 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Although two live oral rotavirus (RV) vaccines, Rotarix and RotaTeq, play a critical role toward reducing disease severity, hospitalization, and death rate in RV infections, regular monitoring of vaccine effectiveness (VE) is yet necessary because the segmented genome structure and reassortment capability of RVs pose considerable threats toward waning VE. In this study, we examined the VE by a test-negative study design against G9P[8]I2 strain during a seasonal outbreak in February–May, 2018, in an outpatient clinic in Kyoto Prefecture, Japan. It remains important because G9P[8]I2 strain remains partially heterotypic to these vaccines and predominating in post-vaccination era. During year-long surveillance, RV infections were detected only from February to May. During this outbreak, 33 (42.3%) children out of 78 with acute gastroenteritis (AGE) remained RV-positive, of which 29 (87.8%) children were infected with G9P[8]I2. Two immunochromatographic (IC) assay kits exhibited 100% sensitivity and specificity to detect G9P[8]I2 strain. Only 23.2% children were found to be vaccinated. Yet, significant VE 69.7% (95% CI: 2.5%-90.6%) was recognized against all RV strains that increased with disease severity. Similar significant VE 71.8% (95% CI: 1%-92%) was determined against G9P[8]I2 strain. The severity score remained substantially low in vaccinated children. Our data reveal that vaccine-preventable G9P[8]I2 strain yet may cause outbreak where vaccination coverage remains low. Thus, this study emphasizes the necessity of global introduction of RV-vaccines in national immunization programs of every country.
Introduction
Rotavirus (RV) group A (RVA) remains the leading cause of severe gastroenteritis (AGE) among children under 5 years of age though two live attenuated oral RV-vaccines, Rotarix (GlaxoSmithKine, Rixensart, Belgium) and RotaTeq (Merck & Co., Whitehouse Station, NJ, USA) have been introduced in many countries since 2006.Citation1 These RV-vaccines are capable to induce broad heterotypic immunity due to their cross-reactivity and thus have played critical role toward reducing disease severity, hospitalizations, and mortality caused by various RV serotypes circulating worldwide.Citation2 Yet, nearly 146,000 children <5 years died globally from RV infections in 2015.Citation3,Citation4 However, this mortality rate remained as high as 453,000 deaths per year in 2008 when RV-vaccines were not implemented widely worldwide.Citation5
In Japan, although the mortality rate in RV infections is very low, the detection rate among hospitalized children <5 years yet remains 2.8 to 13.7 per 1000 child-years, imposing a substantial burden to Japan’s healthcare system.Citation6 Here, Rotarix and RotaTeq were introduced in November 2011 and July 2012, respectively, as a voluntary vaccination.Citation2 Recently, it has been included in the routine vaccination program of Japan from October 1, 2020.Citation7 The average vaccine coverage remained 47.6% until the end of 2014, which has been increased gradually in successive years.Citation8,Citation9
Recently, we reported that some common genotypes of pre-vaccination era like G1P[8] and G3P[8] have been reduced in Japan after the introduction of RV-vaccines, whereas genotypes like G2P[4], G2P[8], G9P[8], and G8P[8] that remained sporadic prior to the introduction of RV-vaccines were emerged/reemerged in post-vaccination period.Citation10 Notably, Rotarix is a live attenuated monovalent vaccine containing G1P[8] human RV strain, while RotaTeq is a live attenuated pentavalent vaccine containing five human-bovine reassortant RV strains of human G1, G2, G3, G4, and P[8].Citation11,Citation12 These vaccines have been reported to provide protection not only to the strains that are present in the vaccines but also to the strains that are not included in the vaccines like G8, G9, and G12.Citation13,Citation14 However, most of these studies were conducted in normal conditions when these strains circulated in limited number. Thus, it yet remains vague whether these vaccines are really capable to provide sufficient protection during an outbreak. Furthermore, several outbreaks and greater genotype diversity have been reported repeatedly in post-vaccination era, which remains an emerging concern in relation to RV vaccination programs.Citation10,Citation15,Citation16
During our study period (July, 2017 to July, 2018), we observed a seasonal outbreak of AGE caused by G9P[8] RVA-strain in an outpatient clinic in Kyoto, Japan. The G9P[8] strain remained antigenically distinct from strains targeted by these vaccines and its outbreaks have been reported in different parts of the world in both pre- and post-vaccination era.Citation10,Citation17 Although these vaccines were found to be effective against G9P[8] strain several years before,Citation18,Citation19 recent evidences suggest that the newly emerging G9P[8] strains have been evolved through inter-genogroup reassortment.Citation20 Furthermore, sudden emergence of G9P[8] strain has been reported not only in JapanCitation10,Citation21 but also in many other countries in post-vaccination era.Citation20,Citation22,Citation23 Thus, it remains tempting to investigate vaccine effectiveness (VE) against this newly emerging G9P[8] strain. Herein, we assessed the VE against G9P[8]-strain during a seasonal outbreak. The clinical characteristics of this outbreak strain and the role of vaccines on clinical outcomes were investigated as well.
Materials and methods
Study sample
This study included a total of 163 children under 12 years old who visited an outpatient clinic in Maizuru City, Kyoto Prefecture, Japan from July, 2017 to July, 2018 due to AGE experiencing >3 times of watery or soft defection per day with or without vomiting, nausea, fever, or abdominal cramps. The disease severity was clinically evaluated by a professional pediatrician using a Vesikari scale of 20 points, in which a score of <7 was regarded as mild, 7–10 as moderate, and ≥11 as severe AGE.Citation24 The vaccination history (vaccination date and vaccine lot number) of a child was confirmed from the record of ‘Maternal and Child Health Handbook’ which is provided to each mother in Japan during pregnancy. The study was approved by the ethical committees of Nihon University School of Medicine (29-9-1).
Detection of RV by immunochromatography (IC) assay and RT-PCR
As per our normal practice, the fecal or rectal swab samples of any suspected AGE children were tested first by IC assay in the clinic using dual detection kits either Norovirus-Rotavirus (IP-Line Duo Noro-Rota immunochromatographic test kit, ImmunoProbe Co., Ltd, Saitama, Japan) or Rotavirus-Adenovirus (RapidTesta Rota-Adeno II immunochromatographic test kit, Sekisui Medical Co., Ltd., Tokyo, Japan) IC kits following manufacturer’s instructions. The samples were then transferred to the laboratory of the Department of Pathology and Microbiology, Nihon University, and processed for Viral RNA extraction, cDNA preparation, and multiplex PCR analysis as described earlier.Citation25 In brief, viral RNA was extracted from 200 µl of supernatant of 10% fecal suspension using QIAamp Viral RNA mini kit (QIAGEN, Hilden, Germany), cDNA was synthesized from 5 µl of viral RNA using random primer (hexa-deoxyribonucleotide mixture) (Takara, Shiga, Japan), and viral RNA was detected by multiplex PCR using specific primers to detect group A, B, and C RVs simultaneously as described elsewhere.Citation25 The PCR product of RVA (395 bp of VP7 gene) was sequenced by Macrogen Japan and nucleotide sequence was blasted to assign G-genotypes. Two most common P-genotypes, P[8] and P[4], and two most common I-genotypes, I1 and I2, were identified by two different multiplex PCRs as described previously.Citation2 The sensitivity and specificity of IC kits were determined based on available IC results of all AGE suspected children who visited the clinic during the RV-epidemic period (February to May 2018).
Vaccine effectiveness (VE) determination and statistical analysis
A test-negative study design was used to investigate VE in a total of 78 children who visited the clinic during the epidemic period of RV from February 2018 to May 2018. The VE was calculated in percentage as [1 − Odds Ratio (OR)] × 100. OR, adjusted for age and sex, was analyzed by logistic regression as shown previously.Citation26 OR was calculated using the formula: OR = (p1q2)/(p2q1); where, p1 is the probability of being vaccinated among cases (RV-positives), q1 = (1 – p1), p2 is the probability of being vaccinated among controls (RV-negatives) and q2 = (1 – p2). To adjust for the potential confounding factors (age and sex), multivariable logistic regression modeling was done in which the dependent variable (RV infection) remained a dichotomous categorical variable, coded as RV-positive = 1 and RV-negative = 0. Logistic regression analysis considered the upper value as the predicted outcome. The multivariable logistic regression model was given by the following equation
where P denotes the probability of the outcome (RV-positives), β0 was the intercept and βi was the regression coefficient of ith variable (i = 1, 2, …, n) in the model. The Exp[B], provided by the model in SPSS, indicates the OR adjusted for all other variables in the model. The detailed explanation of the model can be found elsewhere.Citation27,Citation28
To compare the characteristic of G9P[8]I2-RV-positive AGE with RV-negative AGE, four children infected with other RV-strains were not included for statistical analysis due to small sample number. Groups were compared by Pearson Chi-Square test and medians were compared by Mann–Whitney U Test. All statistical analyses were performed by using SPSS: version 16.
Results
RV infection and seasonal variation
During a year-long survey from July 2017 to July 2018, a total of 163 children visited the clinic with AGE though the RV infections were detected only from February to May 2018, with a peak in April (). During this RV-epidemic period (February to May 2018), 32 (41.0%) out of 78 AGE children were detected RV-positive by IC kits. However, during laboratory investigation by RT-PCR, 33 (42.3%) children were detected RV-positive: 29 (38%) were infected with G9P[8], 2 (6.1%) with G3P[8], 1 (3%) with G3P[9], and 1(3%) with G9P[4]. The analysis of VP6 (I) gene suggested that all G9P[8] strains were of G9P[8]I2 genotype.
Figure 1. Year-long detection RV-positive cases. Percentages among children visited the month with AGE (number, n, has been shown) have been demonstrated
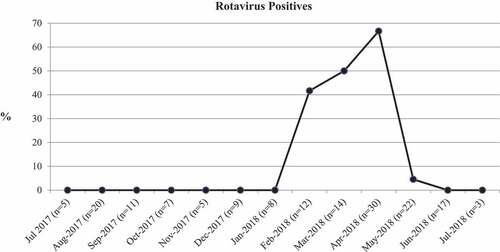
These 33 RV-positive children were found negative for norovirus or adenovirus by dual-detection kits as well as RT-PCR. The sensitivity and specificity of IC kits were 96.9% and 100%, respectively, to detect all RV genotypes. The only one child failed to be detected by IC kit had mild infection (Vesikari score = 5) with G3P[8] strain, implying that both sensitivity and specificity remained 100% in moderately and severely infected children (Vesikari score was ≥7) as well as for G9P[8]I2-infected children.
Characteristics of the outbreak caused by G9P[8]I2 strain
The infection of G9P[8]I2-RV strain was significantly high (P = .009) in the children of 25–36 month age group than those younger (). However, no remarkable difference was noticed in gender distributions between G9P[8]I2-positive and non-RV AGE children. The disease severity score in G9P[8]I2-infected children remained significantly higher (P < .01) than those with non-RV AGE. Majority of children infected with G9P[8]I2 suffered from moderate illness while non-RV infections showed mild symptoms. The detection of G9P[8]I2 remained significantly high (P = .04) in RV-unvaccinated children.
Table 1. Characteristics of RV outbreak
Vaccine effectiveness (VE)
Avoiding repeated visit, the total number of children was 69 of which 16 (23.2%) was vaccinated: 9 with Rotarix and 7 with RotaTeq, all received complete doses. Among 33 RV-positive children, only 5 were vaccinated, suggesting significant VE 69.7% (95% CI: 2.5% to 90.6%) (P = .045) against all mild, moderate and severe RV infections (). For moderate and severe infections (severity score ≥7), VE was estimated 80.7% (95% CI: 3% to 96.2%) (P = .046), while VE was scored 100% (P = .998) in severe children (severity score ≥11). Here, significant P value was not found against severity ≥11 probably because of insufficient sample number. Significant VE 71.8% (95% CI: 1% to 92%) (P = .048) was also determined against G9P[8]I2 strain during the seasonal outbreak ().
Table 2. Vaccine effectiveness (VE) determined at different disease severity
Role of vaccines in disease severity
Finally, we investigated how vaccination may play a role in minimizing disease severity in RV infection. During a year-long survey, 33 children out of 163 were RV-positive. Among them, five were vaccinated and having median severity score 5 which was significantly (P = .047) lower than that of unvaccinated children (median severity score 7) (). On the other hand, disease severity remained usually low (median severity score 5) in both vaccinated and unvaccinated RV-negative AGE children.
Table 3. Differences in Vesikari scores by vaccination status
Discussion
Greater genotype diversity, spreading through the environment, and several outbreaks in post-vaccination era remain an emerging concern in relation to the RV vaccination programs.Citation29 Recent evidences suggest that both Wa-like and DS-1 like G9P[8] genotypes have been emerged abruptly in many countries in post-vaccination era.Citation10,Citation23 Here, all G9P[8] strains having I2 genotype probably have DS-1-like genotype constellation (Gx-P[x]-I2-R2-C2-M2-A2-N2-T2-E2-H2). In fact, DS-1-like strains were found predominatly in post-vaccine eraCitation10,Citation30 even though the reasons for this emergence whether due to vaccine introduction or representing natural epidemiological phenomena yet remain unclear.
Recently, full genome analysis has shown that these newly emerged DS-1-like G9P[8] strains may have acquired the G9-VP7 genes from the co-circulating Wa-like G9P[8] strains with the genetic background of DS-1-like inter-genogroup reassortant strains.Citation20 The impact of such genetic reassortments toward waning VE yet needs to be investigated. If existing RV-vaccines fail to provide sufficient protection against newly emerging G9P[8] strain then new vaccine, for example, Rotavac (Bharat Biotech International Ltd., Hyderabad, India) that has targeted G9 genogroupCitation31 could be an alternative.
In this study, significant VE 71.8% (95% CI: 1% to 92%; P = .048) has been demonstrated against G9P[8]I2 strains (). Previously, VE was estimated 84.5% (95% CI: 23.4% to 96.9%) among hospitalized children in Australia in 2007 during an outbreak of G9P[8] strain.Citation18 Later in 2012, the protection rate of Rotarix was shown 81.8% (95% CI: 36.4–96.6%) against circulating wild-type G9P[8] strains in infants under 2 years of age in Brazil via clinical trial.Citation19 Although the VP6 (I) genotype of G9P[8] strain of earlier studies remained unknown, greater effectiveness is reasonable for severe hospitalized children as well as for infants at earlier ages because these vaccines are taken within the first 6 months of a baby. Together with earlier reports, present data suggest that existing RV-vaccines yet remain significantly effective against newly emerging G9P[8]I2 strains.
Furthermore, herein, significant VE was determined against severity ≥1 when all mild, moderate, and severe RV cases were considered (). Previously, significant VE was not found against severity ≥1 during an outbreak of G8P[8] strain.Citation26 This discrepancy is probably due to the differences in virulence of the outbreak strains. The average severity scores in unvaccinated children remained 7 and 10.9 during the outbreak of G9P[8] and G8P[8] strains, respectively. Thus, RV-vaccines seem to be more effective against less virulent strains.
Low RV-vaccine coverage (23.2%) remained another critical observation of this population. Information collected from local health center and branches of vaccine-companies revealed that 108 and 67 children received Rotarix and RotaTeq, respectively, against 674 total birth during the study period in Maizuru city suggesting nearly 26% RV-vaccine coverage which remained consistent to our findings. RV-vaccines were introduced in Japan since 2011/2012 as a voluntary vaccination that costs around 180 US dollar equivalent for a complete dose.Citation6 Partial or full subsidies in few cities like Nikaho, Yuri-Honjo, and Isumi, etc., have rendered the coverage rate almost 100%.Citation6 Despite knowing the effectiveness of RV-vaccines, lower vaccine coverage may indicate that cost may have mattered toward successful implementation of vaccines worldwide. Therefore, the decision of including RV-vaccines in the routine vaccination program of Japan from 1 October 2020 will remain remarkable to protect Japanese children from severe RV-associated illness in near future.
Noteworthy, unlike G2P[4] or G8P[8], the emergence and spread of G9P[8] or G3P[8] strains were not found everywhere in post-vaccine era.Citation10 This study implies that although RV-vaccines are effective to prevent G9P[8]I2 infections, outbreak may occur where vaccination remains low.
Another major finding of this study revealed that the emerging G9P[8]I2 strains were correctly detected by rapid detection IC assay kits. Two types of IC assay kits, both remained capable for dual detection, exhibited sensitivity and specificity 100% to detect G9P[8] strains. The only one case failed to be detected by IC assay kits was infected with G3P[8] strain. Our data, thus, suggest that existing IC kits are well competent and reliable for rapid diagnosis of RV infections.
RV infections are expected to occur each year. This time the genotype of the RV epidemic in the area has been replaced by the G9P[8]I2 strain while G2P[4]I2 strain predominated here in 2015–2016.Citation10 The G9P[8]I2 strain showed peak in April (). Previously, we showed that RV infection increased rapidly from January/February and lasted till May/June with a peak in April in Japan during 2015–2018.Citation10 In fact, the peak of RV infection in Japan exists in April/May since 2013 while the peak was in March/April during 2003–2013, January/February during 1996–2002 and December/January during 1981–1996.Citation32 Importantly, unlike G8P[8]I2-RV strain, G9P[8]I2 strain neither tended to adapt with high temperature of May–June nor infected older aged children (>60 m).Citation2
Small sample size remained the major limitation of this study that often yielded no significance or weak significance with wide range of 95% CI values. Again, this study was conducted among children of one outpatient clinic only that may have missed many cases of severe patients requiring hospitalization or emergency care. Nevertheless, the sample size in this study remained comparable to that in other outbreak studies.Citation2,Citation18 In addition, the participation of all children represented a complete real scenario of the outbreak suitable to determine VE. This study remains unique from several points of view: VE was determined (1) against G9P[8]I2-RV strain (2) in a low vaccinated population (3) in an outpatient clinic (4) in Japan.
In conclusion, this study has documented two major points: (1) RV-vaccines are significantly effective against G9P[8]I2 strains, (2) These vaccines are capable not only to minimize disease severity but also to provide protection against mild infection if the virulence of the strain remains low. Considering the sudden emergence of G9P[8] strain in recent time and its heterogeneity to the existing vaccines along with probability of acquiring further genetic modification, these data remain important to emphasize the effectiveness of existing RV-vaccines against newly emerging strains even in a setting of low vaccine coverage.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to all parents and children who willingly participated in this study. We also gratefully acknowledge all members of Nishimura clinic and Division of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine, for their generous cooperation throughout this work. This work was supported by Grant-in-Aid for Scientific Research (16H05360) and Public Foundation of the Vaccination Research Center (2018-38, 2019-41).
References
- Abou-Nader AJ, Sauer MA, Steele AD, Tate JE, Atherly D, Parashar UD, Santosham M, Nelson EAS. Global rotavirus vaccine introductions and coverage: 2006–2016. Hum Vaccin Immunother. 2018;14(9):2281–96. doi:10.1080/21645515.2018.1470725. PMID: 29787334.
- Hoque SA, Kobayashi M, Takanashi S, Anwar KS, Watanabe T, Khamrin P, Okitsu S, Hayakawa S, Ushijima H. Role of rotavirus vaccination on an emerging G8P[8] rotavirus strain causing an outbreak in central Japan. Vaccine. 2018;36(1):43–49. doi:10.1016/j.vaccine.2017.11.056. PMID: 29183732.
- Haidong W, Zulfiqar AB, Matthew MC, Megan C, Lalit D, Khassoum D, Elisabeth BF, Maya F, Nancy F, Peter WG, et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1725–74. doi:10.1016/S0140-6736(16)31575-6. PMID: 27733285.
- Carvalho MF, Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother. 2019;15(6):1237–50. doi:10.1080/21645515.2018.1520583. PMID: 30215578.
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–41. doi:10.1016/S1473-3099(11)70253-5. PMID: 22030330.
- Fujii Y, Noguchi A, Miura S, Ishii H, Nakagomi T, Nakagomi O, Takahashi T. Effectiveness of rotavirus vaccines against hospitalisations in Japan. BMC Pediatr. 2017;17(1):156. doi:10.1186/s12887-017-0916-7. PMID: 28693503.
- NIID. Rotavirus, from September 2004 to August 2019, Japan. IASR; 2020 Apr 9 [accessed 2021 Mar 4]. https://www.niid.go.jp/niid/en/a-h7n9-en/865-iasr/9543-478te.html.
- Araki K, Hara M, Sakanishi Y, Shimanoe C, Nishida Y, Matsuo M, Tanaka K. Estimating rotavirus vaccine effectiveness in Japan using a screening method. Hum Vaccin Immunother. 2016;12(5):1244–49. doi:10.1080/21645515.2015.1121337. PMID: 26680277.
- Ministry of Health LaWJ. Committee for immunization and vaccines. Evaluations and analyses on rotavirus vaccines (Report 1–3). 2015.
- Hoque SA, Khandoker N, Thongprachum A, Khamrin P, Takanashi S, Okitsu S, Nishimura S, Kikuta H, Yamamoto A, Sugita K, et al. Distribution of rotavirus genotypes in Japan from 2015 to 2018: diversity in genotypes before and after introduction of rotavirus vaccines. Vaccine. 2020;38(23):3980–86. doi:10.1016/j.vaccine.2020.03.061. PMID: 32307276.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi:10.1056/NEJMoa052664. PMID: 16394299.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi:10.1056/NEJMoa052434. PMID: 16394298.
- Heylen E, Zeller M, Ciarlet M, Lawrence J, Steele D, Van Ranst M, Matthijnssens J. Comparative analysis of pentavalent rotavirus vaccine strains and G8 rotaviruses identified during vaccine trial in Africa. Sci Rep. 2015;5(1):14658. doi:10.1038/srep14658. PMID: 26440913.
- Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, Witte D, Todd S, Louw C, Kirsten M, et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis. 2012;12(1):213. doi:10.1186/1471-2334-12-213. PMID: 22974466.
- Mokomane M, Esona MD, Bowen MD, Tate JE, Steenhoff AP, Lechiile K, Gaseitsiwe S, Seheri LM, Magagula NB, Weldegebriel G, et al. Diversity of rotavirus strains circulating in Botswana before and after introduction of the monovalent rotavirus vaccine. Vaccine. 2019;37(43):6324–28. doi:10.1016/j.vaccine.2019.09.022.
- Markkula J, Hemming-Harlo M, Salminen MT, Savolainen-Kopra C, Pirhonen J, Al-hello H, Vesikari T. Rotavirus epidemiology 5–6 years after universal rotavirus vaccination: persistent rotavirus activity in older children and elderly. Infect Dis (Lond). 2017;49(5):388–95. doi:10.1080/23744235.2016.1275773.
- Kirkwood C, Bogdanovic-Sakran N, Barnes G, Bishop R. Rotavirus serotype G9P[8] and acute gastroenteritis outbreak in children, Northern Australia. Emerg Infect Dis. 2004;10(9):1593–600. doi:10.3201/eid1009.040040.
- Snelling TL, Schultz R, Graham J, Roseby R, Barnes G, Andrews R, Carapetis J. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49(3):428–31. doi:10.1086/600395.
- Justino MCA, Araújo EC, Van Doorn L-J, Oliveira CS, Gabbay YB, Mascarenhas JDP, Miranda YS, Guerra SDFS, Silva VBD, Linhares AC, et al. Oral live attenuated human rotavirus vaccine (RotarixTM) offers sustained high protection against severe G9P[8] rotavirus gastroenteritis during the first two years of life in Brazilian children. Mem Inst Oswaldo Cruz. 2012;107(7):846–53. doi:10.1590/S0074-02762012000700002.
- Fukuda S, Tacharoenmuang R, Guntapong R, Upachai S, Singchai P, Ide T, Hatazawa R, Sutthiwarakom K, Kongjorn S, Onvimala N, et al. Full genome characterization of novel DS-1-like G9P[8] rotavirus strains that have emerged in Thailand. PLoS One. 2020;15(4):e0231099. doi:10.1371/journal.pone.0231099.
- Fujii Y, Oda M, Somura Y, Shinkai T. Molecular characteristics of novel mono-reassortant G9P[8] rotavirus A strains possessing the NSP4 gene of the E2 genotype detected in Tokyo, Japan. Jpn J Infect Dis. 2019;73(1):26–35. doi:10.7883/yoken.JJID.2019.211.
- Tian Y, Chughtai AA, Gao Z, Yan H, Chen Y, Liu B, Huo D, Jia L, Wang Q, MacIntyre CR, et al. Prevalence and genotypes of group A rotavirus among outpatient children under five years old with diarrhea in Beijing, China, 2011–2016. BMC Infect Dis. 2018;18(1):497. doi:10.1371/journal.pone.0231099.
- Kaplon J, Grangier N, Pillet S, Minoui-Tran A, Vabret A, Wilhelm N, Prieur N, Lazrek M, Alain S, Mekki Y, et al. Predominance of G9P[8] rotavirus strains throughout France, 2014–2017. Clin Microbiol Infect. 2018;24(6):660 e661–660 e664. doi:10.1016/j.cmi.2017.10.009.
- Lewis K. Vesikari clinical severity scoring system manual. PATH, A Catalyst for global health; 2011. p. 9–11.
- Thongprachum A, Khamrin P, Pham NTK, Takanashi S, Okitsu S, Shimizu H, Maneekarn N, Hayakawa S, Ushijima H. Multiplex RT-PCR for rapid detection of viruses commonly causing diarrhea in pediatric patients. J Med Virol. 2017;89(5):818–24. doi:10.1002/jmv.24711.
- Hoque SA, Islam MT, Kobayashi M, Takanashi S, Anwar KS, Watanabe T, Khamrin P, Okitsu S, Hayakawa S, Ushijima H, et al. Our response to the letter to the editor. Vaccine. 2018;36(34):5110–11. doi:10.1016/j.vaccine.2018.05.004.
- Chan YH. Biostatistics 202: logistic regression analysis. Singapore Med J. 2004;45:149–53.
- Schlesselman JJ. Case-control studies: design, conduct, analysis. NewYork: Oxford University Press; 1982.
- Hoque SA, Thongprachum A, Takanashi S, Mostafa SM, Saito H, Anwar KS, Nomura A, Hoque SA, Begum R, Sultana UN, et al. Alarming situation of spreading enteric viruses through sewage water in Dhaka City: molecular epidemiological evidences. Food Environ Virol. 2019;11(1):65–75. doi:10.1007/s12560-018-09363-z.
- Takanashi S, Thongprachum A, Okitsu S, Nishimura S, Kobayashi M, Kikuta H, Yamamoto A, Sugita K, Baba T, Hayakawa S, et al. Molecular epidemiological traits of group a rotaviruses in Japanese children during transitional period of rotavirus vaccine implementation, 2011–2014. Clin Lab. 2017;63:961–70. doi:10.7754/Clin.Lab.2017.161216.
- WHO. Immunization, vaccines and biologicals; 2019 [accessed 2021 Mar 4]. https://www.who.int/immunization/diseases/rotavirus/en/ .
- Dey SK, Ushijima H, Phathammavong O, Chanit W, Okitsu S, Mizuguchi M, Ota Y. Seasonal trend and serotype distribution of rotavirus infection in Japan, 1981–2008. Pediatr Infect Dis J. 2010;29(2):166–67. doi:10.1097/INF.0b013e3181b79460.
