ABSTRACT
Objective: At a large public university, we aimed to evaluate an intervention designed to increase serogroup B meningococcal (MenB) vaccine uptake and awareness.
Methods: Using a pretest-posttest design with a double posttest, we evaluated an intervention conducted by a local foundation and the Florida Department of Health that distributed MenB vaccine on campus and conducted an educational campaign. Prior to intervention activities, we recruited students to complete a survey about their MenB knowledge and attitudes. For survey participants who provided contact information, we sent two follow-up surveys and assessed MenB vaccine records. We used chi-square tests, adjusted for nonindependence, to compare preintervention to postintervention (three-month and one-year) vaccination and attitudes.
Results: Among the 686 students with accessible vaccine records, MenB vaccine initiation increased 9% (from 24% to 33%) and completion increased 8% (from 13% to 21%) from before the intervention to one year after the intervention. When restricting to students who completed the relevant follow-up surveys, the percentage of students who heard of the MenB vaccine increased by 15% (p > .001) from before the intervention to three months after (n = 188 students) and maintained a 10% increase (p > .001) one year after the intervention (n = 261 students). Among students that heard of the MenB vaccine, the percentage of students who thought they needed the MenB vaccine even though they received the MenACWY increased 14% (p = .03) by the three-month postintervention survey and up to 18% by the one-year follow-up (p = .002).
Conclusions: A university-wide, on-campus vaccination and educational campaign increased college students’ MenB vaccine initiation, completion, and knowledge.
Clinicaltrials.gov ID: NCT02975596.
Introduction
College students are at risk of both sporadic and outbreak-associated cases of serogroup B meningococcal disease (MenB).Citation1,Citation2 Between 2016 and 2018, MenB incidence was 5 times higher among college students than among 18- to 24-year-olds not attending college.Citation2,Citation3 Once contracted, MenB has a 7% case-mortality rate and 20% of survivors experience long-term disabilities.Citation1,Citation3 Routine vaccination is the recommended strategy to prevent both sporadic and outbreak associated cases.Citation4 As of 2015, the Advisory Community on Immunization Practices (ACIP) recommended the MenB vaccine as a shared clinical decision between patients and their physicians among 16- to 24-year-olds with vaccination preferred at ages 16 to 18 years.Citation5,Citation6 In 2018, however, only 17% of 17-year-olds in the United States received at least one dose of the MenB vaccine, and half of these completed the two to three scheduled doses.Citation6,Citation7 Interventions are needed to increase MenB vaccine uptake among college students.
College student-targeted vaccine interventions frequently include education, on-campus vaccination, and school entry requirements.Citation8–10 When college students received educational interventions, either through mass media or educational sessions, they were more willing to receive vaccines.Citation8,Citation11,Citation12 For example, after a Fall 2011 mass media campaign with the internet and print advertisements, 68% of the students reported an influence of the advertisement on receiving the influenza vaccine.Citation13 When on-campus immunization events were combined with education, college student vaccination increased by 20–30%.Citation8,Citation12,Citation13 One college on-campus vaccine distribution increased influenza vaccination rates by 66% from 2013 to 2014.Citation14 In subsequent years, the addition of peer-to-peer educational events further increased influenza vaccination rates by 27%.Citation14 Lastly, while only 2% of colleges require MenB vaccination for school entry such requirements are particularly effective at increasing vaccination.Citation10,Citation15
Regarding Men B vaccination, most interventions targeting college students have been mass immunization campaigns in response to outbreaks.Citation16–18 Campaigns included providing vaccines at student health centers, targeting subpopulations of students (e.g., students living in dorms, student organizations) or recommending the vaccine based on risk of exposure.Citation16–18 College mass immunization campaigns had a wide range of effectiveness with post campaign MenB vaccine initiation rates ranging from 10% to 95%.Citation4,Citation19,Citation20 The most effective intervention component was the requirement of MenB vaccination for school entry.Citation19
Within college populations experiencing outbreaks, individuals with increased knowledge about MenB vaccine were more likely to be vaccinated.Citation21,Citation22 In 2014, prior to the ACIP recommendation for MenB vaccine, students were more likely to receive the MenB vaccine if they heard it was important to receive the vaccine via official university communication rather than local announcements.Citation21 Within a Canadian university outbreaking 2015, faculty and students who believed MenB posed a health risk were 1150 times more likely than those who did see MenB as a health risk to receive the vaccine.Citation22
Outside of an active outbreak setting, college students’ knowledge of and intention to receive the MenB vaccine is unclear, nor is the effectiveness of an on-campus immunization intervention to increase MenB vaccine uptake. MenB outbreaks result in significant disruption for students and imposes a significant cost to universities.Citation20 Vaccinating a majority of at-risk students during an active outbreak of MenB requires substantial resources including large numbers of staff to administer vaccine doses, trace case contacts, and distribute information to the student body.Citation23 Conducting MenB vaccine campaigns on college campuses before outbreaks can potentially reduce the burden on universities by decreasing the risk of a MenB outbreak.Citation3 In this study, we evaluated an intervention aimed to increase MenB vaccination rates at a large, state-funded, university. The intervention included a MenB educational campaign and two on-campus immunization events. The objectives of this study were to assess the intervention’s effect on (1) MenB vaccine receipt and (2) awareness of meningitis and the MenB vaccine.
Methods
Design
We conducted a pretest-posttest design with a double posttest, to evaluate a university-wide MenB vaccine intervention. The intervention and evaluation were conducted among college students at the University of Florida, a large, public university in the Southeastern United States with 36,000 on-campus students.Citation23 All procedures were approved by the University of Florida Institutional Review Board.
Intervention
The intervention was a campus-wide campaign to increase the number of college students receiving the MenB vaccine. The community-level intervention targeted the student population on a large Southeastern university campus with two components: (1) advertising and educational events conducted by a local foundation focused on increasing MenB vaccination, the Avnee Foundation (www.avnee.org), and (2) on-campus vaccine administration conducted by the state Department of Health.
For approximately one-month prior to each on-campus vaccination administration, the Avnee foundation advertised on Facebook (direct advertising, posting on the college Facebook pages, and boosting posts) and the Avnee foundation website. In the four days (Monday through Thursday) preceding each on-campus vaccine administration, a student vaccine club helped the Avnee Foundation conduct MenB educational events with peer educators at information tables across campus. At residence halls, student peer educators explained MenB and distributed flyers that included the slogan “Beat MenB,” dates of the immunization events, and weblinks to University Student Health Center and Avnee Foundation pages. The Avnee Foundation website has educational information about meningitis, the vaccines, and includes links to other websites with MenB educational information including the Centers for Disease Control and Prevention (CDC) the American Academy of Pediatrics, and the National Meningitis Association.
On September 7, 2018 and October 17, 2018, the Florida Department of Health offered students the 2-dose Bexsero MenB vaccineCitation24 on the University’s student health center lawn. At each immunization event, the student vaccine club was present to provide educational information and answer questions about the vaccine. Educational documents provided included Centers for Disease Control and Prevention’s Meningococcal vaccine information page (https://www.cdc.gov/vaccines/vpd/mening/public/index.html), What you need to know flyer (https://www.cdc.gov/meningococcal/downloads/serogroup-b-factsheet.pdf); MenB Vaccine Information Sheet (https://www.cdc.gov/vaccines/hcp/vis/vis-statements/mening-serogroup.pdf); National Foundation for Infectious Diseases’ serogroup B page (https://www.nfid.org/infectious-diseases/meningococcal-disease-focus-on-serogroup-b/); Immunization Action Coalition Meningococcal questions and answers (https://www.immunize.org/catg.d/p4210.pdf); the National Meningitis Association Facts about Meningococcal Disease and Vaccination; and a flyer created in response to student focus groups conducted to identify the most salient constructs (Supplemental Material). Students who received vaccines were given pizza and t-shirts.
Evaluation survey
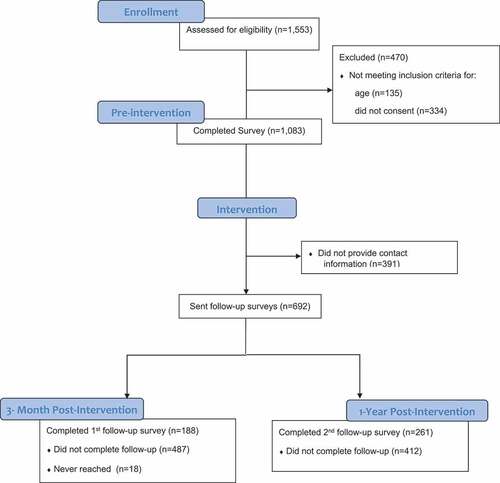
To evaluate the intervention, between August 27 and September 6, 2018, prior to the on-campus immunization events, students (ages 18 to 23 years and enrolled) were invited to complete a survey in REDCap on an iPad or their phone. We invited students to complete the survey by (1) inviting students at several public locations on campus (e.g., near residence halls, outside the student health center), (2) inviting students who visited a department table at an undergraduate prehealth career fair, and (3) an emailed survey link sent to students by college deans. We did not provide information about the on-campus immunization events. A total of 1,553 individuals expressed interest in completing the survey, among whom 1,083 met the eligibility criteria and signed the informed e-consent form (). Students who completed the survey in-person received an incentive valued at approximately 1 USD (ice cream or candy). Participants who responded via the college listserv e-mail did not receive incentives.
If survey participants provided contact information (phone number or e-mail address) on the preintervention survey (n = 692), we sent them invitations via e-mail or text message to complete two postintervention surveys. We coordinated the timing of the first postintervention survey to be after the second immunization event, but before the Spring semester started. The one-year follow-up survey was administered to evaluate any long-term change. On November 27, 2018, we sent the first invitation to complete the three-month postintervention survey. Nonresponders were sent reminders on December 4, 2018 and January 4, 2019. Upon completion of the three-month postintervention survey, students were sent a 2 USD Amazon instant access code. One year after the preintervention survey (September 5, 2019), we sent the students an invitation to complete the one-year postintervention survey. Nonresponders were sent up to five reminders with the last reminders sent on October 31, 2019. Students who completed the one-year postintervention survey received a 5 USD Amazon instant access code.
Outcomes
Primary outcome (receipt of the MenB vaccine)
To evaluate MenB vaccination, Florida Department of Health staff accessed MenB immunization records from the state immunization registry for consented participants. Study staff received records categorized by when students received the MenB vaccines doses either before the immunization event (prior to September 7, 2018) or during and after the immunization event (between September 7, 2018 and October 31, 2019). Record of at least one dose received was considered initiation. Series completion was receipt of the recommended doses for the brand of the first dose: Bexsero (2-dose schedule) or Trumenba (3-dose schedule).Citation24,Citation25 In the survey, students also reported whether they received the MenB vaccine.
Secondary outcomes
To assess the reach of educational events and advertisements, we included survey two questions that asked students to identify if (1) they received information to learn about flu vaccines, MenB vaccine, or obesity and nutrition on campus and (2) they had heard of different health slogans including Beat MenB, Tips from Smokers, Safe to Sleep, or The Heart Truth on campus. We also measured targeted constructs with survey questions on students’ knowledge about meningitis and attitudes toward the MenB vaccines. Items were adapted from previous vaccine surveys including the Health Information National Trends Survey and the National Survey of Children’s Health.Citation22,Citation26,Citation27 Meningitis knowledge questions were patterned after MacDougall et al. and assessed whether students though meningitis could cause each of the following outcomes: loss of limbs, death, brain damage, and cancer.Citation22 Response options included yes, no, and do not know.
To assess the students’ attitudes toward the MenB vaccine, we asked students whether they had heard of the MenB vaccine. Among students who had heard of the MenB vaccine, we measured Health Belief Model constructs associated with receiving the MenB vaccine including perceived benefits (e.g., MenB vaccine is safe), barriers (e.g., side effects) and susceptibility (e.g., need for the MenB vaccine even though MenB is rare).Citation28 Because of potential confusion between the two meningitis vaccines, we asked students whether they had heard of the meningococcal conjugate vaccine (MenACWY) and whether they felt receipt of this vaccine effected their risk of getting MenB.Citation29
Statistical analysis
For preintervention survey responses (n = 1,083), we ran descriptive statistics using frequencies for demographics, awareness of the MenB vaccine, and attitudes toward the MenB vaccine. Across the three time-points, we compared primary and secondary outcomes with chi-square tests adjusted for nonindependence. Consent for access to vaccine records was provided in the first survey. Thus, we were able to compare MenB initiation and completion rates across the three time points for the 686 students who provided contact information. We evaluated pre- to postintervention effects at each wave by restricting the sample to those that responded to both the baseline survey and the applicable follow-up wave. For example, to assess three-month postintervention effects, we restricted analysis to the 188 participants who completed both the preintervention survey and the three-month postintervention survey.
Results
Sample characteristics
Prior to the intervention, 1083 students completed the survey (). We attempted to contact all 692 (65%) who provided contact information for each follow-up survey. The response rate for the three-month postsurvey was 27% (188 of 692) and one-year postsurvey was 37% (261 of 692).
The majority of students who completed the preintervention survey were female (70%), non-Hispanic White (62%), and living off campus (63%) (). Compared to the preintervention cohort, responders to the three-month postsurvey were more likely to be female (70% baseline vs. 76% three months; p = .03) and older (34% age 18 baseline and 27% age 18 three months; p = .02). Compared to the preintervention cohort, responders to the one-year postintervention survey were more likely to be females (70% baseline vs. 79% one year; p = .01), less likely to be Hispanic (10% baseline vs. 4% one year; p = .02), more likely to be older (34% age 18 baseline and 2% age 18 one year; p = .01) and more likely to be living off-campus (63% baseline vs. 82% one year; p = .001).
Table 1. Demographic characteristics of participating students
MenB vaccination (primary outcome)
Among the students who completed the preintervention survey, we were able to assess vaccination records for the 686 (65%) who provided contact information as all had records in the state immunization registry. Prior to the intervention, 168 (24%) had received at least one dose of the MenB vaccine and 89 (13%) had completed the recommended doses. During on-campus vaccine administration events, a total of 840 doses of MenB vaccine were given (433 on the September 7, 2018 and 407 on October 17, 2018). Among the survey responders, MenB vaccine doses were received on intervention days by 29 students on September 7 and 25 students on October 17. By one-year postintervention, October 31, 2019, 224 (33%) students had received at least one dose of the MenB vaccine and 144 (21%) students had completed the recommended doses. When compared with the number of students who received the MenB vaccine preintervention, initiation increased 9% (χ2 = 30.03, p < .001), and completion increased 8% (χ2 = 27.13, p < .001).
Among all survey responders, 17% (189/1,083) of students reported receiving the MenB vaccine prior to the intervention. Among students who responded to the three-month postintervention survey, report of MenB vaccine receipt increased from 22% (42/188) prior to the intervention to 42% (80/188) three months after the intervention (χ2 = 27.69, p < .001). Among students who responded to the one-year postintervention survey, report of MenB vaccine receipt increased from 19% (51/262) to 39% (103/262) one year after the intervention (χ2 = 35.51, p > .001).
Secondary outcomes
Among students that completed the three-month postintervention survey, 36% (68/188) heard about the MenB vaccine on campus, and 12% (23/188) heard of the Beat MenB slogan from the advertisements. At the one-year postintervention survey, 17% (44/261) reported hearing about the MenB vaccine on campus and 11% (28/261) heard of the MenB vaccine slogan from the advertisement.
Among all students prior to the intervention, the vast majority (89%) had heard of meningitis and over half (58%) had heard of the MenACWY. Yet, only 38% had heard of the MenB vaccine. Among the students that heard of the MenB vaccine (n = 371), almost half of students (55%) were concerned about the side effects and less than half (46%) thought they needed vaccine if they received the MenACWY vaccine.
Among students who responded to the three-month postintervention survey, awareness that MenB could cause loss of limbs increased from 32% (60/188) prior to the intervention to 46% (86/188) three months after the intervention (). Among students who responded to the one-year postintervention survey, awareness that MenB could cause loss of limbs increased from 34% (88/261) prior to the intervention to 44% (114/261) one year after the intervention ().
Table 2. Change in vaccine rate, knowledge of meningitis, and awareness of MenB vaccine at three-month postintervention (N = 188)
Table 3. Change in vaccine rate, knowledge of meningitis, and awareness of MenB vaccine at one-year postintervention (N = 261)
Among students who responded to the three-month postintervention survey, awareness of the MenB vaccine increased from 41% (76/188) prior to the intervention to 56% (104/188) three months after the intervention (). A higher percentage of students who responded to the three-month postintervention survey thought they needed the MenB vaccine even though MenB was rare (preintervention 77% vs. three month 87%) and even if they received the MenACWY vaccine (preintervention 44% vs. three month 58%). Among students who responded to the one-year postintervention survey, awareness of the MenB vaccine increased from 32% (96/261) prior to the intervention to 59% (153/261) one year after the intervention (). A higher percentage of students who responded to the one-year postintervention survey thought they needed the MenB vaccine even if they received the MenACWY vaccine (preintervention 47% vs. one year 65%).
To assess effects of attrition, we compared the responses prior to the intervention between the restricted samples (188, 261) and the entire sample (1,083). We did not find differences between the samples. For example, among the 188 students that completed the three-month follow-up, 41% (76/188) heard of the MenB vaccine compared with the 38% from the entire sample. Among the 76 students that heard of the MenB vaccine, 89% thought it was safe compared with the 90% of the entire sample, and 44% thought they did need the MenB vaccine even though they received the MenACWY compared with the 45% from the entire sample. Among the 261 that completed the one-year follow-up, 37% (96/261) heard of the MenB vaccine prior to the intervention compared with the 38% of the entire sample. Among the 96 students that heard of the MenB vaccine, 88% thought the vaccine was safe and 47% thought they needed the MenB vaccine even though they received the MenACWY.
Discussion
An on-campus, university-wide MenB vaccine education and immunization intervention likely increased college students’ MenB vaccine receipt, knowledge, and awareness. The size of the increase in MenB initiation and completion was comparable to mass vaccination campaigns responding to MenB outbreaks on large university campuses.Citation18,Citation30,Citation31 Even up to a year after the intervention, students had increased knowledge about MenB severity, susceptibility and vaccination. MenB vaccine campaigns can be effective on college campuses even without the presence of an outbreak.
Outside the presence of a MenB outbreak, a university-wide MenB vaccine education and immunization intervention was similarly effective at increasing MenB vaccine initiation and completion rates as a large university-based mass immunization campaigns responding to outbreaks.Citation18,Citation19,Citation30 In particular, our postcampaign MenB initiation and completion rates were similar to the postmass immunization campaign following an outbreak at a Oregon university (initiation: current study = 33% versus Oregon = 40% and completion: current study = 21% versus Oregon = 15%).Citation18,Citation32 Another mass immunization campaign at a large university following an outbreak was more successful achieving initiation (80%) with vaccination opportunities offered in multiple locations across campus.Citation31 Still, similar to the current study, only half of the students completed the second dose (completion = 34%).Citation31 While policies mandating vaccines have been effective to increase vaccines, such as HPV vaccine, on college campuses, mandates are often controversial and difficult to enact.Citation10 Furthermore, implications of mandating the MenB vaccine outside of an outbreak, such as increase of students seeking exemptions or increasing antivaccine sentiments, are unclear.Citation33,Citation34 Thus, without effecting sweeping policy changes, effective MenB prevention interventions before outbreaks can potentially save universities the cost and burden of MenB outbreaks.Citation20
Based on theory and prior evidence,Citation28,Citation35–37 the students’ increased knowledge about MenB severity and susceptibility will likely lead to increased vaccinations. The Health Belief Model suggests knowledge about disease severity and a perception of susceptibility to the disease are important influences on the intent to get vaccinated.Citation28 For example, college students were almost twice as likely to intend to receive the H1N1 vaccine if they perceived the disease was severe.Citation11 Similarly, a third of students at a southeast university who had not received the influenza vaccine reported that the flu was not severe enough to warrant vaccination.Citation37 Moreover, college students often do not think they are susceptible to disease.Citation38 Even during a MenB outbreak, the most prominent reason college students did not receive the MenB vaccine was a perception of low risk.Citation21
Our study includes two important limitations. First, our study used a pre-post design with one large university and did not have a control group, thus, limiting our ability to adjust for temporal changes. We are unaware, however, of any concurrent MenB vaccine interventions or other changing temporal influences potentially confounding the results. Second, high attrition limited the analysis comparing the preintervention surveys with the two follow-up surveys. However, our attrition was similar to other studies sampling college students.Citation39 We adjusted for the potential loss to follow-up bias by restricting pre-post analyses to students completing the relevant follow-up surveys.
Our study includes three important strengths. First, to our knowledge, this is the first study of MenB vaccine and college students outside of an outbreak situation. Second, we used the Florida Department of Health registry to assess whether students received the MenB vaccine. Due to the objective measure, we were able to track vaccine uptake from pre- to postintervention regardless of whether the student completed the subsequent follow-up surveys. Finally, we were able to assess student change in knowledge and awareness of the MenB vaccine at three months and one year after the intervention.
Outside of an outbreak setting, combining on-campus vaccination with educational events can likely increase MenB vaccination and students’ perception of MenB vaccine on a large university campus. Effectively preventing MenB outbreaks reduces risk to college students and reduces the burden of mass immunizations responses placed on college health administrators and finances. Our intervention is likely adaptable to other vaccines recommended for college students. Thus, even in the absence of local outbreaks, on-campus immunizations and educational events may be critical interventions to achieving widespread vaccination with other vaccines such as a COVID-19 vaccine.Citation40
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download ()Acknowledgments
The authors would like to thank Justin Tauscher PhD, Cheri Knecht, and Vikasni Mohan for help with survey distribution.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014–2016. Pediatrics. 2019;143(1):e20182130. doi:10.1542/peds.2018-2130.
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease—United States, 1996–2015. Clin Infect Dis. 2017;66(8):1276–81. doi:10.1093/cid/cix993.
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–82. doi:10.1016/j.vaccine.2019.04.020.
- Capitano B, Dillon K, LeDuc A, Atkinson B, Burman C. Experience implementing a university-based mass immunization program in response to a meningococcal B outbreak. Hum Vaccin Immunother. 2019;15(3):717–24. doi:10.1080/21645515.2018.1547606.
- Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine - advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13. doi:10.15585/mmwr.mm6619a6.
- Packnett E, Irwin DE, Novy P, Watson PS, Whelan J, Moore-Schiltz L, Lucci M, Hogea C. Meningococcal-group B (MenB) vaccine series completion and adherence to dosing schedule in the United States: a retrospective analysis by vaccine and payer type. Vaccine. 2019;37(39):5899–908. doi:10.1016/j.vaccine.2019.06.065.
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–23. doi:10.15585/mmwr.mm6833a2.
- Mehta P, Sharma M, Lee RC. Designing and evaluating a health belief model-based intervention to increase intent of HPV vaccination among college males. Int Q Community Health Educ. 2014;34(1):101–17. doi:10.2190/IQ.34.1.h.
- Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college—Rhode Island, 2015–2016. Clin Infect Dis. 2017;64(8):1115–22. doi:10.1093/cid/cix091.
- Oliver SE, Patton ME, Hoban M, Leino V, Mbaeyi SA, Hariri S, MacNeil JR. Evaluation of meningococcal vaccination policies among colleges and universities — United States, 2017. J Am Coll Health. 2019:1–6. doi:10.1080/07448481.2019.1687484.
- Nan X, Kim J. Predicting H1N1 vaccine uptake and H1N1-related health beliefs: the role of individual difference in consideration of future consequences. J Health Commun. 2014;19(3):376–88. doi:10.1080/10810730.2013.821552.
- Barnard M, Cole AC, Ward L, Gravlee E, Cole ML, Compretta C. Interventions to increase uptake of the human papillomavirus vaccine in unvaccinated college students: a systematic literature review. Prev Med Rep. 2019;14:100884. doi:10.1016/j.pmedr.2019.100884.
- Shropshire AM, Brent-Hotchkiss R, Andrews UK. Mass media campaign impacts influenza vaccine obtainment of university students. J Am Coll Health. 2013;61(8):435–43. doi:10.1080/07448481.2013.830619.
- Huang JJ, Francesconi M, Cooper MH, Covello A, Guo M, Gharib SD. Community health workers on a college campus: effects on influenza vaccination. J Am Coll Health. 2018;66(4):317–23. doi:10.1080/07448481.2018.1440582.
- Niccolai LM, Yakely AE, Hansen CE. Up-to-date coverage with meningococcal vaccine among adolescents age 17 years: patterns and correlates in the United States, 2017. Vaccine. 2019;37(40):5934–38. doi:10.1016/j.vaccine.2019.08.015.
- Soeters HM, McNamara LA, Whaley M, Wang X, Alexander-Scott N, Kanadanian KV, Kelleher CM, MacNeil J, Martin SW, Raines N, et al. serogroup B meningococcal disease outbreak and carriage evaluation at a college - Rhode Island, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):606–07.
- Breakwell L, Whaley M, Khan UI, Bandy U, Alexander-Scott N, Dupont L, Vanner C, Chang H-Y, Vuong JT, Martin S, et al. Meningococcal carriage among a university student population – United States, 2015. Vaccine. 2018;36(1):29–35. doi:10.1016/j.vaccine.2017.11.040.
- McNamara LA, Thomas JD, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink SE, Steward-Clark E, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a university serogroup B meningococcal disease outbreak-Oregon, 2015-2016. J Infect Dis. 2017;216(9):1130–40. doi:10.1093/infdis/jix446.
- Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, Mbaeyi SA. Serogroup B meningococcal disease university outbreak group. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25(3):434–40. doi:10.3201/eid2503.181574.
- Alderfer J, Isturiz RE, Srivastava A. Lessons from mass vaccination response to meningococcal B outbreaks at US universities. Postgrad Med. 2020:1–10. doi:10.1080/00325481.2020.1766265.
- Breakwell L, Vogt TM, Fleming D, Ferris M, Briere E, Cohn A, Liang JL. Understanding factors affecting university: students’ decision to receive an unlicensed serogroup B meningococcal vaccine. J Adolesc Health. 2016;59(4):457–64. doi:10.1016/j.jadohealth.2016.06.004.
- MacDougall DM, Langley JM, Li L, Ye L, MacKinnon-Cameron D, Top KA, McNeil SA, Halperin BA, Swain A, Bettinger JA, et al. Knowledge, attitudes, beliefs, and behaviors of university students, faculty, and staff during a meningococcal serogroup B outbreak vaccination program. Vaccine. 2017;35(18):2520–30. doi:10.1016/j.vaccine.2017.02.011.
- Institutional Planning and Research. UF facts: enrollment. Gainesville: University of Florida; 2021 Feb 22 [accessed 2021 Mar 31]. https://ir.aa.ufl.edu/uffacts/enrollment-1/.
- Food and Drug Administration. Bexsero U.S. package insert. US Department of Health and Human Services, Food and Drug Administration.Silver Spring (MD); 2015. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf
- Food and Drug Administration. Trumenba U.S. package insert. Department of Health and Human Services, Food and Drug Administration, Silver Spring (MD); 2014. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf
- National Cancer Institute. Health information national trends survey. National Institutes of Health; 2014 [accessed 2021 Mar 31]. https://hints.cancer.gov/data/survey-instruments.aspx.
- The Child & Adolescent Health Measurement Initiative. National survey of children’s health questionnaire. 2018. [accessed 2021 Mar 31]. https://www.childhealthdata.org/learn-about-the-nsch/survey-instruments.
- Glanz K, Rimer BK, Viswanath K. Health behavior and health education: theory, research, and practice. San Francisco (CA): Wiley; 2008.
- Kurosky SK, Esterberg E, Irwin DE, Trantham L, Packnett E, Novy P, Whelan J, Hogea C. Meningococcal vaccination among adolescents in the United States: a tale of two age platforms. J Adolesc Health. 2019;65(1):107–15. doi:10.1016/j.jadohealth.2019.02.014.
- Mcnamara L, Thomas J, Macneil J, Day M, Fisher E, Martin SW, Poissant T, Wang X, Acosta AM. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a university—Oregon, 2015. Open Forum Infect Dis. 2016;3(suppl_1). doi:10.1093/ofid/ofw172.585.
- Ritscher AM, Ranum N, Malak JD, Ahrabi-Fard S, Baird J, Berti AD, Curtis W, Holden M, Jones CD, Kind J, et al. Meningococcal serogroup B outbreak response University of Wisconsin-Madison. J Am Coll Health. 2019;67(3):191–96. doi:10.1080/07448481.2018.1469502.
- Fisher EA, Poissant T, Luedtke P, Leman R, Young C, Cieslak P. Evaluation of mass vaccination clinics in response to a serogroup B meningococcal disease outbreak at a large, public university-Oregon, 2015. J Adolesc Health. 2018;63(2):151–56. doi:10.1016/j.jadohealth.2018.03.018.
- Bednarczyk RA, King AR, Lahijani A, Omer SB. Current landscape of nonmedical vaccination exemptions in the United States: impact of policy changes. Expert Review of Vaccines. 2019;18(2):175–90. doi:10.1080/14760584.2019.1562344.
- Caleb S, Thompson D, Haimowitz R, Ciotoli C, Dannenbaum M, Fu LY. How colleges intervene to increase student body vaccination coverage. J Am Coll Health. 2020:1–8. doi:10.1080/07448481.2020.1752698.
- Ramsey MA, Marczinski CA. College students’ perceptions of H1N1 flu risk and attitudes toward vaccination. Vaccine. 2011;29(44):7599–601. doi:10.1016/j.vaccine.2011.07.130.
- Maurer J. Inspecting the mechanism: a longitudinal analysis of socioeconomic status differences in perceived influenza risks, vaccination intentions, and vaccination behaviors during the 2009–2010 influenza pandemic. Med Decis Making. 2016;36(7):887–99. doi:10.1177/0272989x15608379.
- Ryan KA, Filipp SL, Gurka MJ, Zirulnik A, Thompson LA. Understanding influenza vaccine perspectives and hesitancy in university students to promote increased vaccine uptake. Heliyon. 2019;5(10):e02604. doi:10.1016/j.heliyon.2019.e02604.
- Bednarczyk RA, Chu SL, Sickler H, Shaw J, Nadeau JA, McNutt L-A. Low uptake of influenza vaccine among university students: evaluating predictors beyond cost and safety concerns. Vaccine. 2015;33(14):1659–63. doi:10.1016/j.vaccine.2015.02.033.
- McDonald B, Haardoerfer R, Windle M, Goodman M, Berg C. Implications of attrition in a longitudinal web-bBased survey: an examination of college students participating in a tobacco use study. JMIR Public Health Surveill. 2017;3(4):e73. doi:10.2196/publichealth.7424.
- Schaffer DeRoo S, Pudalov NJ, Fu LY. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458–59. doi:10.1001/jama.2020.8711.