ABSTRACT
Protection after human papillomavirus (HPV) vaccination can be maximized by optimizing vaccination schedules. We systematically reviewed immunogenicity and effectiveness of HPV vaccines administered 6 months apart compared with longer intervals. Seroconversion to vaccine-type HPV was non-inferior for 12- compared with 6-month intervals, but inconclusive for comparison of 36–96 months with 6 months. A 12-month interval showed non-inferior (margin 0.5) vaccine-type HPV antibody responses compared with a 6-month interval. Compared to 6 months, an interval of 36–96 months resulted in non-inferior antibody responses for HPV6 and high-risk types HPV16 and 18, but did not lead to a non-inferior antibody response for HPV11 (GMR 0.63, 95% CI:0.41–0.97). Data on the effectiveness of extended two-dose schedules were limited. Our findings indicate that HPV immunization programs could adopt a 12-month interval instead of 6 months for increased flexibility without compromising immunogenicity. Further evaluation to confirm the immunogenicity and effectiveness of intervals beyond 12 months is warranted.
Introduction
The human papillomavirus (HPV) is the most common sexually transmitted infection.Citation1,Citation2 It is estimated that almost all cervical cancer cases are attributable to high-risk HPV types.Citation3 Benign anogenital warts are caused by low-risk HPV types and can significantly reduce quality of life.Citation4,Citation5 Immunization using the bivalent, quadrivalent or nonavalent HPV vaccines is effective in protecting against HPV infections and precursor lesions of anal and cervical cancer.Citation6 Immunization is a vital component of the global strategy for elimination of cervical cancer as a public health problem by 2030, and one of the aims is to fully vaccinate 90% of girls worldwide against HPV by age 15.Citation7 Providing as much flexibility as possible in the vaccination schedule, assuming equivalent effectiveness, could help in realizing this global target, to ensure that vaccine schedules meet local public health realities.
Current recommendations for administering two-doses of HPV vaccines to immunocompetent individuals are: 5–13 months apart for the bivalent vaccine if recipient 9–14 years,Citation8 6 or 12 months apart for the quadrivalent vaccine if recipient 9–13 yearsCitation9 and 6–12 months apart for the nonavalent vaccine if recipient 9–14 years,Citation10 for older age groups three-doses are recommended. School-based immunization is widely used for delivery of HPV vaccines to the primary target population who are early adolescents.Citation11 A majority of school-based immunization programs deliver HPV vaccines on a 0,6-month schedule.Citation11,Citation12 Therefore at least two immunization clinics must be held six months apart in each participating school within an already congested nine-month academic calendar. This causes logistical challengesCitation13,Citation14 that limit opportunities for completing HPV vaccination, which have recently been worsened while trying to deliver the program during the COVID-19 pandemic.
In addition to programmatic benefits, there might be immunological benefits from using schedules with an interval longer than 6 months. Indications from Bergman et al are that extension of the two-dose interval up to 12 months, may generate higher antibody levels compared to an interval of two to six months.Citation15 It is not clear whether this trend continues when the two-dose interval extends beyond 12 months. Secor et al did not find higher antibody levels for extended interval schedules longer than 12 months relative to standard intervals. However, the comparisons made by Secor et al were based on pooled data for two and three dose schedules and are not definitive for two dose schedules.Citation16 Further neither study investigated the relative effectiveness of extended and standard interval two dose schedules against HPV-infected and HPV-related disease outcomes. Comparisons of this kind, could help in delimiting optimal two-dose intervals. This systematic review evaluates HPV vaccine immunogenicity and effectiveness for two doses administered six months apart compared with extended intervals.
Methods
Protocol and registration
The protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), under protocol number: CRD42019141959.
Literature search
Systematic literature searches were updated till July 10, 2020. Indexed databases searched were: MEDLINE (Ovid), EMBASE (Ovid), Cochrane Central Register of Clinical Trials, PUBMED, and CINAHL. The original search strategy was developed in Medline (Supplemental Appendix 1) and modified for use with the other platforms, based on their specific syntax and thesaurus. Unindexed databases were searched as follows: the term “HPV vaccine” was used to search GSK study register, MERCK clinical trials, EU Clinical Trials Register and Drugs@FDA, whilst the term “HPV vaccine schedule” was used to search ClinicalTrials.gov and International Clinical Trials Registry Platform.
Study selection
A two-stage selection process was used and consisted of title/abstract screening followed by full-text screening. The screening process was performed in duplicate (ACF and RD). Studies were included if they fulfilled all of the following criteria: study participants were 9–26 years inclusive at first HPV vaccine dose; administered any combination of approved prophylactic HPV vaccines in a two-dose schedule to females and/or males; and study presented data on immunogenicity, effectiveness or efficacy of two doses of HPV vaccine administered at 6 months (± 1 month) as well as two doses administered more than 7 months apart. Studies were excluded if they: included populations with morbidities only; administered a vaccine formulation that is different from the licensed formulation; are reported in a language other than English.
Data extraction and quality assessment
ACF and RD independently extracted data from selected studies into standardized data collection forms, after which ACF checked both sets of extracted data for accuracy. Extracted data included: publication details, general study characteristics, intervention and comparator (HPV vaccine and schedule) and outcomes of interests namely seroconversion, geometric mean concentration or titers (GMC/T), B-cell and T-cell frequencies and rates of HPV infection or HPV-related diseases. The potential for bias and confounding in each study was assessed using the Cochrane Risk of Bias tool version 2 for randomized studiesCitation17 and the Cochrane Risk of Bias in Non-Randomized Studies of Intervention (ROBINS-I) tool for observational studies.Citation18
Analyses and certainty assessment
To validly compare effects across studies, within-study relative measures for the immunogenicity and effectiveness of the extended interval (0,>7 month) versus the standard (0,6 month) interval were obtained. Using the relative within-study effect measures allowed controlling for inter-study methodological variations such as differences in definition of seroconversion or assays of antibody response across studies.Citation19 Immunogenicity was assessed by seroconversion difference as well as antibody response using the geometric mean ratio (GMR) comparing the GMC or GMT for the extended versus the standard interval. For randomized control trials (RCTs), measures for the per protocol populations were used to avoid misclassification of vaccination schedules. Non-inferiority was tested by constructing two-sided 95% confidence intervals (CI) and comparing them to pre-specified non-inferiority criteria. 95% CI for the seroconversion estimates were calculated using Wilsons exact approach without continuity correction. These non-inferiority criteria were previously reportedCitation20 and specify that the lower limit of the 95% CI be > 0.5 for GMR and > −10% for the difference in seroconversion. Where non-inferiority was demonstrated based on ratios or proportions, superiority was assessed to determine if the extended interval was better than 6 months. Superiority required the lower limit of the 95% CI to exceed 1 for ratios and 0 for differences in proportions. Incidence rate difference (IRD) was calculated using incidence count and person years data extracted from study reports. The unavailability of non-inferiority margins for IRD and the intricacies involved in defining meaningful margins for this measure, discouraged such analysis here. The certainty of the evidence available to assess each study objective was determined using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).Citation21
Results
Study selection
The systematic search yielded 1451 unique records, of which 11 publications reporting on four different studies satisfied the selection criteria after full-text screening (). describes the characteristics of included studies.
Table 1. Characteristics of included studies
Seroconversion
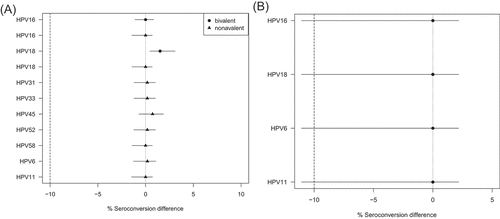
At 1 month post last dose, non-inferior seroconversion to vaccine type HPV was observed for the 12-month interval versus the 6-month interval when using the bivalent and nonavalent HPV vaccines (). For the bivalent vaccine, non-inferiority of a 12-month versus 6-month interval for seroconversion was shown to 1 year post last dose. Non-inferior seroconversion could not be concluded when the mixed quadrivalent/nonavalent regimen was administered at an interval of 36 to 96 months compared with 6 months ().
Figure 2. (A) Difference in seroconversion to HPV vaccine types after two doses of HPV vaccine administered at 12 versus 6 months apart, at 1 month after last HPV vaccine dose. (B) Difference in seroconversion to HPV vaccine types after two doses of HPV vaccine were administered at 36 to 96 versus 6 months apart, at 1 m after last HPV vaccine dose
Antibody response (Supplemental Appendix 2)
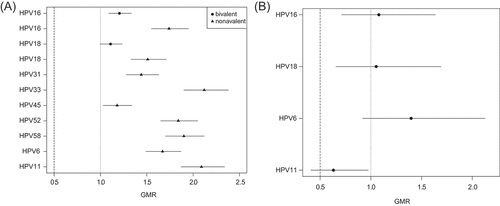
The administration of the bivalent HPV vaccine with a 12-month interval was non-inferior compared to administration using a 6-month interval for HPV18 (GMR 1.11, 95% CI: 1.00 to 1.23) at 1 month post last dose (). GMR comparing the 12-month to the 6-month interval for each individual vaccine type HPV showed substantial heterogeneity for the peak antibody response to HPV16 and 18 at 1 month post last dose (I2 = 76%), precluding pooling of these data. For the bivalent vaccine, the 12-month interval had antibody responses superior to the 6-month interval for both HPV16 (GMR 1.22, 95% CI: 1.09 to 1.37 at 6 months later and 1.27 95% CI: 1.13 to 1.42 at 12 months later) and HPV18 (GMR 1.22, 95% CI: 1.08 to 1.38 at 6 months later and 1.35, 95% CI: 1.19 to 1.55 at 12 months later). At 1 month post last dose, a two-dose interval of 36 to 96 months between two doses was non-inferior to 6 months for HPV6, 16 and 18, but not HPV11 (GMR 0.63, 95% CI: 0.41 to 0.97, ).
Figure 3. (A) Ratios for antibody levels of HPV vaccine-types for two-dose schedules administered at intervals of 12 months versus 6 months at 1 month post last HPV vaccine dose. (B) Ratios for antibody levels of HPV vaccine-types for two-dose schedules administered at intervals of 36–96 months versus 6 months at 1 month post last HPV vaccine dose
Cellular immune response
Memory B-cell and T-cell immune responses were measured in a subset of participants in a study evaluating the bivalent vaccine (NCT01381575). No CD8+ T cell response to HPV16/18 was detectable for any of the evaluated schedules. The memory B-cell and CD4+ T-cell response were of similar magnitude for the 0, 12 m and 0, 6 m schedules, at 36 m post-dose-one. This study however was not powered to detect differences in cellular immune responses after two doses were administered 12 or 6 months apart.Citation22
Efficacy and effectiveness
There were no studies reporting efficacy data for comparing the extended and standard intervals, while a single study provided data for comparing effectiveness. Lamb et al.Citation23 provided incidence data that was used to calculate anogenital wart IRDs for the 8 + m interval schedule relative to the 4–7 m interval schedules. The IRD among females younger than 17 years was 256.21 (95% CI −12.11– 524.53) per 100,000 person-years and among those aged 17–19 years IRD was 448.62 (95% CI – 49.41– 946.64) per 100,000 person-years.
Quality and certainty assessments
Risk of bias assessment indicated a low risk of bias for RCTs evaluating the bivalent and nonavalent vaccines, while the observational studies were assessed to have an overall moderate risk of bias (Supplemental Appendix 3). In applying the GRADE certainty assessment, effect estimates for the bivalent and nonavalent studies were deemed to have high certainty due to them being RCTs with low risk of bias and over 100 participants per study group. The effect estimates based on the observational studies however were assessed to be of low certainty due to their moderate risk of bias and wide confidence intervals (Supplemental Appendix 4). Supplemental Appendix 2 provides an overview of calculated effect estimates and associated certainty for all outcomes assessed in this study.
Discussion
We systematically reviewed the non-inferior immunogenicity and effectiveness of two doses of HPV vaccine when administered at an extended interval of 7 or more months apart, versus a standard interval of 6 months. Our findings extend upon the trend reported by Bergman et al.Citation15 where antibody levels increase as dose intervals increase from 2 to 6 months and from 6 to 12 months. Consistent with this trend, we found that for the bivalent and nonavalent vaccines, increasing the two-dose interval from 6 to 12 months resulted in superior antibody response to all HPV types, except for the bivalent vaccine for HPV18, although an interval of 12 months was non-inferior to an interval of 6 months. Although superiority was shown when an interval of 12 months was compared to 6 months for HPV6, 11 and 16, it is unclear how this finding would translate into effectiveness given the lack of a correlate of protection.Citation24 The non-inferiority of a 12-month interval to a 6-month interval persisted to 1 year post last dose for the antibody response that the bivalent vaccine induces to HPV16 and 18. This is the maximum period for which follow-up data are available.
Although not included in this review, which used the defined minimum required interval of five months as comparator, in line with recommendations of WHO/EMA, several previous studies included shorter intervals. These studies found lower antibody levels when comparing a one or two month interval compared with an interval of six months.Citation25,Citation26
Our findings of non-inferior immunogenicity for the two-dose schedule administered over a year are comparable to an evaluation of extended three-dose schedules, were extended schedules up to 12 and months showed non-inferior antibody schedules one month after the third dose.Citation27 At 29/30 months after the first dose also a schedule extended up to 24 months showed non-inferior antibody responses. Evaluation of this extended dosing schedule (0,12,24 months) at 20 months after the first dose also resulted in non-inferior antibody responses. In addition, for all evaluated schedules the authors compared the antibody responses before administration of the third dose. Interestingly, the antibody titers were comparable or higher after two-doses administered at 0,12 compared to a six month interval for HPV6/11/16/18 before administration of the third dose at 24 and 12 months respectively.Citation28
We did not find a trend of higher antibody levels with increasing interval length to continue when the interval was 36–96 months. Rather, increasing the dose interval from 6 months to between 36 and 96 months resulted in non-inferior antibody response to HPV6 and high-risk HPV types 16 and 18, but not HPV11. It is notable that the single study providing data to compare the 36–96 months interval to the standard interval was not powered to assess superiority of the extended interval. The HPV11 comparison is the only antibody response that failed to demonstrate non-inferiority between extended and standard two-dose intervals. In so doing, it failed to indicate adequate protection by the immunobridging principle, since previous studies showed that vaccine-type antibody responses elicited by the standard (6 months) two-dose schedule in girls were non-inferior to antibody responses elicited by three doses in adolescents and women.Citation29–31 The clinical significance of the HPV11 antibody response failing to achieve non-inferiority is unclear since there is no known minimum antibody level for protection against HPV infection.Citation24 Studies have shown that memory B-cells may trigger a protective anamnestic response, even if plasma antibody levels are low or undetectable.Citation32,Citation33
Only one study reported on the effectiveness of two-dose schedules of six or more months using AGW as the outcome, no studies were found that evaluated the efficacy or effectiveness against HPV infections, precursor lesions or ultimately HPV-associated cancers. Bergman et al.Citation15 also highlighted a lack of available data for comparing HPV vaccine effectiveness when two-dose schedules with extended and standard intervals are used.
Booster vaccine doses mediate their effect through activation of new naïve B cells (not stimulated by the priming dose) and re-activation of memory B cells (elicited by the priming dose). The interval between prime and boost vaccine doses can mediate the interaction between the vaccine antigen and both naïve and memory B cells via the presence of antigen-specific antibody at time of boost.Citation34–36 Extending the interval between doses will likely result in lower antibody levels at the time of booster dose administration, which may allow greater opportunity for stimulation of B cell responses by antigen at this time.Citation36 This may explain the generally superior/higher antibody responses observed for a 12 month interval versus a 6 month interval. The duration of time over which the antibody response remains protective is another important consideration for HPV vaccines. The primary target age for HPV administration is 9–14 years,Citation2 but peak acquisition of HPV extends up to age 25 in many populations.Citation37 Evaluation of the long-term effectiveness of both two-dose schedules with 6-month interval as well as those with extended dose intervals provided by HPV vaccines is therefore an area for further studies, as it is ideal that HPV vaccines remain protective against infection throughout the years of peak HPV exposure.
The higher antibody responses for a 12-month interval suggests that it could be used, instead of 6 months, for school-based HPV immunization programs. With the need to improve the resilience of school-based immunization to disruptions, prolonged intervals could be explored as an option to reduce annual HPV vaccine needs during periods of HPV vaccine shortage. When using a 0,6 month schedule in school-based immunization programs logistical challengesCitation13,Citation14 limit opportunities for completing HPV vaccination since missed school clinics are not easily rescheduled and timing restrictions make it difficult for such programs to adapt to disruptions. Unanticipated school closures can complicate, if not altogether eliminate opportunities for, completion of HPV vaccine schedules as illustrated by the current COVID-19 pandemic.
The 12-month interval could help to streamline series completion. COVID-19 school closures eliminated opportunities to complete the 0,6 month schedule for students in the single target grade for the 2019/2020 school year. Adoption of a 0,12 month schedule allows for two doses to be administered across two consecutive school years. Simultaneous administration of the single HPV vaccine dose to students initiating HPV vaccination in the upcoming school year as well as to those whose schedules were incomplete for the current school year could allow smooth completion of regimen. Further during the academic year, the removal of the 6-month wait between doses that is imposed by a 0,6 month schedule, gives greater flexibility for arranging additional clinics to catch up on missed appointments. In settings where health facility-based immunization is done, a single visit in a year offers convenience and should still be more advantageous than two visits. In implementing a 0,12 month schedule, it will be important to protect against continued extension of the two-dose interval, since data to rigorously evaluate the impact of such longer intervals on vaccine performance is lacking. Considering that HPV vaccines are most effective when administered prior to HPV exposure,Citation38 each jurisdiction should utilize knowledge of its median age of onset of sexual contact, when selecting the consecutive target grades for implementing a 0,12 month schedule for its school-based HPV vaccination program.
Currently, disruptions in the HPV vaccine supply chain is a looming concern that could prevail until 2024.Citation39 This global shortage has led to suggestions for the two-dose interval to be extended to 3–5 years to significantly alleviate current shortfall in HPV vaccine supplies.Citation40 The non-inferiority in HPV16 and 18 antibody response observed for the interval of 36–96 months relative to 6 months is encouraging as it suggests that delaying the second dose for up to 96 months could be as beneficial for cervical cancer prevention as delivery of two doses within six months. Consequently, it supports recommendations that routine HPV immunization programs focus on and prioritize the delivery of at least one dose to all eligible children during the COVID-19 pandemic, with the second dose to be delivered at an opportune time.Citation41 Further well powered studies with comparison to adult women on a 3-dose schedule are however needed to confirm the immunogenicity of the extended interval of 36–96 months. Notably, such long intervals present increased risks for initiation of sexual contact before series completion and will require prudent planning and implementation of strategies to minimize this risk.
This systematic review is the most comprehensive synthesis to evaluate the performance of two-dose intervals of 6 months to intervals longer than 6 months. It included a range of study designs, in contrast to Bergman et al.Citation15 that included only RCTs in assessing intervals of 6 months with longer intervals. Other strengths of this review are the use of time post last dose for true time-matched comparison of schedules and the comparison of relative measures between extended and standard intervals within studies, to minimize the influence of between-study methodological variations. This study was however limited by the paucity of available data on the effect of intervals longer than 12 months on vaccine efficacy and effectiveness. There is a clear need for high quality empirical evidence to assess the impact of extended interval for two-dose schedules on the performance of HPV vaccines in preventing HPV infections and related outcomes.
To conclude, we found that a 12-month interval is non-inferior to a 6-month interval for two-dose HPV vaccine schedules with regard to immunogenicity for all HPV types. Consequently, HPV immunization programs could safely adopt a 12-month interval for more flexibility. An interval extending between 36 and 96 months demonstrated non-inferiority to a 6-month interval with regard to immunogenicity for HPV6, 16 and 18 but not HPV11. This is a promising finding that needs to be further explored in light of recommendations promulgating the use of 3–5 year intervals for HPV vaccine administration during the HPV vaccine shortage. Further studies are needed to confirm the durability of the protection provided by two-dose vaccination schedules with a 12-month interval and the impact of extended intervals on the effectiveness of HPV vaccines. Altogether these are valuable insights for policy and decision makers involved in HPV immunization programs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (608 KB)Acknowledgments
The authors would like to thank Kathryn Hornby, UBC reference librarian for critical review of the search strategy.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1926182.
Additional information
Funding
References
- ICO/IARC. Human papillomavirus and related diseases report. 2019.
- WHO. Human papillomavirus vaccines: WHO position paper, October 2014—recommendations. Vaccine [Internet]. 2015;33:4383–84. http://www.ncbi.nlm.nih.gov/pubmed/25510390.
- De Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–90. doi:10.1016/S2214-109X(19)30488-7.
- Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, Langenberg A, Benedict Yen TS, Ralston R. Human Papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts downloaded from. J Clin Microbiol. 1995;33:2058–63. doi:10.1128/JCM.33.8.2058-2063.1995.
- Sturegård E, Johansson H, Ekström J, Hansson B-G, Johnsson A, Gustafsson E, Dillner J, Forslund O. Human papillomavirus typing in reporting of condyloma. Sex Transm Dis. 2013;40:123–29.
- Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JML, Cummings T, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet Infect Dis. 2019;15:565–80.
- WHO. DRAFT: global strategy towards the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; 2019.
- GSK. Cervarix dosage and administration. Nigeria: GSK group of companies; 2015.
- Merck Canada Inc. GARDASIL product monograph. Canada: Merck Canada Inc; 2017.
- Merck Sharp & Dohme. Highlights of prescribing information. Merck Sharp and Dohme, USA; 2020.
- Paul P, Fabio A. Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program. Vaccine. 2014;32:320–26. doi:10.1016/j.vaccine.2013.11.070.
- Feldstein LR, Fox G, Shefer A, Conklin LM, Ward K. School-based delivery of routinely recommended vaccines and opportunities to check vaccination status at school, a global summary, 2008–2017. Vaccine. 2020;38:680–89. doi:10.1016/j.vaccine.2019.10.054.
- Penny ME, Anaya G, Avila K, Bartolini RM. Challenges in the introduction of nation-wide school-based vaccination against HPV in Peru. Int J Vaccine Res. 2018;4(1):1–9.
- Dubé E, Gagnon D, Clément P, Bettinger JA, Comeau JL, Deeks S, Guay M, MacDonald S, MacDonald NE, Mijovic H, et al. Challenges and opportunities of school-based HPV vaccination in Canada. Hum Vaccines Immunother. 2019;15:1650–55. doi:10.1080/21645515.2018.1564440.
- Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros‐Balta AX, Low N, Henschke N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV‐related disease in females and males. Cochrane Database Syst Rev. 2019;CD013479. 2019.
- Secor AM, Driver M, Kharono B, Hergott D, Liu G, Barnabas RV, Dull P, Hawes SE, Drain PK. Immunogenicity of alternative dosing schedules for HPV vaccines among adolescent girls and young women: a systematic review and meta-analysis. Vaccines. 2020;8:1–13. doi:10.3390/vaccines8040618.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi:10.1136/bmj.d5928.
- Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355;i419.
- Donken R, Knol MJ, Bogaards JA, Van Der Klis FRM, Meijer CJLM, De Melker HE. Inconclusive evidence for non-inferior immunogenicity of two- compared with three-dose HPV immunization schedules in preadolescent girls: a systematic review and meta-analysis. J Infect. 2015;71:61–73. doi:10.1016/j.jinf.2015.02.005.
- Donken R, de Melker HE, Rots NY, Berbers G, Knol MJ. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33:1426–32. doi:10.1016/j.vaccine.2015.01.072.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–26. doi:10.1136/bmj.39489.470347.AD.
- Puthanakit T, Huang L-M, Chiu C-H, Tang R-B, Schwarz TF, Esposito S, Frenette L, Giaquinto C, McNeil S, Rheault P, et al. Randomized open trial comparing 2-dose regimens of the human papillomavirus 16/18 AS04-adjuvanted vaccine in girls aged 9-14 years versus a 3-dose regimen in women aged 15-25 years. J Infect Dis. 2016;214:525–36. doi:10.1093/infdis/jiw036.
- Lamb F, Herweijer E, Ploner A, Uhnoo I, Sundström K, Sparén P, Arnheim-Dahlström L. Timing of two versus three doses of quadrivalent HPV vaccine and associated effectiveness against condyloma in Sweden: a nationwide cohort study. BMJ Open. 2017;7:1–7.
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. Comparison of the immunogenicity and safety of CervarixTM and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–19. doi:10.4161/hv.5.10.9518.
- Safaeian M, Sampson JN, Pan Y, Porras C, Kemp TJ, Herrero R, Quint W, Jan Van Doorn L, Schussler J, Lowy DR, et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. [accessed 2019 Mar 19]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6075614/pdf/djx158.pdf.
- Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared to the licensed 3-dose schedule. Hum Vaccin [Internet]. 2011 [accessed 2019 Aug 14];7:1374–86. http://www.ncbi.nlm.nih.gov/pubmed/22048171 .
- Neuzil KM, Canh DG, Thiem VD, Janmohamed A, Huong VM, Tang Y, Diep NTN, Tsu V, LaMontagne DS. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA [Internet]. 2011 [accessed 2021 Mar 31];305:1424–32. https://jamanetwork.com/ .
- LaMontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. J Infect Dis [Internet]. 2013 [accessed 2021 Mar 31];208:1325–34. https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/jit363 .
- Donken R, Dobson SRM, Marty KD, Cook D, Sauvageau C, Gilca V, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis [Internet]. 2019 [accessed 2019 Oct 16]. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz887/5564371.
- Iversen O-E Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Azurah AGN, Fong SM, Dvorak V, Kim K-H, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys Vs A 3-dose regimen in women. JAMA. 2016;316:2411. doi:10.1001/jama.2016.17615.
- Dobson SR, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, Langley JM, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793. doi:10.1001/jama.2013.1625.
- Olsson SE, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931. doi:10.1016/j.vaccine.2007.03.049.
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–49. doi:10.1016/j.vaccine.2006.06.005.
- Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV VLP vaccines. Expert Rev Vaccines. 2014;13:1027–38. doi:10.1586/14760584.2014.935767.
- Brotherton JML. Rationalizing the HPV vaccination schedule: a long road to a worthwhile destination. Papillomavirus Res. 2019;8:100190. doi:10.1016/j.pvr.2019.100190.
- Whitworth HS, Schiller J, Markowitz LE, Jit M, Brisson M, Simpson E, Watson-Jones D. Continued HPV vaccination in the face of unexpected challenges: a commentary on the rationale for an extended interval two-dose schedule. Vaccine. 2021;39:871–75. doi:10.1016/j.vaccine.2020.12.031.
- Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5.e1–S5.e62.
- Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, Mehlsen J, Chatterjee A, Iversen O-E, Sings HL, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. 2014;134:e657–65. doi:10.1542/peds.2013-4144.
- UNICEF. Human papillomavirus vaccine: supply and demand update. Denmark: UNICEF, 2018.
- WHO. Meeting of the strategic advisory group of experts on immunization, October 2019: conclusions and recommendations. Wkly Epidemiol Rec. 2019;94:541–60.
- JCVI. Joint committee on vaccination and immunisation: statement on the delivery of the HPV vaccine. GOV.UK. UK; 2020.