ABSTRACT
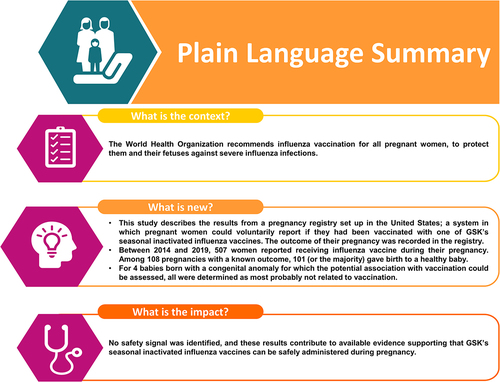
The World Health Organization recommends that all pregnant women receive seasonal influenza vaccine. Under a post-authorization safety study protocol (NCT02148211), a pregnancy exposure registry was established in the United States to monitor spontaneously reported pregnancy outcomes in women vaccinated with GSK’s seasonal inactivated influenza vaccines (IIVs). From 1 June 2014 to 31 May 2019, 507 pregnancies were prospectively reported: 352 (69.4%) were lost to follow-up and 40 (7.9%) were ongoing. Reported outcomes for the remaining 115 were: 101 (87.8%) live births without congenital anomalies; 3 (2.6%) live births with congenital anomalies; 2 (1.7%) spontaneous abortions with no congenital anomalies; 1 (0.9%) spontaneous abortion with a congenital anomaly; 1 stillbirth with no apparent congenital anomaly; 7 (6.1%) ‘Unknown’. Results from 493 prospective reports received via worldwide spontaneous, passive surveillance showed similar outcomes. All cases with congenital anomaly were assessed as not likely/unlikely/unrelated to vaccination. Despite the limited number of cases and outcomes, no safety signal was identified. The study findings are aligned with previously published data and should be confirmed with other robust data sources.
PLAIN LANGUAGE SUMMARY
What is the context?
The pneumococcus bacterium can cause infections of the meninges, blood, lung, middle ear and sinuses.
Two vaccins, Synflorix (GSK) and Prevnar 13 (Pfizer Inc.), are widely used to protect young children against these infections.
The vaccines’ compositions differ: Synflorix includes antigens from 10 pneumococcus strains (or “serotypes”) and Prevnar 13 from 13 serotypes.
However, both have a similar effect on the total pneumococcal disease burden in children.
What does this commentary highlight?
This commentary summarizes the evidence beihnd the two vaccines’ comparable impact on pneumococcal disase.
It also looks at why the vaccines have a similar effect on the total pneumococcal disease burden despite their different compositions.
What is the impact on current thinking?
Given that Synflorix and Prevnar 13 have a comparable impact on pneumococcal disease, a country’s choice between the two vaccines will depend on vaccine supply, cost, logistical factors (e.g., transport, storage, training requirements of health workers) and the local pneumococcal epidemiology.
Influenza infection that occurs during pregnancy can compromise maternal and fetal outcomes.Citation1 In 2012, the World Health Organization recommended that all pregnant women should receive vaccination against influenza.Citation2 Inactivated influenza vaccines (IIVs) have subsequently been administered to millions of women during pregnancy, and a wealth of evidence from large observational studies and meta-analyses suggests that IIV vaccination during pregnancy is not associated with an increased risk of adverse outcomes for mothers or fetuses.Citation3–9
Patient registries can provide prospective, real-world data arising from routine clinical practice. Compared to routine event surveillance, registries can potentially evaluate large numbers of patients and allow more structured data collection.Citation10 Pregnancy registries have provided valuable information in the evaluation of the risk of adverse outcomes in women exposed to vaccines during pregnancy, such as vaccines for human papillomavirus,Citation11,Citation12 anthrax,Citation13 and varicella.Citation14 Between 2011 and 2013, GSK initiated 4 pregnancy registries in the United States (US) to monitor adverse maternal and fetal outcomes after exposure to its licensed seasonal IIVs: Fluarix and Fluarix Quadrivalent vaccines manufactured in Dresden, Germany; and FluLaval and FluLaval Quadrivalent vaccines manufactured in Quebec City, Canada. All 4 IIVs are classified as Pregnancy Category B: Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. GSK’s licensed seasonal IIVs are split virion IIVS consisting of equal amounts of 3 or 4 monovalent viral antigen bulks prepared from influenza strains A/H1N1, A/H3N2 and 1 or 2 B strains (1 B/Yamagata lineage and/or 1 B/Victoria lineage).Citation15 In 2014, the existing registries were transitioned to a combined post-authorization safety study (PASS, NCT02148211). The design of the PASS was the same as the existing registries, but brought them together for administrative reasons and in an attempt to improve enrollment. Here we report the 5-year results of the PASS which commenced on 1 June 2014 to the data lock point of 31 May 2019.
This was an exploratory, prospective, observational cohort study (the IIV pregnancy registry). Patients or healthcare professionals were encouraged to voluntarily and prospectively enroll any pregnant woman who had been exposed to 1 of GSK’s seasonal IIVs. Pregnant women who voluntarily enrolled in the registry had to sign an informed consent form that allowed GSK to contact their healthcare professional around the estimated delivery date (EDD) for follow-up information.
The registry objectives were to describe the proportion and characteristics of prospectively reported pregnancies with abnormal outcomes in women exposed to IIVs during pregnancy or within 28 days preceding gestation. The registry was advertised through the US Prescribing Information and the GSK registry website, which gave a brief summary of the purpose and intent of the registry along with telephone and fax contact information. Additionally, GSK requested that the same information be posted directly on the website of the US Food and Drug Administration.
A woman was included in the registry if she had been exposed to 1 of GSK’s IIVs during pregnancy or within 28 days preceding conception; was a US resident; had an identifiable healthcare professional (including their contact details); and could be identified by GSK or the healthcare professional. Data from registered women were only included in the analysis cohort if the pregnancy was ongoing and the outcome was unknown at the time of the initial report (prospective reporting). Conversely, a retrospective report, defined as when the outcome of the pregnancy (including prenatal test results) was known and abnormal at the time of the initial report, was not included in the analysis cohort.
Initial and follow-up data were collected from pregnant women and/or their healthcare professional using questionnaires. These included an initial notification form, a pregnancy outcome form to be completed within 2 months of the EDD to ascertain outcome of the pregnancy, and a 6 to 12-months post-delivery follow-up form to ascertain the presence of birth defects not previously diagnosed. Follow-up questionnaires could only be completed by healthcare providers and/or their staff. Information about maternal medical and obstetric history, other drug/vaccine exposures, adverse events experienced by the fetus/infant or mother, and infant/neonatal status at birth until 6 and 12 months of age was recorded. Reasonable efforts were made to minimize loss to follow-up, with up to 2 attempts made to obtain additional information from women, healthcare providers and the pediatrician and/or other specialists who had provided healthcare/consultation to the child up until 12 months of age.
Pregnancy outcomes of interest were spontaneous abortion (pregnancy loss before 22 weeks gestation), fetal deaths/stillbirths (loss at or after 22 weeks gestation), elective/therapeutic abortions, and live births. The study outcomes were assessed for the likelihood of a safety signal warranting further investigation against known background rates from external existing systems such as the National Birth Defects Prevention Network, the National Center for Health Statistics and the Metropolitan Atlanta Congenital Defects Program. Potential causal associations between congenital anomalies and vaccination were assessed when data allowed by considering the timing of vaccination in relation to embryogenesis, biological plausibility, and the presence of other potential causal factors.
From 1 June 2014 to 31 May 2019, 507 pregnancies exposed to GSK’s IIVs in the US were prospectively reported. There were 84 exposures (16.6%) that occurred during the first trimester, 113 (22.3%) in the second, 91 (17.9%) in the third trimester, and 219 (43.2%) for which the date of exposure was unknown (). There were 13 pregnancies (2.6%) exposed to FluLaval, 59 (11.6%) to FluLaval Quadrivalent, 112 (22.0%) to Fluarix, and 325 (63.8%) to Fluarix Quadrivalent ().
Table 1. Trimester of exposure and outcomes for IIV-exposed pregnancies in the United States, 2014–2019
Table 2. Trimester of exposure and outcomes for pregnancies exposed to GSK’s seasonal inactivated influenza vaccines
A total of 352 (69.4%) pregnancies were lost to follow-up, 40 (7.9%) women were still pregnant at the time of last contact (no further information available as on 01 April 2020), and a pregnancy outcome was available for 115 (22.7%) women. Of the remaining 115 pregnancies, 101 (87.8%) resulted in a live birth without congenital anomaly; 3 (2.6%) were live births with congenital anomalies; 2 (1.7%) were spontaneous abortions with no apparent congenital anomalies; 1 (0.9%) was a spontaneous abortion with a congenital anomaly; and 1 (0.9%) was a stillbirth with no apparent congenital anomaly. The pregnancy outcome of 7 (6.1%) women in the registry was reported as ‘Unknown’ (usually lost to follow-up) (). Although the sample size was small for some IIVs, the distribution of registered pregnancies by trimester of exposure and outcome was similar for the 4 IIVs combined, and no patterns or trends were observed ().
Among adverse pregnancy outcomes there were 4 infants/fetuses with congenital anomalies. One infant had polycystic kidney disease. The mother of this infant had received IIV at 16 weeks gestation, which is after the period of renal embryogenesis that occurs early in the first trimester,Citation16 and thus a causal association with IIV was deemed unlikely. One infant had a cleft lip and palate at birth. The mother of this infant had received IIV at 16 weeks + 5 days of gestation. The critical period for palatal development is between the 6th and 9th weeks of gestation,Citation17 and a causal association with IIV is unlikely. One woman who received IIV at 23 weeks gestation delivered twins by cesarean section at 31 weeks. A causal association between vaccination and premature delivery seems unlikely and ‘cardiac insufficiency’ found in the case is not suggestive of congenital anomaly because the date of vaccination was after the period of cardiogenesis and was likely due to twin-twin transfusion syndrome. One woman who received IIV at 5 weeks gestation experienced spontaneous abortion at 22 weeks. A congenital anomaly was reported to the registry, but no record of a congenital anomaly was noted in any of the source documents submitted, and the dates are not interpretable.
One woman experienced a stillbirth 188 days after receiving IIV. The cause of death was suspected to be presence of nuchal cord (umbilical cord around neck of baby). Based on the nature of the event and time to onset, a causal association with vaccination was ruled out. There was 1 spontaneous abortion in a woman with a history of extensive recreational drug and alcohol exposure and chickenpox prior to the last menstrual period, which confounded the causality assessment. Finally, a 40-year-old woman spontaneously aborted twins the day after receiving IIV (around week 12 of gestation). There was insufficient information for further assessment.
A total of 692 adverse events were reported for all 507 registered pregnancies, of which the vast majority were ‘exposure during pregnancy’ (504 events) and ‘live birth’ (103 events) (Supplementary Table 1). Other adverse events classified by Medical Dictionary for Regulatory Activities System Organ Class were: Injury, poisoning and procedural complications (29 events), Pregnancy, puerperium and perinatal conditions (24 events), General disorders and administration site conditions (10 events), Musculoskeletal and connective tissue disorders (6 events), Gastrointestinal disorders (5 events), Immune system disorders (2 cases), Infections and infestations (2 cases), Nervous system disorders (2 cases), Psychiatric disorders (1 event), Skin and subcutaneous tissue disorders (1 event), and Surgical and medical procedures (1 event). Two adverse events, both Congenital, familial and genetic disorders, were reported in infants.
In addition to the US pregnancy registry described above, GSK also receives retrospective notification of seasonal influenza vaccine-exposed pregnancies and their outcomes from global sources through its passive spontaneous adverse event reporting system. To complement the registry results and evaluate the outcomes of all pregnancies exposed to GSKs VIIs worldwide, additional reports of exposed pregnancies were extracted from the worldwide safety database until the cutoff date of 31 May 2019. Among an additional 676 spontaneous reports of influenza vaccine exposure during pregnancy received by GSK from global sources, 493 were prospective (). Of these, 307 (62.3%) were reported as lost to follow-up. Of the 186 pregnancies with a known outcome; 66 (35.5%) pregnancies were ongoing, 114 (61.3%) resulted in live births with no birth defects, 5 (2.7%) in spontaneous abortion with no birth defects, and there was 1 (0.5%) elective termination without birth defects. There were no reports of birth defects reported among cases reported prospectively to GSK’s safety database.
Table 3. Pregnancy outcomes from worldwide spontaneous case reporting
Among 54 spontaneous retrospective pregnancy reports with a known outcome, 31 reported still birth, spontaneous abortion or birth defects (). The high proportion of adverse outcomes is not unexpected given that retrospective notification of outcomes following exposure to drugs or vaccines is biased toward reporting the severe and unusual cases.Citation18,Citation19 There were 8 retrospective cases with birth defects including variant polydactyly (causal association not likely), talipes equinovarus (2 cases: causal association not likely for 1 case and unlikely but indeterminate for the second case), congenital cardiac anomalies and aneuploidy with infectious and immune etiologies and chromosomal anomalies (causal association unlikely), Trisomy 21 and chronic chorioamnionitis (causal association ruled out), Trisomy 21 with group B streptococcal colonization (causal association ruled out), gastroschisis with atrial septal defect (causal assessment not possible as timing of vaccination not known), and congenital syphilis (causal association ruled out).
The risk in the general population of all birth defects meeting Centers for Disease Control and Prevention criteria is approximately 3% of live births.Citation20 The Collaborative Prenatal Project, using a broader case definition and prospective ascertainment, reports a frequency of 5% to 7%.Citation21 Most major structural defects originate during the first trimester of pregnancy, which is the critical time for organogenesis.Citation22 For such defects, exposures occurring in the second or third trimester are not likely to be causally associated.
No safety signal was identified among IIV-exposed pregnant women who reported their pregnancy outcomes to the US registry or through GSKs worldwide safety database.
Pregnancy registries can provide real-time insights into the effect of exposures during pregnancy and can collect accurate data around the timing of exposure and a variety of prenatal, perinatal and postnatal outcomes for the mother and infant. However, pregnancy registries face particular challenges because they rely on voluntary reporting and are prone to low rates of recruitment and retention. This is particularly the case because the subjects of interest are usually healthy women who expect a positive pregnancy outcome and have little incentive to participate. The logistics of following up on the health of a pregnant woman and their infant by relying solely on the voluntary submission of information by their obstetrician or pediatrician are complicated, and response rates can be low, contributing to poor retention rates.Citation23 Rates of loss to follow-up vary widely and tend to be higher when the follow-up period is longer.Citation24 Retention rates after at least 6 months of follow-up range from 68%, to as low as 30.6% in our study.Citation12,Citation24 Pregnancy registries lack internal comparators and a population-based denominator, and are unable to deliver estimates of incidence or risk.
The practical challenges of achieving high enrollment and high levels of retention in pregnancy registries mean that their full potential as a prospective data collection tool is infrequently achieved. The use of ‘big data’ to investigate rare events in very large cohorts provides an alternative or complementary approach to inform safety of vaccines in pregnancy. Population-based databases that hold vaccination data or that can be linked to vaccination records can be a powerful tool, providing access to large sample sizes, specific cohorts of interest and control groups that allow estimation of both incidence and risk. A growing number of studies have used population-based databases to investigate associations between exposure to vaccines and adverse pregnancy outcomes including spontaneous abortion, other obstetric events, adverse birth outcomes and diseases of later childhood such as autism spectrum disorder.Citation25–28 Many of these databases allow mother-baby links to be made, and can be used to identify potential congenital disorders not identified at birth, which is difficult, if at all possible, using registries. Pregnancy registries typically follow infants after birth for 6 to 12 months, which also limits their capacity to study diseases of later childhood, in contrast with many databases where the longitudinal medical history of individuals can be tracked. However, as yet, database studies to investigate vaccine safety in women exposed during pregnancy have not been widespread in the post-approval setting, and are often considered ancillary to pregnancy registries.Citation29
GSK’s IIV pregnancy registry was established as part of the pharmacovigilance plan to investigate the effects of exposure to seasonal IIVs during pregnancy. The registry data presented here are limited by the number of pregnancies and outcomes which are not sufficient to reach reliable and definitive conclusions about the risk of IIV exposure during pregnancy or within 28 days of conception. Despite the limitations of the data, these results contribute to the body of evidence supporting that seasonal IIVs can be safely administered during pregnancy. The findings from this study are aligned with previously published data and should be confirmed using robust data sources, such as healthcare databases. Population-based databases can be used to investigate specific research questions and can be powerful alternative or complementary tools to pregnancy registries.
A plain language statement is provided in .
Authors’ contribution statement
Ugo Nwoji has made a substantial contribution to the study, drafting or reviewing the article critically for intellectual content and has approved the final version of the manuscript.
Disclosure of potential conflicts of interest
Ugo Nwoji is employed by and holds shares in the GSK group of companies. He declares no other financial or non-financial relationships and activities.
Trademark statement
Fluarix, Fluarix Quadrivalent, FluLaval and FluLaval Quadrivalent are registered trademark owned by or licensed to the GSK group of companies.
Supplemental Material
Download MS Word (48.1 KB)Acknowledgments
The author thanks the following individuals from GSK: Stéphanie Gilon, Emad Yanni, Jerome Wilson, Harold Silverman, and Chanbin Kim for their involvement in the study conduct; Andrea Sutherland for her involvement in the study conduct and her review of the manuscript and Pooja Jindal for reviewing the manuscript. The author thanks the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Aurélie Roth coordinated manuscript development and editorial support, and Joanne Wolter (independent on behalf of Business & Decision Life Sciences) provided medical writing support.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1932213.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Buchy P, Badur S, Kassianos G, Preiss S, Tam JS. Vaccinating pregnant women against influenza needs to be a priority for all countries: an expert commentary. Int J Infect Dis. 2020;92:1–6. doi:10.1016/j.ijid.2019.12.019.
- World Health Organisation. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012;87:461–76.
- Omer SB, Richards JL, Madhi SA, Tapia MD, Steinhoff MC, Aqil AR, Wairagkar N; for the BMGF Supported Maternal Influenza Immunization Trials Investigators Group. Three randomized trials of maternal influenza immunization in Mali, Nepal, and South Africa: methods and expectations. Vaccine. 2015;33:3801–12. doi:10.1016/j.vaccine.2015.05.077.
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, Van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371:918–31. doi:10.1056/NEJMoa1401480.
- Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17:981–89. doi:10.1016/S1473-3099(17)30252-9.
- McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine. 2015;33:2108–17. doi:10.1016/j.vaccine.2015.02.068.
- Ludvigsson JF, Strom P, Lundholm C, Cnattingius S, Ekbom A, Ortqvist A, Feltelius N, Granath F, Stephansson O. Maternal vaccination against H1N1 influenza and offspring mortality: population based cohort study and sibling design. BMJ. 2015;351:h5585. doi:10.1136/bmj.h5585.
- McHugh L, Marshall HS, Perrett KP, Nolan T, Wood N, Lambert SB, Richmond P, Ware RS, Binks P, Binks MJ, et al. The safety of influenza and pertussis vaccination in pregnancy in a cohort of Australian Mother-Infant Pairs, 2012-2015: the FluMum Study. Clin Infect Dis. 2019;68:402–08. doi:10.1093/cid/ciy517.
- Jeong S, Jang EJ, Jo J, Jang S. Effects of maternal influenza vaccination on adverse birth outcomes: a systematic review and Bayesian meta-analysis. PLoS One. 2019;14:e0220910. doi:10.1371/journal.pone.0220910.
- Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for evaluating patient outcomes: a user’s guide. 3rd ed. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
- Lopez-Fauqued M, Zima J, Angelo M-G, Stegmann J-U. Results on exposure during pregnancy from a pregnancy registry for AS04-HPV-16/18 vaccine. Vaccine. 2017;35(40):5325–30. doi:10.1016/j.vaccine.2017.08.042.
- Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine. 2015;33(29):3422–28. doi:10.1016/j.vaccine.2015.04.014.
- Conlin AM, Bukowinski AT, Gumbs GR. for the Department of Defense, Birth Infant Health Registry Team. Analysis of Pregnancy and Infant Health Outcomes among Women in the National Smallpox Vaccine in Pregnancy Registry Who Received Anthrax Vaccine Adsorbed. Vaccine 2015;33:4387–90. doi:10.1016/j.vaccine.2015.05.054.
- Wilson E, Goss MA, Marin M, Shields KE, Seward JF, Rasmussen SA, Sharrar RG. Varicella vaccine exposure during pregnancy: data from 10 years of the pregnancy registry. J Infect Dis. 2008;197(Suppl 2):S178–184. doi:10.1086/522136.
- Claeys C, Drame M, Garcia-Sicilia J, Zaman K, Carmona A, Tran PM, Miranda M, Martinon-Torres F, Thollot F, Horn M, et al. Assessment of an optimized manufacturing process for inactivated quadrivalent influenza vaccine: a phase III, randomized, double-blind, safety and immunogenicity study in children and adults. BMC Infect Dis. 2018;18:186. doi:10.1186/s12879-018-3079-8.
- Rehman S, Ahmed D. Embryology, kidney, bladder, and ureter. Treasure Island (FL): StatPearls; 2020.
- Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. Lancet. 2009;374:1773–85. doi:10.1016/S0140-6736(09)60695-4.
- Rawlins MD. Pharmacovigilance: paradise lost, regained or postponed? The William Withering Lecture 1994. J R Coll Physicians Lond. 1995;29:41–49.
- Rosenthal S, Chen R. The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health. 1995;85:1706–09. doi:10.2105/ajph.85.12.1706.
- Centers for Disease Control and Prevention. Birth defects; 2021 Mar 05 [Accessed 2021 Apr 12]. http://www.cdc.gov/ncbddd/birthdefects/index.html
- Chung CS, Myrianthopoulos NC. Factors affecting risks of congenital malformations. I. Analysis of epidemiologic factors in congenital malformations. Report from the Collaborative Perinatal Project. Birth Defects Orig Artic Ser. 1975;11:1–22.
- Niebyl JR, Simpson JL. Chapter 8: drugs and environmental agents in pregnancy and lactation: embryology, teratology and epidemiology. In: Gabbe S, Niebyl J, Galan H, Jauniaux E, Landon M, Simpson J, Driscoll D editors. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia (PA): Saunders (Elsevier); 2012. p. 141.
- Sinclair S, Cunnington M, Messenheimer J, Weil J, Cragan J, Lowensohn R, Yerby M, Tennis P. Advantages and problems with pregnancy registries: observations and surprises throughout the life of the International Lamotrigine Pregnancy Registry. Pharmacoepidemiol Drug Saf. 2014;23:779–86. doi:10.1002/pds.3659.
- Bird ST, Gelperin K, Taylor L, Sahin L, Hammad H, Andrade SE, Mohamoud MA, Toh S, Hampp C. Enrollment and retention in 34 United States pregnancy registries contrasted with the manufacturer’s capture of spontaneous reports for exposed pregnancies. Drug Saf. 2018;41:87–94. doi:10.1007/s40264-017-0591-5.
- Baril L, Rosillon D, Willame C, Angelo MG, Zima J, Van Den Bosch JH, Van Staa T, Boggon R, Bunge EM, Hernandez-Diaz S, et al. Risk of spontaneous abortion and other pregnancy outcomes in 15-25 year old women exposed to human papillomavirus-16/18 AS04-adjuvanted vaccine in the United Kingdom. Vaccine. 2015;33:6884–91. doi:10.1016/j.vaccine.2015.07.024.
- Walsh LK, Donelle J, Dodds L, Hawken S, Wilson K, Benchimol EI, Chakraborty P, Guttmann A, Kwong JC, MacDonald NE, et al. Health outcomes of young children born to mothers who received 2009 pandemic H1N1 influenza vaccination during pregnancy: retrospective cohort study. BMJ. 2019;366:l4151. doi:10.1136/bmj.l4151.
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, Omer SB, Hambidge SJ, Lee GM, Jackson ML, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312:1897–904. doi:10.1001/jama.2014.14825.
- Zerbo O, Qian Y, Yoshida C, Fireman BH, Klein NP, Croen LA. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr. 2017;171:e163609. doi:10.1001/jamapediatrics.2016.3609.
- U.S. Food and Drug Administration. Postapproval pregnancy safety studies guidance for industry. Draft Guidance. Rockville (MD); 2019 May. [Accessed 2021 Apr 12] https://www.fda.gov/media/124746/download