not available) were excluded from further analysis. Antigen tests or results of IgG and IgM against SARS-CoV-2 were not analyzed.
The unvaccinated group was observed in the same period (8 weeks) as the vaccinated group and observation was started in September 2020. Observation was started during contact with the health service at that time because prescriptions were continued (without a new health problem) or the individuals were present as companions to other family members. After 8 weeks, the telephone contact was performed and independently analyzed data from the medical base were independently analyzed in terms of eventually SARS-CoV-2 infection according to the established criteria as above.
RT-PCR diagnosis
The SARS-CoV-2 RT-PCR test is a real-time reverse transcription polymerase chain reaction test for the qualitative detection of nucleic acids from SARS-CoV-2 in upper and lower respiratory specimens collected from suspected COVID-19-infected individuals. Medical staff sampled patients by collecting a swab from the throat and nasal vestibules. Total nucleic acids were extracted from specimens using a MagNA Pure 96 system (Roche, Basel, Switzerland). A real-time RT-PCR assay targeting the RdRp/Hel gene of SARS-CoV-2 was conducted using the Quanti Nova Probe RT-PCR Kit (QIAGEN, Germany) in a Light Cycler 480 II Real-Time PCR (Roche, Basel, Switzerland) system or using a virellaSARS-CoV-2 seqc rRT-PCR kit including primers and dual-labeled probes (Kornwestheim, Germany) and the Applied Biosystem (Waltham, MA, USA) system.
Statistics
Baseline characteristics were analyzed using descriptive statistics in Statistica software (Softpol, Cracow, Poland). The χ2 test was used to compare categorical parameters between groups, and an independent t-test was used for continuous parameters. A P-value <0.05 was considered significant.
Cox proportional hazard models were used to assess adjusted hazard ratios comparing vaccinated and unvaccinated patients with respect to SARS-CoV–2 infection diagnosis according inclusion criteria.
Additionally, we adjusted all confounders investigated in the study that were evaluated by a multivariate Cox regression model as follows: comorbidities, subgroup of age (40–50, 51−60), sex, comorbidities mentioned in , high economic status as additional health insurance, influenza infection in the last year, influenza vaccination in the past (minimum in previous season), pro-health behavior: preventive medical checkups in the last year) and BCG vaccination in the past. A hazard ratio of > 1 indicates that adjustment led to a stronger effect on the final effect, which was defined as protection against SARS-CoV-2 infection. The 95% confidence interval was used according to Hrobjartsson.Citation6
For a vaccine effectiveness, to exclude infection soon after vaccination, secondary analysis with excluding the initial 2 weeks of observation after influenza vaccination and the corresponding period of the unvaccinated group were performed and are shown in .
Figure 1. Effect of influenza vaccination on SARS-CoV-2 infections in vaccinated and unvaccinated patients
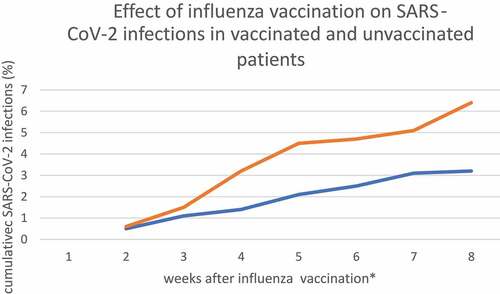
We presented a Kaplan–Meier curve by excluding new SARS-CoV-2 infections within 2 weeks after influenza vaccination to show the trend in vaccinated and unvaccinated patients.
Therefore, the follow-up period for the vaccinated group had two variants: 6 weeks (presented only in ) and 8 weeks (all other presented results).
Results
A total of 1098 patients who received influenza vaccination and 1205 unvaccinated patients were analyzed due to the presence of their complete medical data. A total of 245 unvaccinated patients were excluded due to lack of contact with them or insufficient medical data in documentation after the whole period of observation. The initial characteristics of the analyzed patients are presented in .
SARS-CoV-2 infection
During the 8 week retrospective observation, 42 (3.8%) patients after influenza vaccination and 87 (7.2%) unvaccinated had confirmed SARS-CoV-2 infection based on PCR and ICD-10. Vaccinated patients had significantly fewer SARS-CoV-2 infections in the studied age group (p = .017) than the unvaccinated control group. The hazard ratio (HR) of SARS-CoV–2 infection in patients after influenza vaccination was 0.74 (95% CI: 0.54−0.89). The hazard ratios of all possible confounding factors revealed moderate importance on final observation, and detailed results are presented in .
Table 2. Hazard ratios (HRs) for protection against SARS-CoV–2 infection comparing vaccinated beneficiaries with unvaccinated patients in relation to the analyzed confounding factors
In the vaccinated group, 6 (14%) of 42 patients needed hospitalization for to respiratory problems, due to a decrease in oxygen saturation over the course of SARS-CoV-2 infection.
A total of 4 patients exhibited comorbidities (all of them cardiovascular and one cardiovascular and diabetes), but none required mechanical ventilation. In the control group, 15 (17%) patients were hospitalized due to the same symptoms of SARS-CoV-2 infection, this accounted for statistically more patients in comparison to those who were vaccinated (p = .013). Two unvaccinated patients needed mechanical ventilation. The HR of the hospitalization caused by SARS-CoV–2 for patients after influenza vaccination was 0.48 (95% CI: 0.36−0.77). Detailed information about hospitalized patients is included in .
Table 3. Patients hospitalized due to SARS-CoV-2 based on PCR confirmation
Discussion
There is no consensus about the relationship between influenza vaccination and the risk of SARS-CoV-2 infection. However, there is evidence that vaccines may induce a positive nonspecific immune response to other pathogens.Citation1 Generally, there is an open discussion about the potential benefits or risks of influenza vaccination on the risk of COVID–19.Citation7–10
The data suggest that influenza vaccination could partially protect against SARS-CoV-2 in middle-age patients with or without comorbidities. The data are partially compatible with the results of Ragni et al.Citation11 In their study, influenza vaccination was associated with a less frequent SARS-CoV-2 infection diagnosis. A protective effect was seen in elderly individuals receiving the influenza vaccination almost in parallel with the SARS-CoV-2 outbreak. However, the study design and the target group were different than those in our study. Additionally, Noale et al. confirmed a decreased probability of a SARS-CoV-2- positive test in people <65 years old who received vaccinated influenza and pneumococcal vaccination.Citation12 However, in each of these studies, other factors may have influenced the final results.
We also analyzed confounding factors involved in our study including sex, age, comorbidities, economic status, pro-health behavior, influenza and BCG vaccination in the past which were adjusted however their roles were not significant except for the moderate importance of comorbidity as reported by other authors in a similar study with IV but with different analyzed endpoints.Citation13
An explanation of the effect of vaccination is that the cellular immune response against one virus may produce unspecific protection against other infections, but this effect has not been studied.Citation9 However, influenza hemagglutinin, which is included in influenza vaccines, is not related to any of the SARS-CoV-2 antigens. Therefore, cross-immunity between specific antibodies against both viruses is not expected.Citation4,Citation7
Different observations were noted by Martinez-Baz I et al.Citation14 They conducted an evaluation of whether influenza vaccination in the 2019–2020 season had any effect on the risk of SARS-CoV-2 confirmed infection in a cohort of health workers. The authors concluded that influenza vaccination did not significantly modify the risk of SARS-CoV-2 infection. However, these observations, which were similar to the present study, were not performed in a sufficiently large populations to draw a final conclusion.
Some experimental studies proposed reducing the impact of COVID‐19 by means of one other non-COVID-19 vaccine.Citation3,Citation15 This method has been proposed for the bacillus Calmette–Guérin (BCG) vaccine, the oral polio vaccine and the measles, mumps and rubella vaccine.Citation16,Citation17 The proposed mechanism is described as trained immunity, where exposure to one agent alters the epigenetic profile of innate immune cells, potentially increasing the production of cytokines.Citation3
There were some limitations of this study. First, this study was only a retrospective observational study, and many possible confounding factors could influence on the final results, as it was described above. There was a small group of patients under observation, and there was a lack of a precise immunological and general health assessment of the studied patients. However, this is only a preliminary study, and the observed number of patients vaccinated in this group and in other age groups is constantly being increased.
Conclusion
Influenza vaccination may reduce the risk of SARS-CoV-2 infection and associated hospitalization in the middle-aged portion of the standard population. Prospective studies on a larger group are needed to confirm this conclusion.
Authors’ contributions
Andrzej Bozek: concept of the project, data collection, manuscript preparation; Renata Kozłowska: data collection, data analysis; Beata Galuszka: data collection, data analysis, review of the literature and responsibility for designing the study. All authors read and approved the final manuscript; Alicja Grzanka- data recalculation, statistical analysis.
Data sharing statement
The data for this manuscript are available from the corresponding authors upon reasonable request.
Disclosure of potential conflicts of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript.
Ethical conduct of research
This study was reviewed by the Ethics Committee (Katowice, Poland), but no special permission was needed for this project; however, written informed consent was obtained from the enrolled patients.
Acknowledgments
The authors thank Dr. M. Lodek and Dr. I. Zlotek for their cooperation and for sharing the data.
References
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, Van Der Meer JWM, Mhlanga MM, Mulder WJM, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. doi:10.1038/s41577-020-0285-6.
- Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2020;17:108634.
- Tregoning JS, Brown ES, Cheeseman MH, Flight KE, Higham SL, Lemm NM, Pierce BF, Stirling DC, Wang Z, Pollock KM. Vaccines for COVID‐19. Clin Exp Immunol. 2020 Nov;202(2):162–92. Published online 2020 Oct 18. doi:10.1111/cei.13517
- World Health Organization. Immunization, vaccines and biologicals. Influenza in the time of COVID (2020). [accessed 2021 Feb 15]. https://www.euro.who.int/immunization/health-topics/commoable-diseases/influenza/news.
- Main Statistical Office in Poland. 2020. [accessed 2021 Feb 20]. https://stat.gov.pl/obszary-tematyczne/ludnosc/.
- Hróbjartsson A, Skou Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, Ravaud P, Brorson S. Observer bias in randomized clinical trials with binary outcomes systematic review of trials with both blinded and non-blinded outcome assessors. BMJ. 2012;344:e1119. doi:10.1136/bmj.e1119.
- Sultana J, Mazzaglia G, Luxi L, Cancellieri A, Capuano A, Ferrajolo C, de Waure C, Ferlazzo G, Trifirò G. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks and public health considerations. Expert Rev Vaccines. 2020;6:1–18.
- Consortium E. COVID-19 severity in Europe and the USA: could the seasonal influenza vaccination play a role? Soc Sci Res Netw. 2020;1:1–8.
- Li Q, Tang B, Bragazzi NL, Xiao Y, Wu J. Modeling the impact of mass influenza vaccination and public health interventions on COVID-19 epidemics with limited detection capability. Mat Biosc. 2020;325.
- Amato M, Werba JP, Frigerio B, Coggi D, Sansaro D, Ravani A, Ferrante P, Veglia F, Tremoli E, Baldassarre D, et al. Relationship between influenza vaccination coverage rate and COVID-19 outbreak: an Italian ecological study. Vaccines. 2020;8(3):535. doi:10.3390/vaccines8030535.
- Ragni P, Marino M, Formisano D, Bisaccia E, Scaltriti S, Bedeschi E, Grilli R. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines (Basel). 2020;8(4):E675. doi:10.3390/vaccines8040675.
- Noale M, Trevisan C, Maggi S, Antonelli Incalzi R, Pedone C, Di Bari M, Adorni F, Jesuthasan N, Sojic A, Galli M. The association between influenza and pneumococcal vaccinations and SARS-CoV-2 infection: data from the EPICOVID19 web-based survey. Vaccines. 2020;8(3):471. doi:10.3390/vaccines8030471.
- Zhang HT, McGrath LJ, Wyss R, Alan R, Ellis AR, Stürmer T. Controlling confounding by frailty when estimating influenza vaccine effectiveness using predictors of dependency in activities of daily living. Pharmacoepidemiol Drug Saf. 2017 Dec;26(12):1500–06. doi:10.1002/pds.4298.
- Martínez-Baz I, Trobajo-Sanmartín C, Arregui I, Navascués A, Adelantado M, Indurain J, Fresán U, Ezpeleta C, Castilla J. Influenza vaccination and risk of SARS-CoV-2 infection in a cohort of health workers. Vaccines (Basel). 2020;8(4):E611. doi:10.3390/vaccines8040611.
- O’Neill LA, Netea MG. BCG‐induced trained immunity: can it offer protection against COVID‐19? Nat Rev Immunol. 2020;20:335–37. doi:10.1038/s41577-020-0337-y.
- Chumakov K, Benn CS, Aaby P, Kottilil S, Gallo R. Can existing live vaccines prevent COVID‐19? Science. 2020;368:1187. doi:10.1126/science.abc4262.
- Fidel PL, Noverr MC, Gilmore MS. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? mBio. 2020;11(3):e00907–20. doi:10.1128/mBio.00907-20.
