ABSTRACT
A systematic review was conducted in Mexico to consolidate and evaluate evidence after 15 years of rotavirus vaccination, according to the National Immunization Program. Five databases were screened to identify published articles (January 2000–February 2020) with evidence on all clinical and epidemiological endpoints (e.g. immunogenicity, safety, efficacy, impact/effectiveness) of rotavirus vaccination in Mexico. Twenty-two articles were identified (observational studies including health-economic models: 17; randomized controlled trials: 5). Fourteen studies evaluated a human attenuated vaccine (HRV), four studies evaluated both vaccines, and only two evaluated a bovine-human reassortant vaccine, with local efficacy data only for HRV. Local evidence shows vaccines are safe, immunogenic, efficacious, and provide an acceptable risk-benefit profile. The benefits of both vaccines in alleviating the burden of all-cause diarrhea mortality and morbidity are documented in several local post-licensure studies. Findings signify overall benefits of rotavirus vaccination and support the continued use of rotavirus vaccine in Mexico.
Introduction
Diarrhea is among the leading causes of mortality in young children under 5 years of age, especially in low- and middle-income countries.Citation1,Citation2 Every year, estimated 1.5 million children under 5 years of age die of diarrhea worldwide.Citation3 Vaccine-preventable rotavirus infection is one of the most frequent causes of gastroenteritis and diarrhea, which can lead to rapid dehydration in children under 5 years of age.Citation4,Citation5 Since 2006, two live, orally administered rotavirus vaccines have been available and licensed for the prevention of rotavirus gastroenteritis: Rotarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium), a two-dose human attenuated vaccine (HRV), and RotaTeq® (Merck & Co. Inc., West Point, PA, USA), a three-dose bovine-human reassortant vaccine (BHRV).Citation6 Based on the World Health Organization (WHO) recommendation, rotavirus vaccines have been introduced into the National Immunization Programs (NIP) of several countries, and licensed in more than 100 countries.Citation6 Since then, diarrhea-associated mortality has decreased markedly over time, attributable to the widespread use of rotavirus vaccines with other contributing factors such as improvements in diarrhea treatment, sanitation and provision of safe drinking water, and other aspects related to nutrition (breastfeeding practices, vitamin A supplementation).Citation7
Yet, rotavirus is still responsible for high levels of diarrhea-related morbidity globally, especially in low- and middle-income nations.Citation8 In Latin America and the Caribbean (LAC) region, during the pre-vaccination era, it was estimated that rotavirus caused about 75,000 hospitalizations and 2 million clinic visits per year.Citation9 The majority of this burden peaked during the cool winter months.Citation9,Citation10 After rotavirus vaccination, in the LAC region, it was observed that in children <5 years of age the number of diarrheal deaths decreased from 32,780 in 2000 to 8,750 in 2013; and deaths due to rotavirus decreased from 11,631 in 2000 to 2,288 in 2013.Citation9 In the LAC region, the most common G type of rotavirus is G1, which is responsible for almost half of the rotavirus diarrhea burden, followed by G4, G3, and G9, although regional and temporal variations are significant.Citation11 The Pan American Health Organization (PAHO) Technical Advisory Group on vaccine-preventable diseases recommends that countries in this region should continue making efforts to administer rotavirus vaccines as part of their routine vaccination schedules, at the recommended ages according to the vaccine used, usually at 2 and 4 or 2, 4 and 6 months of age. Both of these schedules, particularly the two-dose with HRV which can be completed by 24 weeks of age, foster the early protection for children at the highest risk of severe disease due to rotavirus diarrhea.Citation10
Several Latin American countries participated and led the way in pivotal pre-licensure clinical trials. This led to a comprehensive evidence base from a substantial number of rotavirus-specific studies that are available to guide and inform vaccine policy development in the region.Citation12 In July 2004, HRV was first registered in Mexico after which it was introduced for routine use into the NIP of several countries in the region.Citation11 Brazil and Mexico were among the first to implement childhood rotavirus vaccination into their NIP.Citation13 In the Mexican NIP, the two-dose HRV was used from 2006 to 2011 and the three-dose BHRV has been offered since 2011.Citation14 The Mexican Social Security Institute (IMSS) partially re-introduced HRV into the NIP in 2019, and distributed both the vaccines through the NIP. It has been over a decade since the licensing and first introduction of rotavirus vaccination into the NIP of Mexico. Numerous studies have been published since, on the impact of vaccination on diarrheal disease burden and safety, testifying to the success of the vaccination programs. Therefore, we conducted a systematic literature review to appraise the available evidence on rotavirus vaccination in Mexico: First we describe findings on the clinical effectiveness, safety, burden of disease, cost-effectiveness of vaccination, and compliance to the recommended vaccine schedule. Then, the results are evaluated to assess the overall impact of rotavirus vaccination on diarrhea-associated mortality, morbidity, and hospitalization since the implementation of the vaccine in the NIP of Mexico (see Plain Language Summary).
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) guidelines.Citation15,Citation16 In line with these guidelines, we developed a search strategy and established study eligibility criteria prior to conducting the review. Following this, searches were performed and retrieved articles were assessed for eligibility in a two-phase screening process and full-text review by two reviewers. From the final list of eligible publications, data were extracted based on the scope which was established a priori. A risk of bias assessment was conducted for all included studies independently by two authors.
Search sources and strategy
The search was conducted in five electronic databases (Medline [via PubMed], EMBASE, Scopus, Latin American and Caribbean Health Sciences Literature [LILACS], and Scientific Electronic Library Online [SciELO]) using a comprehensive set of search terms. The search strategy was developed in Medline, utilizing a combination of both free-text and medical subject headings (MeSH) terms ((Rotavirus) AND (vaccine OR vaccination OR vaccine) AND (Mexico) AND (effectiveness OR impact OR compliance OR safety OR efficacy OR immunogenicity)) and then adapted to the other databases (Supplementary Tables 1). The databases were searched over a 20-year period capturing studies published between January 1, 2000, and February 1, 2020. Articles published in English and Spanish were included in this review and the geographic scope was restricted to Mexico.
Article selection, data extraction, and reporting
The identified articles were screened in two phases by two reviewers using the inclusion and exclusion criteria provided in . The retrieved articles were initially screened by title and abstract for eligibility by two reviewers followed by a second step which included screening of the full-text of articles using the eligibility criteria specified in . Any discrepancies were discussed and resolved with the other review authors.
Table 1. Inclusion and exclusion criteria
From each of the eligible articles, relevant information established a priori with all authors was extracted using a customized extraction form that included the following items: reference, author, journal and year, region/city, main study objectives, study type/design, study period, sample size, age group, clinical outcomes, and measures of vaccine impact.
A descriptive analysis of the extracted data was performed to summarize the main outcomes of this review.
Risk of bias assessment
A risk of bias assessment was conducted for all included observational studies and randomized controlled trials (RCTs). The risk of bias for observational studies was assessed using the Strengthening the Reporting of Observational studies in Epidemiology (STROBE)Citation17 checklist of essential items, modified according to Sanderson S et al. and Fowkes FG & Fulton PM (Supplementary Table 2).Citation18,Citation19 An algorithm programmed into a spreadsheet was used to estimate a summary assessment of risk of bias considering five criteria: methods for selecting study participants, methods for measuring exposure and outcome variables, and methods to control confounding, design-specific sources of bias, and statistical methods. The risk of bias of each study was rated as high, moderate, low, or doubtful. The Cochrane risk of bias tool was used to assess RCTs and clinical controlled trials (Supplementary Table 3).Citation20 The criteria for judging risk of bias were adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. The risk of bias of each study was rated as high, moderate and low. The Cochrane Effective Practice and Organization of Care (EPOC) quality criteria were used to assess the risk of bias of the controlled before and after studies and interrupted time series.Citation21 The risk of bias assessment was conducted independently by two authors and any disagreements were resolved by consensus through discussion with the authors.
Results
Characteristics of included studies
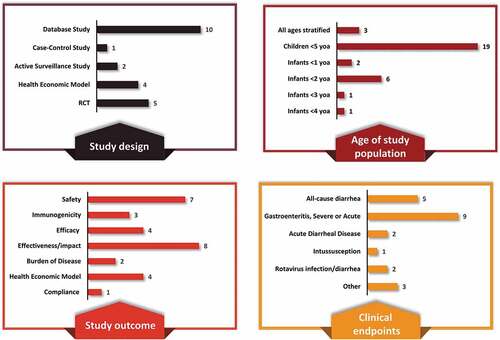
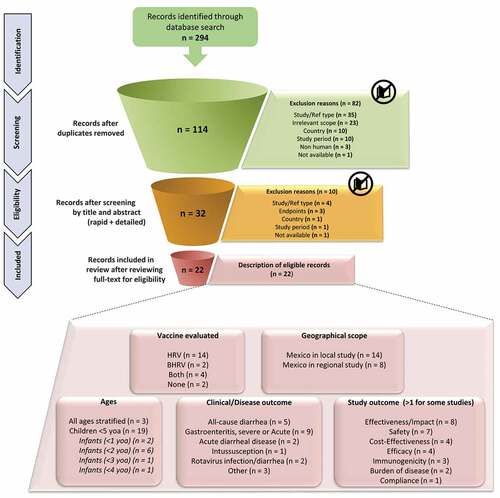
The literature search yielded 294 articles; of these 114 articles were screened at the title, the abstract phase, and finally 32 articles were screened at the full-text phase (). After full-text screening, 22 articles were included in this review (; ).Citation14,Citation22–43 Among these 22 articles, 14 studies were conducted only in Mexico, whereas eight studies were conducted in the Latin America region and included Mexico (; ).
Table 2. Overview of main results from the included studies (N = 22)
Figure 1. PRISMA flowchart
An overview of the study characteristics is presented in with individual study details provided in . Of the 22 studies, 17 were observational studies and 5 were RCTs. A majority of the studies included children ≤5 years of age (n = 19 studies) among which the distribution is as follows: infants <2 years (n = 6 studies), <1 year (n = 2), and <3 and <4 years each (n = 1 each).
Eligible studies reported evidence for disease burden (n = 2), immunogenicity (n = 3), efficacy (n = 4), safety (n = 7), impact on all-cause/acute diarrhea mortality and morbidity (n = 7), cost-effectiveness (n = 4), clinical effectiveness (n = 1), and vaccination compliance (n = 1). The majority of studies considered rotavirus gastroenteritis as the clinical endpoint followed by all-cause diarrhea, acute diarrheal disease, rotavirus infection/diarrhea and intussusception.
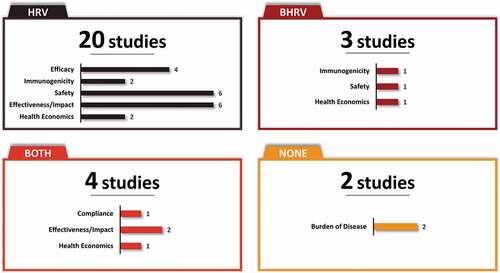
Fourteen and two studies evaluated HRV and BHRV, respectively, and four studies evaluated both vaccines. The distribution of studies by vaccine and type of study outcome is provided in and . Both vaccines, HRV and BHRV, had data for all outcomes with the exception of local efficacy data which were not identified for BHRV.
Summary of main findings
Burden of disease
One study reported estimates of the burden of disease prior to the implementation of rotavirus vaccination in Mexico in 2006. It estimated the percentage of rotavirus gastroenteritis cases among all-cause acute gastroenteritis cases in children <3 years of age at 59% (2003).Citation30 A second study estimated the effect of rotavirus diarrhea on disability-adjusted life-years (DALYS) and diarrhea treatment costs in hypothetical cohorts of infants who were followed from birth up to 5 years of age.Citation28 From birth to the age of 5 years, the estimated DALYs were 19,426 in 2001 and decreased by 28.9% in 2006, meanwhile costs of treatment were relatively constant, estimated at US$ 38.7 million and increased only by 5% ().Citation28
Immunogenicity and efficacy of rotavirus vaccine
Five RCTs with Mexican participantsCitation24,Citation31,Citation38–40 provided evidence on the immunogenicity (n = 3)Citation24,Citation38,Citation40 and efficacy (n = 4)Citation31,Citation38–40 of rotavirus vaccination (; ). The immunogenicity of HRV was reported in two studies which showed that most of the infants had seroprotective levels of antibodies when co-administered with the oral polio vaccine and other routine vaccinations.Citation39,Citation40 In the first phase 2b, randomized, dose–response study, range of seroconversion rates was 34.2–63.9% 2 months after the first dose and increased to 50%–70.6% two months after second dose in infants 6–12 weeks of age. Geometric mean titers were high and sustained after the completion of two doses.Citation38 In a second randomized, placebo-controlled study, the efficacy of different concentrations (10,4.Citation7 105.Citation2 or 105.Citation8 focus-forming units [FFU]) of HRV was evaluated in infants of 6–13 weeks of age. The study reported seroconversion rates of 38% (104.Citation7) to 43% (105.Citation8) two months after the first dose and ranged between 61% (104.Citation7) and 65% (105.Citation8) two months after the second dose.Citation40 The immunogenicity of BHRV was reported in one study which lends support to the concomitant use of BHRV and the oral poliovirus vaccine.Citation24 While the immunogenicity of OPV did not change when co-administered with BHRV, there was a reduction in the anti-RV IgA titers when given at the same time as OPV, yet children still met the criteria for seroconversion.
Vaccine efficacy was investigated in four studies for HRV.Citation31,Citation38–40 Overall, the evidence from RCTs shows that HRV is efficacious in preventing against severe and any rotavirus gastroenteritis, with efficacy ranging from 77%–100% and 70%–80%, respectively.Citation31,Citation38–40 The efficacy of rotavirus vaccination against hospitalizations due to any cause of gastroenteritis and severe rotavirus gastroenteritis was 42% and 85%, respectively.Citation39 In one study among infants <2 years of age vaccine efficacy was high against severe rotavirus gastroenteritis and sustained up to the third year of life (82.1%–100%).Citation31
Safety of rotavirus vaccine
A total of seven studies carried out in Mexico provided local evidence of an adequate safety profile of both rotavirus vaccines. While six of the seven studies reported safety data for HRV (randomized [n = 5]; non-randomized[n = 1]),Citation22,Citation31,Citation38–40,Citation42 only one provided safety data for BHRV (randomized)Citation24 (). Overall, both vaccines were well tolerated among vaccine recipients with low rates of serious adverse events including a low risk of intussusception. Both vaccines showed an acceptable safety profile when co-administered with the oral polio vaccine and other routine vaccinations.
Significantly fewer serious adverse events were reported among infants who received HRV compared to those who did not receive the vaccine (i.e. placebo).Citation22,Citation38,Citation40 While the majority of studies showed that HRV was not associated with an increased risk of intussusception during a 31-day window after administration of the first or second dose versus placebo,Citation31,Citation38,Citation39 other studies indicate a low risk of intussusception, specifically a temporal increase in the risk for intussusception within 7 days of administration of the first vaccine dose.Citation22,Citation42 In the largest surveillance study for intussusception after rotavirus vaccination to date, the relative incidence of intussusception within 31 days of vaccination was 1.75 (p = .001) after the first dose and 1.06 (p = .75) after the second dose; and within 7 days of vaccination, the relative incidence was 6.49 (p < .001) after the first dose and 1.29 (p = .29) after the second dose.Citation42 The health benefits of vaccination, in terms of absolute number of deaths and hospitalizations averted, far outweigh the risk of short-term probable side effects which rarely have complications.Citation44
A randomized study that evaluated the concomitant use of BHRV with the oral poliovirus vaccine compared to BHRV alone in infants showed a similar safety and tolerability profile between both regimens ().Citation24
Health economics of rotavirus vaccination
The cost-effectiveness of rotavirus vaccination in Mexico has been elucidated in four publications (HRV [n = 2]; BHRV [n = 1]; both vaccines [n = 1]).Citation23,Citation25,Citation34,Citation41 Overall, the two-dose vaccination schedule with HRV or the three-dose vaccination schedule with BHRV was associated with higher net savings and gain in quality-adjusted life-years (QALY) compared with no vaccination ().Citation25,Citation34,Citation41 Only one analysis directly compared HRV and BHRV.Citation23 For both vaccines, the economic evaluation projected a reduction in rotavirus events by 39% for HRV and 30% for BHRV, a reduction in the frequency of cases seeking medical advice by 58% for HRV and 45% for BHRV, and a decrease in hospital admissions by 67% for HRV and 53% for BHRV. The two-dose vaccination schedule with HRV was associated with a net savings of 74 million Mexican pesos (MXN) plus a gain of 553 QALY when compared with the three-dose schedule, with BHRV indicating that vaccination with HRV was the most cost-effective strategy ().Citation23
Impact/effectiveness of rotavirus vaccination on mortality and morbidity
Evidence on the impact of rotavirus vaccination on acute diarrheal disease mortality was reported in five studies (HRV [n = 4]; both vaccines [n = 1]) ().Citation26,Citation27,Citation32,Citation35,Citation37 Overall, a substantial decline in all-cause diarrhea mortality rate was observed in children under 5 years of age after the implementation of rotavirus vaccination in Mexico,Citation26,Citation27,Citation32,Citation35,Citation37 regardless of the choice of vaccine. The majority of the studies provide evidence of vaccine-specific impact for HRV and one study assessed the overall impact on mortality for a period of 10 years without a differentiation in the vaccine used. During the time when HRV was implemented in the NIP in Mexico, a significant decline in all-cause diarrhea mortality and deaths due to acute diarrheal disease among children under 5 years of age was observed.Citation26,Citation27,Citation35,Citation37 Only one study provided evidence of the impact of vaccination with HRV in the different regions of Mexico: across the regions, mortality due to all-cause diarrhea among children aged under 5 years of age declined by 43%–55% in all regions after the implementation of vaccination with HRV (2003–2006) ().Citation27
Evidence on the impact of rotavirus vaccination on all-cause diarrhea morbidity was reported in three studies (HRV [n = 2]; both vaccines [n = 1]) ().Citation29,Citation33,Citation37 Overall, the numbers of new cases and hospitalizations due to all-cause diarrhea including acute diarrhea were reduced during 2006–2017. The first evidence of this comes from a 10-year observational study which showed that rotavirus vaccination resulted in a 15.5%–46% reduction in morbidity (new cases and hospitalizations) resulting from acute diarrheal disease of any cause in children under 5 years old during the post-vaccination period (2008–2017) compared to the pre-vaccination period (2006). This decline was clearly more pronounced (28.7%–64.7%) during the rotavirus season (November–March) in the post-vaccination period.Citation37 A study by Leboreiro et al. report a reduction in the risk of severe episodes (odds ratio: 0.18, p = .01) in children >2 years old, attributable to rotavirus vaccination (both vaccines).Citation29 These trends of declining levels of morbidity due to rotavirus vaccination were confirmed in a second study that assessed the vaccine-specific impact of HRV vaccination: a decline of 11%–40% in all-cause diarrhea hospitalizations was observed during 2008–2009 with the greatest reduction reported in infants <12 months of age (25%–52%). In addition, among children 12–23 months of age, a 43% decline in all-cause diarrhea hospitalizations was reported during the 2009 season.Citation33
Vaccine effectiveness data were identified only for HRV. In an observational case–control study, a completed 2-dose schedule with HRV resulted in an effectiveness of 94% against hospitalization due to laboratory-confirmed G9P[4] rotavirus infection.Citation43
Compliance of rotavirus vaccination
Evidence on compliance with the recommended vaccination schedule, including timeliness of vaccination, was reported in one study based on a registry provided by the IMSS. In this registry, there were 659,249 and 780,483 infants eligible for HRV (2010) and BHRV (2012), respectively.Citation14 Among these infants, compliance with full vaccine series was reported in 93.7% of infants who received HRV compared to 71.1% who received BHRV (p < .001). Likewise, the percentage of infants who completed the full vaccination series according to the recommended schedule (age and interval between doses) was higher with HRV (75.5%) compared to BHRV (70.9%) (p = .105).Citation14
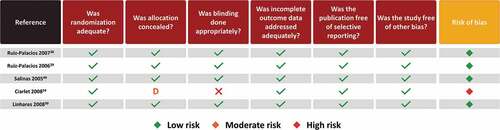
Risk of bias
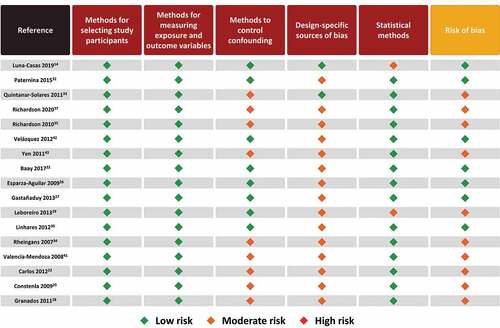
The results of the risk of bias appraisal for observational studies are shown in . The majority of studies (10/17) were regarded as presenting a moderate risk of bias and the remaining studies presented a low risk of bias. The moderate risk of bias of individual studies was driven mainly by a lack of methods to control confounding and design-specific source of bias which can be attributed to the nature of observational studies, specifically those using passive surveillance and laboratory data (with non-probabilistic sampling methods). Observational studies have inherent biases, particularly since they are not randomized. Yet we classified most as having low-to-moderate risk of biases overall. The specific categories that contained higher bias were mostly around design-specific sources of bias (i.e. recall bias, loss to follow-up, no blinding, retrospective databases from passive surveillance systems, underreporting) and in most studies the methods for controlling confounding (i.e. appropriate design or analytical methods) were unclear/not reported. For almost all of these studies, most endpoints were descriptive with no adjustment for multiple comparisons.
Figure 4. Risk of bias assessment of observational studies using STROBE checklist.Citation18,Citation19
The results of the risk of bias appraisal for RCT studies are shown in . The majority of studies (4/5) were regarded as presenting a low risk of bias and one study was associated with a high risk of bias.Citation24 This was driven by the fact that the concealment of allocation was unclear, and it was not a blinded trial. Adding to this was the small sample size considering loss of follow-up and adherence.Citation24
Figure 5. Risk of bias assessment of RCTs using Cochrane risk bias of tool.Citation20
Discussion
In this review, we summarize evidence on the burden of rotavirus gastroenteritis in Mexico, regional and local immunogenicity, efficacy and safety data of the available rotavirus vaccines, health economics, and the impact of the rotavirus vaccination program in Mexico.
In 2006, rotavirus vaccination for children was added into the Mexican NIP; HRV was used from 2006 to 2011 and partially re-introduced in 2019 until present, and BHRV has been used since 2011. Along with several Latin American countries, Mexico was one of the countries that led the accelerated clinical development of rotavirus vaccines. Since the beginning of the rotavirus vaccination program in Mexico, several studies have been conducted to assess the local immunogenicity, efficacy, and safety of rotavirus vaccines. Local immunogenicity data available only for HRV show that infants had seroprotective levels of antibodies after both vaccine doses,Citation38,Citation40 whereas immunogenicity data to support the use of rotavirus vaccines with the oral polio vaccine and other routine vaccinations were available for both HRV and BHRV.Citation24,Citation38,Citation40 Local efficacy data were reported in five studies, all of which were specific to HRV.Citation31,Citation38–40,Citation43 Overall, the local efficacy of HRV among children <5 years of age is high against severe (77%–100%) and any rotavirus gastroenteritis (70%-80%),Citation31,Citation38–40 including hospitalizations (all-cause: 42%; severe rotavirus gastroenteritis-related hospitalizations: 85%).Citation39 According to local studies, both rotavirus vaccines show an acceptable safety profile without a severe risk of intussusception. However, a temporal increase in the risk for intussusception was observed within 7 days of receipt of the first vaccine dose.Citation22,Citation42 Whether rotavirus vaccination has any impact on the overall incidence of intussusception is yet to be determined.Citation22,Citation42 Importantly, this finding should be interpreted along with the well-documented benefits of rotavirus vaccination, demonstrating a high benefit versus risk profile.
Over more than 15 years after implementation of the childhood rotavirus vaccination program in Mexico, a substantial reduction in the diarrheal disease burden primarily among children <5 years of age has been documented. These findings correspond with the trends observed from other Latin American countries such as Brazil and Panama which were also early in their implementation of a national rotavirus vaccination program.Citation45,Citation46
In Mexico, G9, a strain fully heterotypic from the vaccine strain, has emerged as an important serotype causing severe rotavirus gastroenteritis.Citation11,Citation47 We identified one study that showed high vaccine effectiveness (94%) against laboratory-confirmed G9P[4] rotavirus infection,Citation43 indicating that the strain predominance in Mexico was unrelated to vaccine pressure. Because variations in rotavirus types can occur independently of vaccination, the role of vaccination in observed strain changes requires cautious interpretation.Citation11
In 2017, a systematic review and meta-analysis was conducted to analyze efficacy, safety, and effectiveness of BHRV and HRV rotavirus vaccines used in the LAC region. This review highlights that the risk of any-severity rotavirus-related gastroenteritis was reduced by 65% following rotavirus vaccination; both vaccines significantly reduced the risk of hospitalization and emergency visits by 85%-90% and did not increase the risk of death, intussusception, or severe adverse events.Citation48 Our review reaffirms these previous findings from the region that rotavirus vaccination was effective with a good risk-benefit profile in children. Evidence on compliance to the HRV and BHRV vaccination schedule shows a better compliance (age and interval between doses) with two-dose HRV throughout Mexico, while regional differences were observed with BHRV.Citation14
In the majority of health economic evaluations for Mexico, rotavirus vaccination was compared with no vaccination. Only one study that directly assessed the cost-effectiveness of HRV and BHRV was identified in this review; this analysis suggests that vaccination with HRV is a much more cost-effective strategy when compared to vaccination with BHRV.Citation23 Findings from health economic evaluations of the rotavirus vaccination program in Mexico underscore the benefit of continuing the rotavirus vaccination program in Mexico. Notably, extensive economic evaluations were performed in the LAC region during the time vaccine introduction decision-making processes were ongoing.Citation11 As more data on the vaccine-specific effectiveness of rotavirus vaccination programs become available, further economic analyses are needed to make evidence-based decisions for universal use of rotavirus vaccinations. These analyses would support the ongoing discussions on changing vaccine policy in Mexico based on new epidemiological data or the availability of new rotavirus vaccines.Citation49,Citation50
With regard to new rotavirus vaccines, recently, ROTAVAC™ (Bharat Biotech, Hyderabad, India) and RotaSIIL, (Serum Institute of India, Pune, India) received WHO prequalification.Citation51 These vaccines are anticipated to expand the global reach of rotavirus vaccines by improving on certain programmatic aspects of HRV and BHRV, like heat stability, reduction of cold-chain footprint, and potentially providing more cost-effective options.Citation52 The initial Phase 3 clinical studies of both ROTAVAC and RotaSIIL reported no intussusception events in the first month following any dose of vaccine or placebo; however, these studies are of limited size and geographic scope and thus do not have extensive safety results nor an established risk-benefit profile.Citation53,Citation54 These efficacy results are similar to the results from the clinical trials of BHRV and HRV which showed a lower efficacy in low- and middle-income nations with high diarrhea-related mortality. Based on the limited clinical trial data available for these new vaccines, the vaccine efficacy against severe rotavirus disease was 56% for ROTAVAC (in 3 sites in India) and ranged from 37% (in 6 sites in India) to 67% (1 site in Niger) for RotaSIIL.Citation53–55 Currently, these vaccines have not been evaluated in Latin American or Mexican populations, and their three-dose schedule might limit their utilization in these countries.
A few limitations of this review are worth noting in the interpretation of the overall findings. Systematic reviews are high in the hierarchy of evidence generation, but they always have specific (inherent) biases such as publication bias. To deal with these biases, we had two reviewers during the screening, and eligibility process and all discrepancies were discussed among the reviewers to reach consensus on the outcome. Additionally, the risk-of-bias evaluations were done as part of the quality assessment of each article in order to reduce biases during the interpretation (i.e. putting less weight on the articles with high risk of bias/lower quality). For this review, we had a wide scope covering a diverse array of clinical and epidemiological endpoints with different time periods considered in the studies. This may have led to the dilution of the individual findings. However, the focus on a single country which has licensed use of both rotavirus vaccines allowed us to meet our review objective to consolidate and integrate all existing evidence on the situation of rotavirus diarrhea in Mexico. Consequently, generalizability to other countries in the region or middle-income countries is limited.
Conclusions
This systematic review underscores the documented benefit of the childhood rotavirus vaccination program in Mexico more than 15 years after its implementation, specifically in terms of good efficacy/immunogenicity, clinical and real-life effectiveness, a favorable safety and tolerability profile, and substantial reductions in diarrhea-related mortality and hospitalizations. Both HRV and BHRV vaccines have been widely used, and this review highlights that rotavirus vaccines have a large and robust evidence base in Mexico, extending from clinical trials to real-world evidence, and the high compliance rate of HRV with the two-dose schedule, provides confidence in its continued use in all Mexican infants.
Disclosure of potential conflicts of interest
AGH, EO, MYCA, DC, and RC are employed by the GSK group of companies. MYCA, EO, and RC hold shares in the GSK group of companies. AAF is an external GSK employee hired by Randstad. All authors declare no non-financial relationships and activities.
Trademark
Rotarix is a trademark owned by or licensed to the GSK group of companies.
RotaTeq is a trademark of Merck Sharp & Dohme Corp.
ROTAVAC is a trademark of Bharat Biotech International Limited.
RotaSIIL is a trademark of the Serum Institute of India.
Contributorship
All authors comply with the ICMJE criteria for authorship. All authors were involved in the conception and/or the design of the study. AGH conducted the study and the review analysis; both AGH and AAF participated in the data collection/generation of the study data. All authors were involved in the interpretation of the data. All authors reviewed and approved the final manuscript.
Supplemental Material
Download PDF (184.8 KB)Acknowledgments
The authors would also like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Maxime Bessières coordinated manuscript development and editorial support and Amrita Ostawal (Arete Communication UG) provided writing support for this literature review.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1936859.
Additional information
Funding
References
- Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PLoS One. 2012;7:e29151. doi:10.1371/journal.pone.0029151.
- World Health Organization. Diarrhoeal disease: fact sheet. 2017; [accessed 2020 Nov 6]. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
- World Health Organization. Diarrhea: why children are still dying and what can be done. Geneva. 2009; [accessed 2020 Nov 6]. https://apps.who.int/iris/bitstream/handle/10665/44174/9789241598415_eng.pdf;jsessionid=E855D6F11A7121F159AD3467672D2BD2?sequence=1.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World Health Organization-coordinated global rotavirus surveillance N. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, Diaz Z, Parashar UD, Kirkwood CD. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Hum Vaccin Immunother. 2019;15:1215–27. doi:10.1080/21645515.2018.1553593.
- World Health Organization. Rotavirus vaccines. WHO position paper January 2013. Weekly Epidemiol Rec. 2013;88:49–64.
- Black R, Fontaine O, Lamberti L, Bhan M, Huicho L, El Arifeen S, Masanja H, Walker CF, Mengestu TK, Pearson L, et al. Drivers of the reduction in childhood diarrhea mortality 1980-2015 and interventions to eliminate preventable diarrhea deaths by 2030. J Glob Health. 2019;9:020801. doi:10.7189/jogh.09.020801.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172:958–65. doi:10.1001/jamapediatrics.2018.1960.
- de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7:345–53. doi:10.1586/14760584.7.3.345.
- Pan American Health Organization. About Rotavirus. [accessed 2020 June 21]. https://www.paho.org/hq/index.php?option=com_content&view=article&id=1861:2009-about-rotavirus&Itemid=1621&lang=en.
- Perez Schael I, O’Ryan M, Sáez-Llorens X, Linhares AC, Velázquez FR, Colindres RE, Breuer T, Ortega-Barria E. Clinical development, registration, and introduction of human rotavirus vaccine: the Latin American experience. Trials in Vaccinology. 2012;1:10–20. doi:10.1016/j.trivac.2012.01.001.
- Oliveira L, Camacho LAB, Coutinho ESF, Ruiz-Matus C, Leite JPG. Rotavirus vaccine effectiveness in Latin American and Caribbean countries: a systematic review and meta-analysis. Vaccine. 2015;33:A248–A254. doi:10.1016/j.vaccine.2014.11.060.
- Vesikari T. Rotavirus vaccination: a concise review. Clin Microbiol Infect. 2012;18(Suppl 5):57–63. doi:10.1111/j.1469-0691.2012.03981.x.
- Luna-Casas G, Juliao P, Carreño-Manjarrez R, Castañeda-Prado A, Cervantes-Apolinar MY, Navarro-Rodriguez R, Sánchez-González G, Cortés-Alcalá R, DeAntonio R. Vaccine coverage and compliance in Mexico with the two-dose and three-dose rotavirus vaccines. Hum Vaccin Immunother. 2019;15:1251–59. doi:10.1080/21645515.2018.1540827.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W264. doi:10.7326/0003-4819-151-4-200908180-00135.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–49. doi:10.1016/j.jclinepi.2007.11.008.
- Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302:1136–40. doi:10.1136/bmj.302.6785.1136.
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–76. doi:10.1093/ije/dym018.
- Higgins J, Green S, (editors). Cochrane handbook for systematic reviews of interventions. Version 5.0.2. The Cochrane Collaboration; 2008.
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC resources for review authors. EPOC Resources. 2017; [accessed 2020 Sept 29]; epoc.cochrane.org/resources/epoc-resources-review-authors.
- Baay M, Bollaerts K, Struchiner C, Verstraeten T. Background rates of disease in Latin American children from a rotavirus vaccine study. Hum Vaccin Immunother. 2017;13:1916–20. doi:10.1080/21645515.2017.1320007.
- Carlos F, Gomez JA, Anaya P, Standaert B, Carreño-Manjarrez R. Health economics assessment of rotavirus vaccines in Mexico. Value Health. 2013;16:A72. doi:10.1016/j.jval.2013.03.324.
- Ciarlet M, Sani-Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, Arredondo JL, Schodel F. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J. 2008;27:874–80. doi:10.1097/INF.0b013e3181782780.
- Constenla D, Velázquez FR, Rheingans RD, Antil L, Cervantes Y. Economic impact of a rotavirus vaccination program in Mexico. Rev Panam Salud Publica. 2009;25:481–90. doi:10.1590/S1020-49892009000600003.
- Esparza-Aguilar M, Bautista-Márquez A, MdC G-A, Richardson-López-Collada VL. Mortalidad por enfermedad diarreica en menores, antes y después de la introducción de la vacuna contra el rotavirus. Salud publica de Mexico. Salud Publica Mex. 2009;51:285–90. doi:10.1590/S0036-36342009000400004.
- Gastañaduy PA, Sánchez-Uribe E, Esparza-Aguilar M, Desai R, Parashar UD, Patel M, Richardson V. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics. 2013;131:e1115–1120. doi:10.1542/peds.2012-2797.
- Granados-García V, Velázquez FR, Salmerón J, Homedes N, Salinas-Escudero G, Morales-Cisneros G. Burden of disease and costs of treating rotavirus diarrhea in Mexican children for the period 2001-2006. Vaccine. 2011;29:6712–19. doi:10.1016/j.vaccine.2011.03.020.
- Leboreiro JI, Zapata IB, Montessoro GM, Aguilar CEL, Macías MER. Anti-rotavirus vaccination and the demand for hospital care of children with diarrhea. Rev Mex Pediatr. 2013;80:15–21.
- Linhares AC, Macias-Parra M, Sáez-Llorens X, Vergara R, Jimenez E, Raúl Velázquez F, Cervantes Y, Abate HJ, Rivera L, Ruttimann R, et al. Rotavirus gastroenteritis in Latin America: a hospital-based study in children under 3 years of age. Trials in Vaccinology. 2012;1:36–41. doi:10.1016/j.trivac.2012.07.002.
- Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, Lopez P, Macias-Parra M, Ortega-Barria E, Rivera-Medina DM, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–89. doi:10.1016/S0140-6736(08)60524-3.
- Paternina-Caicedo A, Parashar UD, Alvis-Guzman N, De Oliveira LH, Castano-Zuluaga A, Cotes-Cantillo K, Gamboa-Garay O, Coronell-Rodriguez W, De la Hoz-Restrepo F. Effect of rotavirus vaccine on childhood diarrhea mortality in five Latin American countries. Vaccine. 2015;33:3923–28. doi:10.1016/j.vaccine.2015.06.058.
- Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J. 2011;30:S11–15. doi:10.1097/INF.0b013e3181fefb32.
- Rheingans RD, Constenla D, Antil L, Innis BL, Breuer T. Potential cost-effectiveness of vaccination for rotavirus gastroenteritis in eight Latin American and Caribbean countries. Rev Panam Salud Publica. 2007;21:205–16. doi:10.1590/S1020-49892007000300003.
- Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi:10.1056/NEJMoa0905211.
- Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med. 2011;365:772–73. doi:10.1056/NEJMc1100062.
- Richardson-López Collada V, Bautista-Márquez A, Sánchez-Uribe E, Esparza-Aguilar M. [Population impact of rotavirus vaccination in Mexico after 10 years]. Salud Publica Mex. 2020;62:6–13. doi:10.21149/9936.
- Ruiz-Palacios GM, Guerrero ML, Bautista-Márquez A, Ortega-Gallegos H, Tuz-Dzib F, Reyes-González L, Rosales-Pedraza G, Martínez-López J, Castañón-Acosta E, Cervantes Y, et al. Dose response and efficacy of a live, attenuated human rotavirus vaccine in Mexican infants. Pediatrics. 2007;120:e253–261. doi:10.1542/peds.2006-2630.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi:10.1056/NEJMoa052434.
- Salinas B, Perez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzabal JP, Cervantes Y, Costa Clemens S, Damaso S, Hardt K, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24:807–16. doi:10.1097/01.inf.0000178294.13954.a1.
- Valencia-Mendoza A, Bertozzi SM, Gutierrez JP, Itzler R. Cost-effectiveness of introducing a rotavirus vaccine in developing countries: the case of Mexico. BMC Infect Dis. 2008;8:103. doi:10.1186/1471-2334-8-103.
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31:736–44. doi:10.1097/INF.0b013e318253add3.
- Yen C, Figueroa JR, Uribe ES, Carmen-Hernández LD, Tate JE, Parashar UD, Patel MM, Richardson López-Collado V. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis. 2011;204:783–86. doi:10.1093/infdis/jir390.
- Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi:10.1056/NEJMoa1012952.
- Bayard V, DeAntonio R, Contreras R, Tinajero O, Castrejon MM, Ortega-Barria E, Colindres RE. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis. 2012;16:e94–98. doi:10.1016/j.ijid.2011.09.003.
- Santos VS, Berezin EN, Gurgel RQ. Rotavirus in Latin America: current situation and perspectives. J Pediatric Infect Dis Soc. 2017;6:1–2. doi:10.1093/jpids/piw071.
- Felix-Valenzuela L, Cooley-García DP, Cano-Rangel MA, Durazo-Arvizu MA, Mata-Haro V. Predominance of G9P[4] Rotavirus from Children with Acute Gastroenteritis in Northwestern Mexico. Intervirology. 2016;59:228–33. doi:10.1159/000464132.
- Velazquez RF, Linhares AC, Munoz S, Seron P, Lorca P, DeAntonio R, Ortega-Barria E. Efficacy, safety and effectiveness of licensed rotavirus vaccines: a systematic review and meta-analysis for Latin America and the Caribbean. BMC Pediatr. 2017;17:14. doi:10.1186/s12887-016-0771-y.
- Debellut F, Clark A, Pecenka C, Tate J, Baral R, Sanderson C, Parashar U, Kallen L, Atherly D. Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. The Lancet Global Health. 2019;7:e1664–e1674. doi:10.1016/S2214-109X(19)30439-5.
- Pan American Health Organization. PAHO revolving fund. [accessed 2020 Jun 21]. https://www.paho.org/en/resources/paho-revolving-fund#:~:text=The%20PAHO%20Revolving%20Fund%20is,in%20the%20annals%20of%20vaccination.&text=Through%20the%20fund%2C%2041%20countries,populations%20at%20the%20lowest%20price.
- World Health Organization. WHO prequalifies new rotavirus vaccine. [accessed 2020 Nov 6]. https://www.who.int/medicines/news/2018/prequalified_new-rotavirus_vaccine/en/. Geneva, 2018.
- Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018;20:223–33. doi:10.1007/s40272-018-0283-3.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. The Lancet. 2014;383:2136–43. doi:10.1016/S0140-6736(13)62630-6.
- Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo A, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in niger. N Engl J Med. 2017;376:1121–30. doi:10.1056/NEJMoa1609462.
- Kulkarni PS, Desai S, Tewari T, Kawade A, Goyal N, Garg BS, Kumar D, Kanungo S, Kamat V, Kang G, et al. A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35:6228–37. doi:10.1016/j.vaccine.2017.09.014.