ABSTRACT
Currently, most licensed vaccines against SARS-CoV-2 infection are approved for adults and not for children. We conducted a test negative case-control study to assess the effectiveness of Measles Containing Vaccines (MCVs) against SARS-CoV-2 infection in Pune, India, in children who were ≥1 year and <18 years of age and were tested for SARS-CoV-2 infection by Reverse transcription polymerase chain reaction (RT-PCR). The enrolled participants included 274 SARS-CoV-2 positive cases (216 vaccinated and 58 unvaccinated) along with 274 SARS-CoV-2 negative controls (265 vaccinated and 9 unvaccinated). Of the 274 cases, 180 (65.7%) were asymptomatic while 94 (34.3%) were symptomatic, all with mild severity. The number of participants with symptomatic SARS-CoV-2 infection was significantly lower in the vaccinated group compared to the unvaccinated group (p < .0001). The unadjusted overall Vaccine Effectiveness (VE) in the vaccinated group compared to unvaccinated group was 87.4% (OR = 0.126, 95% CI of VE: 73.9–93.9) while the adjusted overall VE after adjusting for age and sex was 87.5% (OR = 0.125, 95% CI of VE: 74.2–94.0). MCVs reduced incidence of laboratory confirmed SARS-CoV-2 infection in children. Number of symptomatic cases were also lower in the vaccinated group compared to the unvaccinated group. Results of our study have provided strong preliminary evidence that MCVs have a good effectiveness against SARS-CoV-2 infection in the pediatric population, which needs to be confirmed further through prospective randomized clinical trials.
Introduction
SARS-CoV-2 infection emerged in Wuhan, China, in December 2019. It was officially declared as a global pandemic by the World Health Organization (WHO) on 11 March 2020.Citation1 A striking feature seen during the pandemic has been that children have been less affected as compared to adults, both in terms of morbidity and mortality.Citation2 Most of the pediatric cases have been mild compared to the elderly population.Citation3 In the US, the incidence of SARS-CoV-2 infection in children aged 9 years or younger was 51.1 cases per 100,000 population compared to 401.6 cases per 100,000 population in adults aged 20–29 years, and 902 cases per 100,000 population in adults aged 80 years or older.Citation4
Multiple reasons have been proposed for the above findings. The first reason is that studies have shown that there are lower levels of angiotensin converting enzyme-2 (ACE-2) in the respiratory tract of children compared to adults.Citation5 Second, it is believed that due to cross-reactive T-cell immunity and cross-reactive antibody immunity between common coronaviruses and SARS-CoV-2, coronavirus associated with common colds in children may offer some protection against SARS-CoV-2 infection.Citation5 Children may also be protected against SARS-CoV-2 infection by nonspecific immunity provided by live attenuated vaccines like Measles Containing Vaccines (MCVs) and Bacillus Calmette–Guérin (BCG).Citation2,Citation3 A recent study indicated that participants who had received polio, Hemophilus influenzae type-B (HIB), measles-mumps-rubella (MMR), varicella, pneumococcal conjugate (PCV13), geriatric flu, and hepatitis A/hepatitis B (HepA/HepB) vaccines in the past 1, 2, and 5 years had lower rates of SARS-CoV-2 infection.Citation6 It has also been shown that there are proportionately less cases, milder illness, and a lower death rate due to SARS-CoV-2 infection in countries with BCG-vaccinated population as compared to those without BCG-vaccinated populations.Citation7
In India, measles vaccine became part of the Universal Immunization Program (UIP) in 1985 as a single dose at 9 months of age. A mass immunization campaign was conducted in the country in 2017–2018 with the measles and rubella (MR) vaccine, targeting 410 million children aged 9 months to 15 years.Citation8 Since then, measles vaccine in UIP has been replaced by MR vaccine, in a two-dose schedule at 9–12 months and 16–24 months, respectively. During 2019-2020, the coverage of the first dose of MCV in the UIP ranged between 72.5% to 96.2% across different states in India while that for the second dose ranged between 11.7% to 44.4%.Citation9 Studies have estimated that the coverage during the MR vaccination campaign of 2017-2018 ranged between 60% to 95% across different parts of India.Citation10–14
Children are important facilitators of the SARS-CoV-2 virus transmission.Citation15 Currently, there is only one licensed vaccine (Pfizer-BioNTech) for prevention of SARS-CoV-2 infection in children. We conducted a case-control study to assess the effectiveness of MCVs in preventing SARS-CoV-2 infection and reducing its severity.
Methods
Study design and population
The present test negative case-control study was conducted at Byramjee Jeejeebhoy Government Medical College (BJGMC), a tertiary care center in Pune, India. Participants ≥1 years of age and <18 years of age with documented evidence for testing for SARS-CoV-2 infection by reverse transcription polymerase chain reaction (RT-PCR) during pandemic season of SARS-CoV-2 between 1 February 2020 till 31 July 2020 were included in this study.
Cases were defined as participants who has tested positive for SARS-CoV-2 infection with positive RT-PCR using throat swabs, nasal swabs or any other swabs as directed by the treating physician till the time of enrollment. Controls were participants who had tested negative by RT-PCR.
Participants were classified as being vaccinated if they had a history and documented evidence for MCV immunization. The documents considered valid were Maternal & Child Health (MCH)/Immunization Card, School certificate of MR campaign, anganwadi/health center data, Pune Municipal Corporation MR campaign data.
They were classified as being unvaccinated if their parents did not give history of having received a MCV as well as they did not have any documented evidence. Participants who had no documented evidence of their immunization record for any vaccine including MCV and whose parents gave history of them not having received MCV were also classified as unvaccinated. The participants, who had received the first dose of an MCV but the time between vaccination and SARS-CoV-2 infection testing was less than 4 weeks, were also considered as unvaccinated. Participants who were tested for SARS-CoV-2 infection within four weeks of administration of 2nd dose of MCV were considered to be vaccinated and deemed to have received only 1 dose of MCV.
Participants with an unknown vaccination status, that is, those who gave a history of having received a MCV but had no documented evidence of having received a MCV, were not enrolled in the study. Participants who underwent testing by Rapid Antigen test were not included in this study.
Study procedures
The study protocol was approved by the Institutional Ethics Committee (IEC) of BJGMC, Pune before starting the study. A detailed list of participants tested for SARS-CoV-2 infection between 1 February 2020 till 31 July 2020 using RT-PCR was obtained from the Pune Municipal Corporation (PMC), Pune. From the list, 274 cases and controls each (participants ≥ 1 years of age and < 18 years of age) were selected by simple random sampling technique. Their parents/guardians were contacted telephonically by the study team.
A verbal consent was obtained from the parents/guardians using an IEC approved informed consent script. The process of verbal consent was documented in the source notes.
Participants who fulfilled all the inclusion/exclusion criteria were enrolled in the study. The parents/legal guardians of all participants were again contacted telephonically. The MCV status was documented for each participant. The history included details of SARS-CoV-2 infection for severity and outcome. Demographic and clinical information including date of birth, age, gender, medical history, symptoms (fever, dry cough, tiredness, breathlessness, sore throat, diarrhea, anosmia, ageusia etc.), virological testing on specimens of nasal or throat swabs for detection of SARS-CoV-2 infection and results of these tests and any history of contact with known SARS-CoV-2 positive cases were collected from the hospital records/discharge summaries/PMC records. The BCG and MCV immunization history of all the participants was noted. The investigator transcribed all such information in an electronic Case Record Form (eCRF).
Statistical analysis
Assuming odds ratio of 0.5, 90% power, and probability of risk exposure (probability of receiving MCV) of 0.8, the target sample size calculated was 548 (274 participants in case group and 274 in control group).Vaccine effectiveness (VE) is the percentage reduction of incidence of SARS-CoV-2 among vaccinated participants compared to participants unvaccinated. It was calculated as (1-OR) x 100, where OR is the odds ratio for cases (i.e. incidence of SARS-CoV-2 infection) among vaccinated vs. unvaccinated. Potential confounders were age, sex, concomitant medical history, and BCG vaccination status. The unadjusted and adjusted VE with 95% CI was reported using logistic regression. The adjusted VE considered the potential confounders. A p-value based on a chi-square test was used to compare differences in rates of asymptomatic cases and mild cases of COVID-19 between the vaccinated and unvaccinated groups. All statistical analyses were performed using SAS version 9.4.
We also compared the duration (years) between the last dose of MCV received by the participants and the date of testing for SARS-CoV-2 infection (we used the date of the test report as a proxy for onset of infection since the date of swab collection was not available). The difference was compared using the Mann-Whitney test.
Results
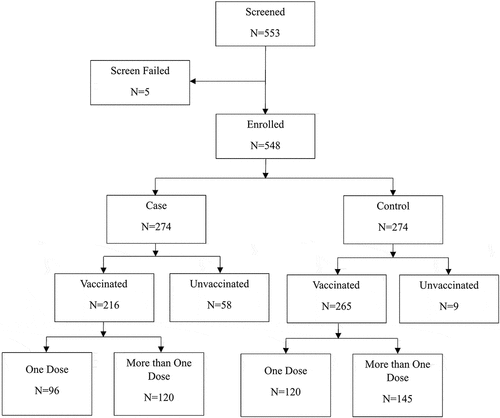
Data were collected from 548 participants who had undergone testing for SARS-CoV-2 infection ( and ). No screened participant had an unknown vaccination status. There were 274 participants each in the case and control group, respectively. The case group had 216 vaccinated participants (78.8%) and 58 unvaccinated participants (21.2%), while the control group had 265 (96.7%) vaccinated and 9 (3.3%) unvaccinated participants. All 274 cases received the BCG vaccine at birth while all except 1 participant in the control group received the BCG vaccine at birth.
Table 1. Demographics and baseline characteristics
Vaccinated participants had received 1, 2, or 3 doses of Measles, MR or MMR vaccines, either individually or in combination. Out of the 214 vaccinated participants in the case group, 96 received 1 dose, 98 received 2 doses while 22 received 3 doses of MCVs. The number of participants in the control group who received 1, 2, and 3 doses of MCVs were 120, 136, and 9, respectively ().
Table 2. Details of the Measles Containing Vaccine (MCV) received by the participants in the case and the control group
The unadjusted overall VE was 87.4% (OR = 0.126, 95% CI of VE: 73.9–93.9). Potential confounders were age, sex, concomitant medical history, and BCG vaccination status. Only 2 participants out of 548 reported a medical history event and only 1 out of the 548 participants was unvaccinated for BCG. Hence, they were not included in the analysis due to lack of data. The overall VE after adjusting for age and sex was 87.5% (OR = 0.125, 95% CI of VE: 74.2–94.0) ().
Table 3. Vaccine effectiveness
Among the cases, there were 180 (65.7%) asymptomatic participants and 94 (34.3%) symptomatic participants, all with mild disease. There were no participants with moderate, severe or critical or life-threatening disease, including deaths in the case group. The number of participants with symptomatic SARS-CoV-2 infection was significantly lower (p < .0001) in the vaccinated group compared to the unvaccinated group ().
Table 4. Summary of vaccination status by severity of COVID-19
The time interval between the last vaccine dose administration and the onset of infection was not significantly different between the case and the control group (median duration was 4.26 years vs 3.39 years, p > .05) ().
Table 5. Duration (Years) between last dose of measles containing vaccine and SARS-CoV-2 testing. All enrolled and vaccinated participants
Along with MCVs, BCG, Oral Polio Virus (OPV) and Rota virus vaccine are the commonly administered live attenuated vaccines as per the UIP in India. All participants in our study had received OPV and all except one had received BCG. The number of participants who received rotavirus vaccine was also similar across both the groups i.e. 69 in case group and 72 in control group.
Discussion
This was a test negative case-control study to assess effectiveness of MCVs in prevention of SARS-CoV-2- in children. The overall unadjusted VE of MCVs against SARS-CoV2 was 87.4%. Age and sex did not influence the protection provided by MCVs. Moreover, there were significantly fewer symptomatic cases in the vaccinated group compared to the unvaccinated group.
Two doses of MCV are recommended by the WHO.Citation16 Participants had received up to 3 doses of MCV which included either measles, MR or MMR vaccine. Although our study was not powered to do either a dose-wise comparison or comparison of different MCVs, results indicate that the vaccines give protection irrespective of the combination or the number of doses used. All live attenuated vaccines are supposed to provide cross-reactive immunity. In our study apart from MCVs, almost all the children had received other live attenuated vaccines like BCG and OPV while around 26% children in each group received the rotavirus vaccine. Therefore, we do not believe that these vaccines played any role in giving protection against COVID-19.
Previous studies have proposed the benefits of MCVs against SARS-CoV-2.Citation17,Citation18 It was found that people who had recently received various routine vaccines, including MMR vaccine, had lower SARS-CoV-2 infection rates, though this was not a peer-reviewed study.Citation6 An inverse relation was seen between mumps IgG titers and severity of SARS-CoV-2 infection in patients who had previously received MMR vaccine.Citation18 A 29% amino acid sequence homology has been identified between the macro domains of SARS-CoV-2 and rubella virus leading to the hypothesis that rubella antibodies could potentially provide long-term cross-immunity against SARS-CoV-2.Citation3 However, none of these studies assessed actual effectiveness of MCVs. To the best of our knowledge, ours is the first study in the world to calculate the effectiveness of MCVs in preventing SARS-CoV-2 infection in children.
Majority of the participants in the case arm (65.7%) were asymptomatic. Most of them had a contact history with a known case of SARS-CoV-2. There were no moderate or severe cases or deaths due to COVID-19 seen in the study. The symptomatic cases were significantly lower in the vaccinated arm compared to the unvaccinated arm, indicating that MCVs may also reduce the severity of COVID-19.
We compared the time interval between the last dose of MCV received and the diagnosis of infection between the case and control group to evaluate if this time interval was shorter in the control group than the case group. There was no significant difference in the time interval between the two groups, thereby suggesting that MCVs might offer a long-term protection against SARS-CoV-2 infection.
In the last decade, MR vaccine campaigns have been conducted by GAVI in children in many developing countries including Bangladesh,Citation19 Rwanda, Ghana, Senegal, Tanzania, Indonesia,Citation20 and India.Citation8 Interestingly, the COVID-19 deaths per 100,000 population in these countries are much lower than countries like the United Kingdom, United States, France, Spain, and Italy where MMR vaccine is administered only in routine immunization.Citation21 It is not known whether the mass campaigns had any role to play in the varying mortality.
Two doses of measles containing vaccines are known to give a long-lastingimmunity, including in adulthood.Citation22–24 It will be interesting to check if MCVs received in childhood also offer protection against SARS-CoV-2 in the adult population and needs to be confirmed by further additional studies in the adult population.
The impact of the SARS-CoV-2 pandemic has been milder in the pediatric population than in adults.Citation25 However, children can be important carriers for transmission of the virus.Citation26 Our data suggests that MCVs may also be helpful in blocking the transmission of the infection to adults by reducing the total incidence of SARS-CoV-2 positive cases in children.
This study has many strengths. It was conducted in a real life situation at a place, Pune which was one of the SARS-CoV-2 hotspots in India. The test negative design afforded a right control group. Use of the test-positive case vs. test-negative control methodology has the additional advantage of controlling for difficult to measure factors associated with both illness severity and the propensity to seek care when ill.Citation27,Citation28 We only used the RT-PCR laboratory confirmed cases, which gave a validated result.
Our study also had some limitations. Calculating the VE in the adult population would have been useful. However, we did not include the adult population due to the challenge in obtaining documented immunization records in that population. The study was not powered to compare the efficacy between the different MCVs (MR, MMR, Measles) and nor was it powered to compare the dose-wise effectiveness of MCVs. In case-control studies, it is difficult to determine the impact of all potential biases. The demographic and baseline characteristics were similar between the cases and controls, indicating similarities between the two groups, but unknown sources of bias may still occur.
To conclude, MCVs were found protective against laboratory confirmed SARS-CoV-2 in participants ≥ 1 to < 18 years of age. They also reduced the severity of COVID-19 in vaccinated participants. Randomized clinical trials are currently underway in the US and Egypt, which are evaluating the role of MCVs against SARS-CoV-2.Citation29,Citation30 Results from these trials will be helpful in further strengthening the evidence regarding the benefit of MCVs against SARS-CoV-2.
Disclosure of potential conflicts of interest
Prasad S. Kulkarni, Abhijeet Dharmadhikari and Anand Lakhkar are employed by Serum Institute of India Pvt. Ltd. which is a manufacturer of Measles, MR and MMR vaccines. Sarah Anderson is employed by a CRO which was contracted by the sponsor for statistical analysis.
Additional information
Funding
References
- Ng Kee Kwong KC, Mehta PR, Shukla G, Mehta AR. COVID-19, SARS and MERS: a neurological perspective. J Clin Neurosci. 2020;77::13–16. doi:10.1016/j.jocn.2020.04.124.
- Samaddar A, Gadepalli R, Nag VL, Misra S. The Enigma of low COVID-19 fatality rate in India. Front Genet. 2020 Jul 28;11:854. doi:10.3389/fgene.2020.00854.
- Franklin R, Young A, Neumann B, Fernandez R, Joannides A, Reyahi A, Modis Y. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv. 2020 Apr 10;20053207. accessed 2020 Apr 10. doi:10.1101/2020.04.10.20053207.
- Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. Morb Mortal Wkly Rep. 2020 Jun 19;69(24):759–65. doi:10.15585/mmwr.mm6924e2.
- Steinman JB, Lum FM, Ho PP, Kaminski N, Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):24620–26. doi:10.1073/pnas.2012358117.
- Pawlowski, C., Puranik, A., Bandi, H. et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci Rep 11, 4741 (2021). https://doi.org/10.1038/s41598-021-83641-y
- Ozdemir C, Kucuksezer UC, Tamay ZU. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy. 2020;75(7):1824–27. doi:10.1111/all.14344.
- Introduction of Measles Rubella Vaccine (Campaign and Routine Immunization). National Operational Guidelines 2017. Ministry of Health and Family Welfare. Government of India. Accessed 1 Jun 2021. https://main.mohfw.gov.in/sites/default/files/195431585071489665073.pdf
- National Family Health Survey (NFHS-5) 2019-2020. Ministry of health and family welfare. Government of India. Accessed 2 June 2021. http://rchiips.org/NFHS/NFHS-5_FCTS/NFHS-5%20State%20Factsheet%20Compendium_Phase-I.pdf
- Newtonraj A, Vincent A, Selvaraj K, Manikandan M. Status of coverage of MR vaccination, after supplementary immunization activities in a rural area of South India: a rapid immunization coverage survey. Rural Remote Health. 2019 Sep;19(3):5261. doi:10.22605/RRH5261.
- Scobie HM, Ray A, Routray S, Bose A, Bahl S, Sosler S, Wannemuehler K, Kumar R, Haldar P, Anand A, et al. Cluster survey evaluation of a measles vaccination campaign in Jharkhand, India, 2012. PLoS One. 2015;10(5):e0127105. doi:10.1371/journal.pone.0127105.
- Priyadharshini JA. Coverage survey of Measles-Rubella mass vaccination campaign in a rural area in Tamil Nadu. J Family Med Prim Care. 2019;8(6):1884–88. doi:10.4103/jfmpc.jfmpc_319_19.
- Rajkumari B, Keisam A, Haobam DS, Thounaojam T. Evaluation of vaccination coverage of measles-rubella campaign in Imphal East District, Manipur: a cross-sectional study. Indian J Public Health. 2020;64(2):173–77. doi:10.4103/ijph.IJPH_361_19.
- Patle RA, Wankhade NR. Measlesrubella vaccination campaign: evaluation of coverage in rural area of Central India. Int J Med Sci Public Health. 2019;8:765–68.
- Kelvin AA, Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis. 2020 Jun;20(6):633–34. doi:10.1016/S1473-3099(20)30236-X.
- World Health Organization. Measles vaccines: WHO position paper, April 2017 - Recommendations. Vaccine. 2019 Jan 7;37(2):219–22. doi:10.1016/j.vaccine.2017.07.066.
- Elhusseiny KM, Abd-Elhay FA, Kamel MG. Possible therapeutic agents for COVID-19: a comprehensive review. Expert Rev Anti Infect Ther. 2020 Oct;18(10):1005–20. doi:10.1080/14787210.2020.1782742.
- Gold JE, Baumgartl WH, Okyay RA, Licht WE, Fidel PL, Noverr MC, Tilley LP, Hurley DJ, Rada B, Ashford JW, et al. Analysis of Measles-Mumps-Rubella (MMR) titers of recovered COVID-19 patients. mBio. 2020 Nov;11:6. doi:10.1128/mBio.02628-20.
- Sarma H, Budden A, Luies SK, Lim SS, Shamsuzzaman M, Sultana T, Rajaratnam JK, Craw L, Banwell C, Ali MW, et al. Implementation of the World’s largest measles-rubella mass vaccination campaign in Bangladesh: a process evaluation. BMC Public Health. 2019;19(925). doi:10.1186/s12889-019-7176-4.
- Pronyk P, Sugihantono A, Sitohang V, Moran T, Kadandale S, Muller S, Whetham C, Kezaala R. Vaccine hesitancy in Indonesia. Lancet Planet Health. 2019 Mar;3(3):e114–e115. doi:10.1016/S2542-5196(18)30287-0.
- [accessed 2021 Jan 22]. John Hopkins Corona Virus Resource Centre. https://coronavirus.jhu.edu/data/mortality
- Deshpande S, Balaji S. MMR Vaccine and Covid-19: a Myth or a low risk-high reward preventive measure? Indian Pediatr. 2020;57(8):773. doi:10.1007/s13312-020-1941-4.
- World Health, Organization. Immunological basis for immunization: rubella. World Health Organization; Geneva, Switzerland. Accessed on 1 June 2021. https://apps.who.int/iris/bitstream/handle/10665/43922/9789241596848_eng.pdf?sequence=1&isAllowed=y
- Mclean, Huong Q, Hickman, C. J, Seward, Jane F, World Health Organization & Centers for Disease Control and Prevention (U.S.). 2010. The immunological basis for immunization series: module 16: mumps. World Health Organization, Geneva, Switzerland. Accessed on 2 Jun 2021. https://apps.who.int/iris/handle/10665/97885
- Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. Dec 1 2020. doi: 10.1136/archdischild-2020-320338
- Han MS, Choi EH, Chang SH, Jin B-L, Lee EJ, Kim BN, Kim MK, Doo K, Seo J-H, Kim Y-J, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2021;175(1):73–80. doi:10.1001/jamapediatrics.2020.3988.
- Fleming DM, Andrews NJ, Ellis JS, Bermingham A, Sebastianpillai P, Elliot AJ, Miller E, Zambon M. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64(12):1062–67. doi:10.1136/jech.2009.093450.
- Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, Orenstein WA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36(3):623–31. doi:10.1093/ije/dym021.
- [accessed 2021 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT04475081?cond=MMR+COVID-19&draw=2&rank=
- [accessed 2021 Jan 22]. https://clinicaltrials.gov/ct2/show/NCT04357028