ABSTRACT
Following a rubella outbreak in 2011, Vietnam implemented a mass measles-rubella vaccination campaign for children aged 1–14 years in 2014–2015, further expanding the target age to 16–17 years in 2016; routine vaccination was introduced in 2014. However, there was concern that a substantial proportion of women of child-bearing age were still susceptible to rubella, with the fear of congenital rubella emergence. Thus, we conducted a prospective cohort study in Nha Trang, Vietnam, from 2017–2018 to investigate pregnant women’s susceptibility to rubella infection, the incidence of congenital rubella infection, and factors associated with susceptibility. Cord blood was tested for rubella-specific immunoglobulin M (IgM) and IgG; neonatal saliva and cord blood specimens were examined for rubella-RNA. We analyzed 2013 mother-baby pairs. No baby was rubella-IgM or rubella-RNA positive. Overall, 20.4% of mothers were seronegative (95% confidence interval, 18.6%–22.1%). The seronegativity was significantly low among mothers aged <35 years. We found that maternal age groups of 20–24 and 25–29 years, and the lack of self-reported vaccination history were significantly associated with seronegativity. Many pregnant women who were not covered by the vaccination campaign are still at risk of rubella infection.
Rubella infection in pregnant women can result in miscarriage, fetal death, or congenital disabilities known as congenital rubella syndrome (CRS).Citation1,Citation2 In 2009–2010, when rubella-containing vaccines (RCVs) were not part of the national vaccination programme, 29% (95% confidence interval [CI], 27%–31%) of pregnant women in Nha Trang, Vietnam, were susceptible to rubella.Citation3 In 2011, a large-scale rubella outbreak occurred throughout Vietnam, and many CRS cases emerged.Citation4 After experiencing this outbreak and estimating the number of CRS cases,Citation3,Citation5 Vietnam conducted a mass measles-rubella (MR) vaccination campaign in 2014–2015, targeting children aged ≤14 years (born between January 2000 and June 2013). Furthermore, MR vaccination was introduced into the routine vaccination programme in 2014 for 18-month-old children and added MR vaccination campaign for adolescents aged 16–17 years in 2016 (born in 1998–1999).Citation6 The MR vaccination campaign and the routine vaccination programme were initiated with MR-VAC (Serum Institute of India Pvt. Ltd., Pune, India) and subsequently, the routine vaccination programme was continued using MRVAC (Polyvac, Hanoi, Vietnam) that was produced and licensed in Vietnam in 2018. The MR vaccination campaign covered 98.2% and 94.9% of the target population in 2014–2015 and 2016, respectively, in the entire country. The coverage was 97.6% and 93.1%, respectively, in the Khanh Hoa province, where Nha Trang is located.Citation7 Vietnam has maintained 90%–95% MR routine vaccination coverage between 2014 and 2018. Despite introducing the vaccines in Vietnam, 59–798 rubella and 1–19 CRS cases were reported annually between 2014 and 2018.Citation8 While such preventive strategies could prevent rubella outbreaks among children, it is a serious concern that a substantial proportion of women of child-bearing age (not covered by the catch-up vaccination) may remain susceptible and get infected in case of an outbreak.
This study investigated the susceptibility of pregnant women to rubella infection, the incidence of CRS/congenital rubella infection among new-born infants, and the factors associated with them in 2017–2018, in a transition period from pre- to post-RCV status, when it had been six years after the previous outbreak and three years after the catch-up campaign and introduction of the MR vaccine into the routine vaccination programme.
A prospective cohort study was launched at Khanh Hoa General Hospital (KHGH) in Nha Trang, the capital city in Khanh Hoa province, central Vietnam. This study, a survey using the birth cohorts, was a variant of a previous study published before the introduction of MR vaccinations in 2014.Citation3 KHGH is a provincial hospital with approximately 6000 deliveries per year. We enrolled women aged ≥18 years, living in 16 target communes in Nha Trang, who delivered a live singleton baby at KHGH, between July 2017 and September 2018. Women with multiple births, stillbirths, or serious complications before pregnancy were excluded. We provided a structured questionnaire before delivery and collected demographic, socioeconomic, and clinical information, including self-reported RCV history and symptoms of rubella or other infections during pregnancy. The symptoms included fever, rash, arthralgia/arthritis, lymphadenopathy, and conjunctivitis. Perinatal and neonatal information was obtained from medical charts. The neonate’s symptoms were also recorded, focusing on those suspected of congenital infection that included birthweight (<the 10th percentile), suspected meningoencephalitis (bulging or enlarged anterior fontanel), microencephaly (head circumference <30 cm), hydrocephalus/ventriculomegaly, congenital heart disease, and suspected hearing loss (lack of Moro reflex in response to auditory stimuli or failed automated auditory brainstem response test (if performed)). Other symptoms included cataracts, glaucoma, hepatosplenomegaly, jaundice (<24 hours after birth or requiring exchange transfusion), purpura, and/or petechiae (“blueberry muffin” lesions), lymphadenopathy, anemia (hemoglobin <13 g/dL), and thrombocytopenia (platelet <150,000/μL). The process was conducted by two trained research staff-nurses at KHGH and two trained field workers from Khanh Hoa Health Service, under the supervision of a research clinician in KHGH.
Cord blood was collected immediately after delivery. The plasma was tested for rubella-specific immunoglobulin M (IgM) using an enzyme immunoassay kit (Denkaseiken, Tokyo, Japan) and immunoglobulin G (IgG) using an enzyme-linked immunosorbent assay kit (IBL INTERNATIONAL, Hamburg, Germany). Anti-rubella IgM levels were categorized as negative for antibody index of <0.80, equivocal for antibody index of 0.80–1.20, and positive for antibody index of >1.20, following the manufacturer’s recommendations. IgG levels were categorized as negative for titers <10 IU/mL and positive for titers ≥10 IU/mL.Citation9,Citation10
Saliva samples were obtained from the infants by retaining a swab under the tongue for approximately 30 seconds to absorb sufficient saliva on birth or the next day. The swab was placed into a sample tube, and the saliva was spun down. The saliva and whole blood samples were tested for rubella virus RNA using real-time polymerase chain reaction (RT-PCR), which was considered negative if viral RNA was not detected (<2 x 10^3 copies/mL).Citation11
Demographic, socioeconomic, and clinical characteristics of the enrolled mothers and babies, who were rubella-specific IgG-positive/negative, were described using frequency and percentages. The mothers’ age was divided into six groups: 18–19, 20–24, 25–29, 30–34, 35–39, and ≥40 years. Overall and age group-specific proportions of those negative for rubella-specific IgG, such as rubella seronegativity (susceptibility), were calculated. The number of neonates with positive rubella-specific IgM or rubella-RNA, as congenital rubella infection, was determined. The odds ratio (OR) of rubella seronegativity for every mother’s or baby’s demographic, socioeconomic, or clinical factor was estimated using logistic regression. Each OR, except for the OR for the mother’s age group, was adjusted for the mother’s age group (adjusted ORs [aORs]). Rubella seronegativity in the total cohort and each mother’s age group were compared with those in a previous study,Citation3 using the chi-squared test. P values of <0.05 were considered statistically significant. Statistical analyses were conducted using STATA version 14.0 (StataCorp LLC, TX, USA).
The institutional ethical review boards of the National Institute of Hygiene and Epidemiology, Hanoi (IRB-VN01057-30/2015) and the Institute of Tropical Medicine, Nagasaki University (160908158), approved this study. Written informed consent was obtained from all respondents before participation.
Overall, 3223 deliveries from mothers living in the catchment area were carried out at KHGH. Of those, 2015 women (62.5%) were eligible and enrolled in the study, and 2013 cord blood samples were collected and tested. We could not collect the samples from two of the 2015 enrollments. The mean age of the 2013 mothers was 28.4 years (standard deviation [SD] 4.9), ranging from 18–46 years. A majority (1407/2013, 69.9%) of the women had obtained high school or higher-level education, and 60% (1199/2013) lived in urban areas. Overall, 12.6% (253/2013) of the mothers reported receiving RCV. The proportions of self-reported RCV administration were 0% (0/53), 7.6% (29/382), 15.2% (119/785), 15.6% (88/564), 8.3% (16/193), and 2.8% (1/36) in the age groups 18–19, 20–24, 25–29, 30–34, 35–39, and 40–46 years, respectively. The babies’ mean birthweight was 3282.8 g (SD 419.9), range 1700–5300 g, at the median gestational age of 39.3 weeks (interquartile range 38.6–40.0), range 32.7–41.4. The characteristics of the mothers and babies are shown in .
Table 1. Rubella seronegativity in each characteristic of mothers and babies
Further, 20.4% (410/2013, 95% CI, 18.6–22.1) of the mothers were rubella seronegative. The highest (104/382, 27.2% [95% CI, 22.7–31.7]) and the lowest (4/36, 11.1 [95% CI, 0.3–21.9]) were observed in the age groups 20–24 and ≥40 years, respectively (). Rubella seronegativity was associated with women aged 20–24 years (OR 1.89 [95% CI, 1.38–2.60]) and 25–29 years (OR 1.35 [1.02–1.79]), compared to those aged 30–34 years; and those who reported to have not been administered RCV previously (aOR 5.85 [3.24–10.58]), compared with those that reported RCV history.
Figure 1. Rubella seronegativity in total and each mothers’ age group in Nha Trang, Vietnam, 2009–2010 and 2017–2018
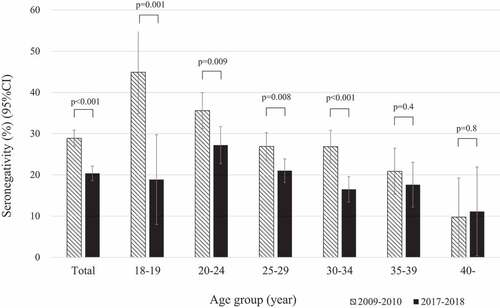
Rubella seronegativity was significantly lower in the overall (p < .001) and in age-groups 18–19 (p = .001), 20–24 (p = .009), 25–29 (p = .008), and 30–34 years (p < .001) (), compared to the 2009–2010 birth cohort study.Citation3 Younger mothers had higher seronegativity during 2009–2010; however, the peak shifted to age-group 20–24 years during 2017–2018.
Symptoms, such as fever and arthralgia/arthritis were reported in 101 and 1 mother, respectively; however, none experienced rash, lymphadenopathy, or conjunctivitis during pregnancy. In this study, 38 infants had at least one symptom suspected of congenital infection, including purpura/petechiae (only) in 17, jaundice within 24 hours after birth (only) in 6, light-for-dates (only) in 6, thrombocytopenia with/without light-for-dates in 6, PDA (only) in one, PDA, microcephaly, and light-for-dates in one, PDA and purpura/petechiae in one, hepatosplenomegaly in one, and microcephaly in one. However, all the study subjects tested, including the above mentioned 38 infants, were negative for rubella-specific IgM and rubella-RNA; therefore, no congenital rubella infection was detected in this birth cohort. Hence, we regarded the 38 babies as non-CRS.
Although there were no congenital rubella infection cases in this study, we found that a high proportion (20%) of women of child-bearing age (WCBA) are still susceptible to rubella infection after the 2011 outbreak.
The introduction of RCV may have contributed to the suppression of congenital rubella infection, as detected in this study, by reducing the rubella virus circulating in the community and suppressing an epidemic even 6 years after the previous outbreak. Before the introduction of the vaccination, large epidemics occurred every 3–8 years.Citation12
The previous birth cohort study, with the same setting, found that 28.9% of WCBA were seronegative to rubella, in which 3 of 1988 babies had congenital rubella infection.Citation3 The study was conducted in 2009–2010, before the rubella outbreak (2011) and the introduction of RCV (2014). In comparison, seronegativity decreased significantly in the current study owing to infection and/or vaccination. The 2014–2016 MR vaccination campaign could be implemented in the women aged 18–20 years in 2017–2018, most of the youngest age group and a part of age 20–24 group in this study, when they were 14–17 years old. Assuming that the rubella virus can equally infect susceptible people of all age groups, the substantial reduction in seronegativity among the youngest age group could be explained by vaccination. Meanwhile, 18.9% of women aged 18 to 19 were still seronegative, and the reduction in seronegativity seemed less than expected from the high immunogenicity of RCVs and the high officially-reported coverage rate (>90%) of the campaign in Khanh Hoa. Without individual vaccination record information, however, it will be difficult to discuss the gap in detail.
Globally, a wide range of rubella seronegativity among WCBA has been reported after implementing the RCV programme. It ranged from 1.6% in Iran, in 2012,Citation13 1.7% in Poland, in 2015,Citation14 and 3.0% in Brazil, between 2009–2010,Citation15 to 24% in Sri Lanka, in 2017,Citation16 33.8% in China, in 2012,Citation17 and 41.6% in China, in 2017.Citation18 These rates may differ due to the vaccination strategy differences, coverage, time after its introduction, and timing of rubella epidemic. To introduce RCVs in a country, the World Health Organization recommended a campaign to promote RCV, targeting individuals of varying ages (e.g., 9 months–15 years), followed immediately by the introduction of RCV into the routine programme. Additionally, it emphasized that countries should make efforts to vaccinate all non-pregnant WBCA who are not already vaccinated or who are seronegative for rubella by 1 dose of RCV.Citation19,Citation20 Robertson et al. warned against the strategy of a mass campaign for 1–14-year-olds with routine RCV vaccination for children, resulting in the resurgence of rubella in teens/adults, leading to CRS.Citation21 In a systematic review, Mongua-Rodríguez et al. compared the impact of rubella vaccination strategies on acquired rubella and CRS rates in the Americas.Citation22 In the review, vaccination strategies for children, similar to those conducted in Vietnam, reduced the rate of acquired rubella incidenceCitation23,Citation24 and prolonged the epidemic cycles.Citation25 However, this strategy was insufficient to control the endemic rates because outbreaks among young adults continued to occur.Citation24 Combined vaccination strategy with a risk population approach for adults (WCBA, college students, health care workers, teachers, military personnel, government employees, and industry employees) and universal vaccination for boys and girls could be more efficient in reducing the incidence of acquired rubella and CRS as observed in Brazil, the US, and Cuba.Citation26–29 The review concluded that a combined vaccination strategy with routine vaccination for boys and girls and universal vaccination for adolescents and adults (both men and women), aged 15–39 years, through “speed-up” campaigns, was the most effective strategy.Citation22 This strategy reduced rubella incidence by more than 98%, and no CRS was reported since 2008 in Mexico and Costa Rica.Citation30,Citation31 A universal or risk population approach to adults should also be considered in Vietnam and other countries, initiating a rubella elimination programme to protect WCBA directly and indirectly from exposure to rubella, depending on the feasibility of such approaches and the available resources.
We found that age groups 20–24, 25–29, and having no self-reported RCV history increased seronegativity. The youngest age group showed the highest seronegativity in previous studies during the pre/post-RCV era,Citation3,Citation17,Citation32–34 indicating cumulative opportunities to be exposed to rubella virus or vaccination by age. However, younger age did not influence increased seronegativity in other studies, both before and after RCV initiation,Citation15,Citation35,Citation36 depending on the timing of rubella epidemics and the difference in vaccine coverage of each age group after changing the vaccine strategy in each country. Our study shifted the peak age from <20 to 20–29 years, probably due to mass vaccination campaigns discussed above.
Some factors, including maternal education, maternal occupation, residential area, para, number of antenatal care visit, mode of delivery, baby’s sex, low birth weight, and preterm birth, might be less relevant for rubella seronegativity in this study, while some of these were discussed as the relevant factors in previous studies.Citation3,Citation17,Citation34,Citation36–39
A limitation of the present study is that we could not discuss the effect of RCVs on the rubella seronegativity sufficiently due to the unavailability of individual vaccination record to confirm the self-reported RCV history. This study described the CRS incidence and rubella seronegativity that was reported a relatively short time after the previous rubella outbreak and vaccine introduction. Further studies are required to monitor the changes in the CRS incidence and rubella seronegativity in WCBA and all generations in Vietnam to assess the risk and potential source of rubella infection/transmission. The study site, Nha Trang, was not necessarily representative of Vietnam as a whole. Considering the 2011 rubella outbreak throughout Vietnam and the high coverage of MR vaccination campaign in both Nha Trang and all of Vietnam, however, the situation might be similar in other areas in Vietnam.
In conclusion, no babies with CRS were detected in this study; however, a substantial proportion of WCBA who were not covered by the MR vaccination campaign are at risk of rubella infection during pregnancy, potentially contributing to the CRS burden in Vietnam, three years after introducing the vaccine. This susceptible proportion will drop over time if a high vaccination rate among children is maintained. Moreover, a universal or risk population approach to adults should be considered until the routine vaccination programme immunizes most of the population.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the participants and the staff in Vietnam who cooperated in the study. We are also grateful to Dr. Kiyoko Okamoto and Dr. Makoto Takeda (Department of Virology III, National Institute of Infectious Diseases, Tokyo, Japan) who provided rubella virus RNA as control, Ms. Taeko Akase (Department of Pediatrics, Nagasaki University Hospital, Nagasaki, Japan) who supported checking the samples and extracting the virus RNA, and Editage [http://www.editage.com] for providing English language editing service.
Additional information
Funding
References
- World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011; 86:301–16.
- Plotkin SA. Rubella eradication. Vaccine. 2001;19:3311–19. doi:10.1016/S0264-410X(01)00073-1.
- Miyakawa M, Yoshino H, Yoshida LM, Vynnycky E, Motomura H, Tho LH, Thiem VD, Ariyoshi K, Anh DD, Moriuchi H. Seroprevalence of rubella in the cord blood of pregnant women and congenital rubella incidence in Nha Trang, Vietnam. Vaccine. 2014;32:1192–98. doi:10.1016/j.vaccine.2013.08.076.
- Toda K, Reef S, Tsuruoka M, Iijima M, Dang TH, Duong TH, Nguyen VC, Nguyen TH. Congenital rubella syndrome (CRS) in Vietnam 2011-2012-CRS epidemic after rubella epidemic in 2010-2011. Vaccine. 2015;33:3673–77. doi:10.1016/j.vaccine.2015.06.035.
- Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar AM, Simons E, Yoshida LM, Brown DWJ, Jackson C, Strebel PM, et al. Using seroprevalence and immunisation coverage data to estimate the global burden of Congenital Rubella Syndrome, 1996-2010: a systematic review. PLoS One. 2016;11:e0149160. doi:10.1371/journal.pone.0149160.
- Vynnycky E, Yoshida LM, Thanh Huyen DT, Trung ND, Toda K, Van Cuong N, Hong DT, Ariyoshi K, Miyakawa M, Moriuchi H, et al. Modeling the impact of rubella vaccination in Vietnam. Hum Vaccin Immunother. 2016;12:150–58. doi:10.1080/21645515.2015.1060380.
- Ministry of health, Vietnam; 2021 Feb 1 [accessed 2021 Feb 1]. https://www.moh.gov.vn/en_US/web/ministry-of-health .
- WHO vaccine-preventable disease: monitoring system. 2020 global summary; 2020 July 15 [accessed 2021 Feb 1]. https://apps.who.int/immunization_monitoring/globalsummary .
- Skendzel LP. Rubella Immunity: defining the level of protective antibody. Am J Clin Pathol. 1996;106:170–74. doi:10.1093/ajcp/106.2.170.
- Best JM, Reef S. The immunological basis for immunisation series. Module 11: rubella. In: Immunization, Vacccines and Biologicals. Geneva (Switzerland): World Health Organization; 2008.
- Okamoto K, Fujii K, Komase K. Development of a novel TaqMan real-time PCR assay for detecting rubella virus RNA. J Virol Methods. 2010;168:267–71. doi:10.1016/j.jviromet.2010.05.016.
- Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet. 2015;385:2297–307. doi:10.1016/S0140-6736(14)60539-0.
- Zahraei SM, Mokhtari-Azad T, Sabouri A, Khazaei S, Karami M. Sero-epidemiological evaluation of rubella immunity among pre-marriage women in Iran. Hum Vaccin Immunother. 2019;15:2117–20. doi:10.1080/21645515.2018.1504527.
- Plewik D, Tokarska-Rodak M, Paszkiewicz J, Szepeluk A. Seroprevalence of rubella and cytomegalia in young women from biała podlaska district. Pol J Microbiol. 2017;66:543–45. doi:10.5604/01.3001.0010.7103.
- Costa GB, De Oliveira MC, Gadelha SR, Albuquerque GR, Teixeira M, Raiol MRDS, Sousa SMB, Marin LJ. Infectious diseases during pregnancy in Brazil: seroprevalence and risk factors. J Infect Dev Ctries. 2018;12:657–65. doi:10.3855/JIDC.9492.
- Palihawadana P, Wickremasinghe AR, Perera J. Seroprevalence of rubella antibodies among pregnant females in Sri Lanka. Southeast Asian J Trop Med Public Health. 2003;34:398–404.
- Liu F, Zhang S, Liu J, Wang Q, Shen H, Zhang Y, Liu M. Sociodemographic and economic characteristics of susceptibility to rubella among women preparing for pregnancy in rural China. Int J Infect Dis. 2017;62:112–18. doi:10.1016/j.ijid.2017.07.013.
- Zhou Q, Wang Q, Shen H, Zhang Y, Zhang S, Li X, Acharya G. Rubella virus immunisation status in preconception period among Chinese women of reproductive age: a nation-wide, cross-sectional study. Vaccine. 2017;35:3076–81. doi:10.1016/j.vaccine.2017.04.044.
- World Health Organization. Rubella vaccines: WHO position paper—Recommendations. Vaccine. 2011;29:8767–68. doi:10.1016/j.vaccine.2011.08.061.
- World Health Organization. Rubella vaccines: WHO position paper – July 2020. Wkly Epidemiol Rec. 2020; 95:306–24.
- Robertson SE, Cutts FT, Samuel R, Diaz-Ortega JL. Control of rubella and congentital rubella syndrome (CRS) in developing countries, part 2: vaccination against rubella. Bull World Health Organ. 1997;75:69–80.
- Mongua-Rodriguez N, Díaz-Ortega JL, García-García L, Piña-Pozas M, Ferreira-Guerrero E, Delgado-Sánchez G, Ferreyra-Reyes L, Cruz-Hervert LP, Baez-Saldaña R, Campos-Montero R. A systematic review of rubella vaccination strategies implemented in the Americas: impact on the incidence and seroprevalence rates of rubella and congenital rubella syndrome. Vaccine. 2013;31:2145–51. doi:10.1016/j.vaccine.2013.02.047.
- Herrmann KL, Halstead SB, Brandling Bennett AD, Witte JJ, Wiebenga NH, Eddins DL. Rubella immunization: persistence of antibody four years after a large-scale field trial. J Am Med Assoc. 1976;235:2201–04. doi:10.1001/jama.1976.03260460021015.
- Castillo-Solórzano C, Carrasco P, Tambini G, Reef S, Brana M, De Quadros CA. New horizons in the control of rubella and prevention of congenital rubella syndrome in the Americas. J Infect Dis. 2003;187:S146–S152. doi:10.1086/368034.
- Man C, Umido V, Bakir J, Caparelli M, Copiz A, Castillo C, Gentile A. Evaluation of the immunisation impact on the epidemiology of rubella and congenital rubella syndrome in Argentina. Rev Hosp Niños (B Aires). 2005;47:205–10.
- Lanzieri TM, Pinto D, Prevots DR. Impact of rubella vaccination strategy on the occurrence of congenital rubella syndrome. J Pediatr (Rio J). 2007;83:415–21. doi:10.2223/JPED.1692.
- Menegolla IA, Bercini MA, Schermann MT, Nunes ZMA, Segatto TC, Siqueira MM, Flannery B. Outbreak of rubella after mass vaccination of children and adult women: challenges for rubella elimination strategies. Rev Panam Salud Publica. 2011;29:243–51. doi:10.1590/S1020-49892011000400005.
- Orenstein WA, Herrmann KL, Holmgreen P, Bernier R, Bart KJ, Eddins DL, Fiumara NJ. Prevalence of rubella antibodies in Massachusetts school children. Am J Epidemiol. 1986;124:290–98. doi:10.1093/oxfordjournals.aje.a114387.
- De Los Angeles Ribas M, Galindo M, Torres G, Valcarsel M, Cancio R, Mg G, Rosario D, Resik S, Rodriguez C, Garcia D, et al. Role of the virology diagnosis laboratory in the surveillance of rubella virus: cuba 1988/2000. Vaccine. 2004;22:4287–90. doi:10.1016/j.vaccine.2004.04.023.
- Díaz-Ortega JL, Meneses-Reyes CD, Palacios-Martínez M. Incidence and transmission patterns of rubella in Mexico. Salud Publica Mex. 2007;49:337–44. doi:10.1590/S0036-36342007000500004.
- Jiménez G, Ávila-Aguero ML, Morice A, Gutiérrez H, Soriano A, Badilla X, Reef S, Castillo-Solórzano C. Estimating the burden of congenital rubella syndrome in Costa Rica, 1996-2001. Pediatr Infect Dis J. 2007;26:382–86. doi:10.1097/01.inf.0000260000.84792.9e.
- Snell LB, Smith C, Chaytor S, McRae K, Patel M, Griffiths P. Screening for potential susceptibility to rubella in an antenatal population: a multivariate analysis. J Med Virol. 2017;89:1532–38. doi:10.1002/jmv.24818.
- Dayan GH, Panero MS, Urquiza A, Molina M, Prieto S, Del Carmen Perego M, Scagliotti G, Galimberti D, Carroli G, Wolff C, et al. Rubella and measles seroprevalence among women of child-bearing age, Argentina, 2002. Epidemiol Infect. 2005;133:861–69. doi:10.1017/S0950268805004437.
- Pegha Moukandja I, Ngoungou EB, Lemamy GJ, Bisvigou U, Gessain A, Toure Ndouo FS, Kazanji M, Lekana-Douki JB. Non-malarial infectious diseases of antenatal care in pregnant women in Franceville, Gabon. BMC Pregnancy Childbirth. 2017;17:185. doi:10.1186/s12884-017-1362-0.
- Muliyil DE, Singh P, Jois SK, Otiv S, Suri V, Varma V, Abraham AM, Raut C, Gupta M, Singh MP, et al. Sero-prevalence of rubella among pregnant women in India, 2017. Vaccine. 2018;36:7909–12. doi:10.1016/j.vaccine.2018.11.013.
- Sasmaz T, Kurt AO, Ozturk C, Bugdayci R, Oner S. Rubella seroprevalence in women in the reproductive period, Mersin, Turkey. Vaccine. 2007;25:912–17. doi:10.1016/j.vaccine.2006.09.033.
- Gilbert NL, Rotondo J, Shapiro J, Sherrard L, Fraser WD, Ward BJ. Seroprevalence of rubella antibodies and determinants of susceptibility to rubella in a cohort of pregnant women in Canada, 2008-2011. Vaccine. 2017;35:3050–55. doi:10.1016/j.vaccine.2017.04.057.
- Lao TT, Sahota DS, Law LW, Leung TY. Maternal rubella immunity status and pre-eclampsia. Am J Reprod Immunol. 2017;78:e12677. doi:10.1111/aji.12677.
- Tamirat B, Hussen S, Shimelis T. Rubella virus infection and associated factors among pregnant women attending the antenatal care clinics of public hospitals in Hawassa City, Southern Ethiopia: a cross-sectional study. BMJ Open. 2017;7:e016824. doi:10.1136/bmjopen-2017-016824.
