ABSTRACT
The aim of this study was to examine the risk factors for pharyngeal carriage of meningococci in third-level students using an unsupervised machine learning approach. Data were gathered as part of meningococcal prevalence studies conducted by the Irish Meningitis and Sepsis Reference Laboratory (IMSRL). Pharyngeal swab cultures for meningococcal carriage were taken from each student once they had completed a single-page anonymous questionnaire addressing basic demographics, social behaviors, living arrangements, vaccination, and antibiotic history. Data were analyzed using multiple correspondence analysis through a machine learning approach.
In total, 16,285 students who had a pharyngeal throat swab taken returned a fully completed questionnaire. Overall, meningococcal carriage rate was 20.6%, and the carriage of MenW was 1.9% (n = 323). Young Irish adults aged under 20 years and immunized with the meningococcal C vaccine had a higher MenW colonization rate (n = 171/1260, 13.5%) compared with non-Irish adults aged 20 years or older without the MenC vaccine (n = 5/81, 6%, chi-square = 3.6, p = .05). Unsupervised machine learning provides a useful technique to explore meningococcal carriage risk factors. The issue is very complex, and asked risk factors only explain a small proportion of the carriage. This technique could be used on other conditions to explore reasons for carriage.
Introduction
Meningococcal disease has been one of the leading causes of meningitis and sepsis in children and young adults in Ireland, although the incidence of disease has been decreasing since the introduction of successful vaccination programmes.Citation1,Citation2 Studies investigating risk factors for carriage in third-level students have demonstrated that smoking, multi-occupancy settings, and overcrowding have been associated with an increased risk of carriage.Citation3–5 These studies used traditional statistical methods to assign and describe the risk; however, by using an exploratory data mining technique, novel risk factors or combinations of factors may be identified. In this study, we have examined an existing dataset to determine if novel risk factors could be identified using artificial intelligence and specifically machine learning.
Machine learning is an emerging technology, where computational power can be used to identify risk factors using techniques such as multiple correspondence analyses. An advantage of these techniques are that they can detect subtle inter-relationships between risk factors, which may be missed by more standard statistical approaches. This technique has been used to examine risk factors for a diverse range of conditions, such as obesity, maxilla-facial trauma, and rheumatological disease.Citation6–8 Machine learning is starting to become part of reverse vaccinology and has been considered for meningococcal vaccines.Citation9
The data used in this study was from meningococcal carriage prevalence surveys that were performed by the Irish Meningitis and Sepsis Reference Laboratory (IMSRL), under the remit of examining vaccine effectiveness and impact on asymptomatic carriage at a national level. Meningococcal carriage is highest in young adults, and for logistical reasons third-level students only were included in this study and young adults, who were not in third-level education, were not included in the study. There was a specific focus on MenW carriage risk factors as there has been a recent rise in the incidence of invasive disease across Europe and North America.Citation10–15
The aim of this study was to examine risk factors for pharyngeal carriage of meningococci in third-level students using an unsupervised machine learning approach. The advantages of this type of approach above multivariate analysis are that it allows for novel patterns to potentially be found without making reference to known labels, in this study the labels being carriage or not of meningococcus.
Materials and methods
Participants
Since 2000 up until 2017, the IMSRL conducted meningococcal carriage prevalence studies on students attending third-level educational institutions, with the purpose of monitoring trends in carriage relating to the introduction of the meningococcal C (MenC) vaccine in to the routine childhood immunization schedule, in October 2000. Third-level educational institutions, and students within them, were selected by contacting the different organizations and requesting permission to swab students. It was a convenience sample and not a verified nationally representative sample. It was approved by the Research and Ethics Committee of the Children Health Ireland at Temple Street initially in 1999, extended in 2008 (08/025) and renewed in 2016. Once students gave informed verbal consent, a throat swab was taken by a health-care provider or trained scientist, which was immediately placed into the accompanying swab transport medium. Collected swabs were immediately plated onto New York City selective agar medium (E&O Laboratories, Bonnybridge, Scotland, UK) where possible, and occasionally were plated within a maximum delay of threehours. Following 48 h, incubation at 37°C in the presence of 5% CO2 putative N. meningitidis colonies were tested for cytochrome c oxidative production (Medical Wire & Equipment Co Ltd, Wiltshire, UK). Subsequent examination by PCR confirming species identity included a ctrA-PCR and a serogroup-specific PCR previously described.Citation16
Each participant was also invited to complete a one-page questionnaire requesting details on basic demographics (age, gender, location of family home (rural/urban) and college, accommodation situation (including dormitory, shared room or shared apartment), nationality and vaccination history) and other factors understood to be associated with meningococcal carriage or disease (smoking, upper respiratory tract infection (URTI), children in the house, recent travel and recent antibiotics). They were asked where they lived, and those in the Leinster region were classified as the East region, and the rest of the country as non-East. Responses were entered in to an electronic spreadsheet (Excel, Microsoft, Redmond WA, USA) and linked with the carriage result and serogroup results (groupable, nongroupable and MenW) of the meningococcus if isolated from throat swab.
Statistical methods
Data for all participants were initially included, and then records with missing data were excluded. Analysis was performed to compare three different hypotheses; 1, colonized with meningococcus or noncolonized; 2, colonized with serogroupable/encapsulated or nongroupable meningococcus and 3, colonized with the MenW or with other groupable meningococcus. For each situation, the risk factors were initially compared in two-by-two tables, with the chi-square value and p-value calculated. Both these tests and traditional logistic regression was done using univariate and multivariate analysis in MedCalcversion 15 [MedCalc Software, Ostend, Belgium], and odds ratios with 95% confidence intervals were reported.
Only when the p-value was statistically significant (p ≤ 0.003), taking into account a Bonferroni correction, were the variables included in the multiple correspondence analysis (MCA) for comparing colonized to noncolonized people. Bonferroni correction was not used for selecting risk factors when examining colonization with MenW as the overall numbers of cases was lower, and a p-value of 0.05 was used. MCA was not performed for the group comparing being colonized by a groupable meningococcus against non-groupable meningococcus as there was no significant risk factor identified. MCA was performed using Microsoft Excel and the Addinsoft plug-in XLStat [Version 2019.3.2, New York, USA], and all risk factor variables were expressed as binary options/categorical. Initially, the scree plots were constructed from derived eigenvalues, along with the squared cosine, contribution and principle components. Eigenvalues are the variances of the linear combinations defined by corresponding eigenvectors in a covariance matrix and represent the amount of information (inertia) summarized in each dimension.Citation17 The principle components were displayed in a visual map form for easier visual interpretation in a two-dimensional format.
Results
There were 17,955 individual students included across the ten carriage surveys, which spanned from 2000 to 2017. Records that had missing data were removed (n = 1,669), leaving a final cohort of 16,285 and the distribution by college and year is shown in Table S1. Descriptive analyses of the prevalence of risk factors between different groups are shown in with traditional univariate and multivariate analysis for the different groups detailed in . For meningococcal colonization (n = 3,367, 20.6%) compared with non-colonization (n = 12,918, 79.3%), smoking, male gender and Irish nationality were independently associated with colonization; whereas, factors associated with lower meningococcal colonization were living at home, aged 20 years or more, recent antibiotic use, living in the East region and recent travel.
Table 1. Overview of the 16,285 indiviuals included in the study. The three sections reflect a) colonized versus noncolonized, (b) colonized with groupable meningococcal versus a nongroupable meningococcus and (c) colonized with meningococcal W135 against other groupable meningococcus
Table 2. Univariate and multivariate analysis of the risk factors for meningococcal carriage. Statistically significant items put in bold
For colonization with a serogroupable/encapsulated (n = 2212, 71.3%) versus a non-groupable (n = 891, 28.7%) meningococcus, only smoking was identified as a risk factor in the univariate analysis; however, this was not deemed significant in the multivariate analysis. For MenW carriage (n = 323, 1.9%), in comparison with carriage of another groupable (n = 2790, 17.1%) meningococcus multivariate analysis suggested that being aged 20 years and over was associated with less frequent MenW carriage, while MenC vaccination was associated with higher carriage.
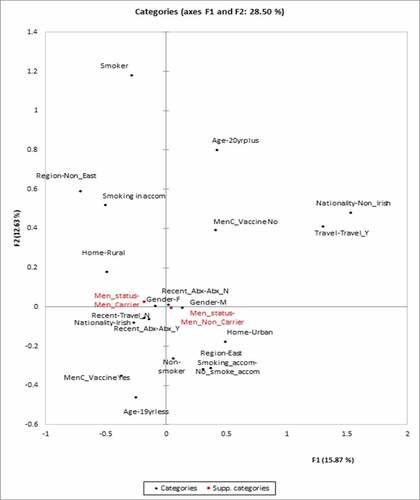
For MCA comparing colonized versus non-colonized, region, age group, gender, location of family home, nationality, recent travel, smoking, smoking in accommodation, recent antibiotics, and MenC vaccination were included as the categories for analysis to create the principle component analysis map. Meningococcal carriage included as a supplementary variable and not as part of the analysis, but plotted on to the PCA map. Approximately 27% of the variance in terms of carriage of meningococcus is explained by the first two factors F1 and F2 as shown in .
Figure 1. Principle component map of the risk factors for colonization versus noncolonized with any meningococcus strain. The x-axis represents Factor 1 and the y-axis represents Factor 2
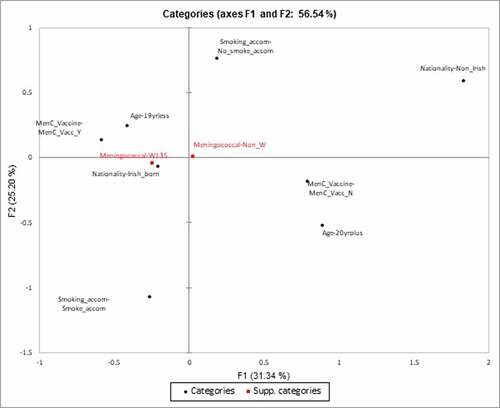
MCA was not performed for risk factors for colonization with groupable meningococcus against colonization with a non-groupable meningococcus as there were no statistically significant risk factors on traditional analysis. MCA was performed to examine the carriage of MenW over carriage of other groupable meningococcus strains. The only risk factors that were included as they were significant were age (19 years or less/ 20 years and over), nationality (Irish or non-Irish), smoking in accommodation (yes/no) and MenC vaccination (yes/no) with MenW carriage as a supplementary variable (). As a subgroup, we examined individuals who were colonized with any groupable meningococcus, and we found young Irish adolescents aged 19 years or less who had been vaccinated with the MenC vaccine had a higher colonization rate with MenW (n = 171/1260, 13.5%), as opposed to non-Irish adults aged 20 years of more without the MenC vaccine (n = 5/81, 6%, chi-square = 3.6, p = .05).
Figure 2. Principle component map of risk factors for colonization with MenW against colonization with other groupable meningococcus strains. The x-axis represents Factor 1 and the y-axis represents Factor 2
With this issue raised regarding MenC vaccine and the colonization with MenW, the colonization with groupable meningococci was examined across four groups depending on age, nationality, and a history of MenC vaccine (). This demonstrated that a MenC vaccine history in Irish adults aged 19 years or less was associated with a higher MenW colonization rate (n = 171/1260, 13.6% versus n = 60/686, 8.7%, chi-square = 9.8, p-value = 0.002). The 20 years and older Non-Irish group conversely had a higher MenB carriage rate when compared to 19 years and younger Irish group whether they had the menC vaccine (n = 30/58,51.7% vs. 442/1260, chi-square = 6.6, p-value = 0.01) or the individuals without the MenC vaccine (n = 38/81,46.9% vs. n = 239/686, 34.8%, chi-square = 4.5, p = .003). This higher MenB carriage trend was driven by higher carriage rates earlier in the study, which decreased since 2003.
Table 3. Colonization rates with groupable meningococci depending on age, nationality, and MenC vaccination history
Discussion
This study used an unsupervised machine learning approach to examine risk factors for carriage of meningococcus, and particularly pathogenic MenW, in students attending Irish third-level educational institutions. The sum of the variation in meningococcal carriage is only 28% for feature 1 (F1) and feature 2 (F2) as per . This means that there are other risk factors, not examined in this study, that explain meningococcal carriage in certain individuals, and that the risk factors examined only explain a small part of the issue of carriage. This is an important finding as it highlights the multifactorial nature of meningococcal carriage, and potentially there are host factors which were not examined here.
Smoking or exposure to smoking (active or passive), does seem to be related to meningococcal carriage as the direction of the component is the same as for carriage. Smoking has previously been described as a risk factor, thus validating the MCA approach.Citation4,Citation18–21 The risk factor of residing in the Non-East part of the country, rural family home and smoking are all likely related. The non-East part of the country has a higher percentage of people living in a rural area, as opposed to the capital city Dublin which is located on the East part of the country. In conclusion, smoking appears to be a modifiable risk factor for creating an ecological niche for meningococcus in the pharynx, although there may be relevant socio-cultural reasons for higher smoking rates such as living away from home. When that ecological niche is created, there does not appear to be a risk factor which increases a person’s risk of carrying an encapsulated meningococcus or a non-groupable one.
To have a comparison for the machine learning approach, traditional univariate and multivariate analysis was also performed. Carriage of any meningococcus was associated with male gender, Irish nationality and smoking. These risk factors have been reported widely in other studies showing the comparability of our study.Citation4,Citation18,Citation22,Citation23 Ireland has also had a higher rate of IMD in comparison with other countries with outbreaks occurring in different regions and ethnic groups.Citation2,Citation24,Citation25
The outcomes of both multivariate and MCA carriage analysis have different outcomes, which shows the utility of using these methods side-by-side, rather than exclusively focusing on one output from a particular method. For colonization with any meningococcus, both methods identify living at home, East region, age and smoking as risk factors. On multivariate analysis male gender, Irish nationality, recent antibiotics, and recent travel were also significant. The interplay between these excluded risk factors may explain why there were not as important in MCA.
For risk factors associated with MenW carriage, it is interesting to compare the machine learning approach to the traditional multivariate logistic regression approach. The multivariate approach identified being 19 years or less, and MenC vaccination as risk factors for increase carriage of MenW. The respective PCA map has also clearly shown these risk factors are related to MenW carriage but also have identified smoking in the accommodation (passive/sharing accommodation with a smoker) and Irish nationality. Smoking in accommodation had an odds ratio of 1.22 (95%CI 0.96–1.55) and was removed at univariate analysis stage, as was Irish nationality as it had an odds ratio of 1.58(95% CI 0.98–2.55). In this way, machine learning has highlighted two risk factors that were eliminated previously and emphasized their importance. It may be the interplay and multiplicative effect of these risk factors that is important (i.e. MenW carriage associated with Irish adolescents aged 19 years or less, who have received the MenC vaccine and who share accommodation with a smoker). A previous post-vaccination study of students vaccinated with MenC had shown a rise in MenW carriage from 6.3% to 7.5% in the following year returning to 7.14% the year after.Citation26 This may be due to strain capsule replacement; however, it is not possible to be certain from this work about the exact mechanism, and further investigation is required.
A hypervirulent MenW135 strain has emerged in the UK at an increased prevalence, and its spread is being monitored closelyCitation27–29 In this historical cohort, taken prior to the emergence of MenW135 in the UK, we sought to identify associated risk factors for MenW carriage. We found that colonization was highest in the 19 years or younger Irish cohort who had received the MenC vaccine at 13%, while it was lowest in the 20 years or older non-Irish unvaccinated cohort who had a colonization rate of 3%. A limitation of this study is the small number of people colonized with MenB and MenW, and so the findings would need to be explored in a larger study as part of ongoing surveillance. Conversely, the older non-Irish cohort had a higher colonization rate with MenB irrespective of whether or not they had received the MenC vaccine. The reasons for this difference with respect to MenW and MenB carriage between young Irish students and older non-Irish students are unclear, but may be related to differences in meningococcal carriage patterns in the countries of origin of recently arrived non-Irish students.
There was potential for bias in this study as it used a historical cohort of third-level college students rather than a national representative sample of children and adults. There could also have been potential for bias in clustering of cases within certain colleges, as well as changing patterns of colonization when considering that the study took place over 18 years, although no invasive meningococcal outbreak was declared in any of the colleges during the study period. This was not a nationally representative sample; however, the over-weighted presence of samples from students in the East would help to identify carriers in a region which has the highest density of population with more mixing between colleges. However, the aim of this paper was to review if an unsupervised machine learning approach could identify unusual patterns of risk factors associated with meningococcal carriage, which would warrant further examination rather than conclusively prove cause and effect. It has shown that college students cannot be treated as a homogenous group and that there is a different predominance of meningococcal strains when comparing young Irish students to older non-Irish students. The low eigenvalue for the first two components for meningococcal carriage shows that the risk factors examined only reflect a small part of why a particular student is colonized or not and thus there must be other factors at play. The level at which a person had a particular risk factor was not examined in detail at the time of survey collection, so no data were available on type of tobacco, marijuana use, electronic cigarette use, duration of antimicrobial use, and external validation of vaccination status.
Meningococcal carriage, and differences in carriage between certain groups, is a complex area that has many drivers. Only a small part of the reason why a person is colonized with meningococcus is captured with the risk factors detailed in this study and thus other environmental reasons and genetic factors must play a significant part. Unsupervised machine learning has been shown to be a useful tool when examining large databases to identify clusters of people who have the same characteristics and may help improve our understanding of patterns in datasets. We have shown that meningococcal carriage is associated with smoking and also identified a subset of individuals (Irish, aged 19 years or less, MenC vaccinated) who have a higher than expected MenW colonization rate. This should help to direct future studies to focus on certain groups to help to better understand colonization and also the emergence of hypervirulent clones.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (21.3 KB)Acknowledgments
The authors would like to thank the staff and students of the universities/third-level educational institutions who participated in this study.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1940651
Additional information
Funding
References
- Bennett D, O’Lorcain P, Morgan S, Cotter S, Cafferkey M, Cunney R. Epidemiology of two decades of invasive meningococcal disease in the Republic of Ireland: an analysis of national surveillance data on laboratory-confirmed cases from 1996 to 2016. Epidemiol Infect. 2019;147:e142. doi:10.1017/S0950268819000396.
- O’Lorcain P, Bennett DE, Morgan SL, Cunney RJ, Cotter SM, Cafferkey MT, O’Flanagan DM. A retrospective assessment of the completeness and timeliness of meningococcal disease notifications in the Republic of Ireland over a 16-year period, 1999-2015. Public Health. 2018;156:44–51. doi:10.1016/j.puhe.2017.11.027.
- Cassio de Moraes J, Kemp B, de Lemos AP, Outeiro Gorla MC, Lemes Marques EG, Ferreira Mdo C, Sacchi C, Carvalhanas TR, Ribeiro AF, Ferreira CM, et al. Prevalence, risk factors and molecular characteristics of meningococcal carriage among Brazilian adolescents. Pediatr Infect Dis J. 2015;34:1197–202. doi:10.1097/INF.0000000000000853.
- Peterson ME, Mile R, Li Y, Nair H, Kyaw MH. Meningococcal carriage in high-risk settings: a systematic review. Int J Infect Dis. 2018;73:109–17. doi:10.1016/j.ijid.2018.05.022.
- Thomas JC, Bendana NS, Waterman SH, Rathbun M, Arakere G, Frasch CE, Wenger JD, Magsombol V, Clark JH. Risk factors for carriage of meningococcus in the Los Angeles County men’s jail system. Am J Epidemiol. 1991;133:286–95. doi:10.1093/oxfordjournals.aje.a115873.
- de Macedo Bernardino Í, Santos LM, Ferreira AVP, de Almeida Lima TLM, da Nóbrega LM, d’Avila S. Multiple correspondence analysis as a strategy to explore the association between categories of qualitative variables related to oral-maxillofacial trauma and violent crimes at the community level. Int J Oral Maxillofac Surg. 2018;47:339–44. doi:10.1016/j.ijom.2017.08.001.
- Han L, Benseler SM, Tyrrell PN. Cluster and multiple correspondence analyses in rheumatology: paths to uncovering relationships in a sea of data. Rheum Dis Clin North Am. 2018;44:349–360.e29. doi:10.1016/j.rdc.2018.01.013.
- Van Horn A, Weitz CA, Olszowy KM, Dancause KN, Sun C, Pomer A, Silverman H, Lee G, Tarivonda L, Chan CW, et al. Using multiple correspondence analysis to identify behaviour patterns associated with overweight and obesity in Vanuatu adults. Public Health Nutr. 2019;22:1533–44. doi:10.1017/S1368980019000302.
- Heinson AI, Gunawardana Y, Moesker B, Hume CC, Vataga E, Hall Y, Stylianou E, McShane H, Williams A, Niranjan M, et al. Enhancing the biological relevance of machine learning classifiers for reverse vaccinology. Int J Mol Sci. 2017;18:312. doi:10.3390/ijms18020312.
- Campbell H, Parikh SR, Borrow R, Kaczmarski E, Me R, Sn L. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016. Euro Surveill. 2016;21. doi:10.2807/1560-7917.ES.2016.21.12.30175.
- Eriksson L, Hedberg ST, Jacobsson S, Fredlund H, Mölling P, Stenmark B. Whole-genome sequencing of emerging invasive neisseria meningitidis serogroup W in Sweden. J Clin Microbiol. 2018;56:e01409–17. doi:10.1128/JCM.01409-17.
- Tsang RS, Deeks SL, Wong K, Marchand-Austin A, Jamieson FB. Invasive serogroup W Neisseria meningitidis (MenW) in Ontario, Canada shows potential clonal replacement during the period January 1, 2009 to June 30, 2016. Can Commun Dis Rep. 2016;42:263–66. doi:10.14745/ccdr.v42i12a06.
- Tsang RS, Hoang L, Tyrrell GJ, Minion J, Van Caeseele P, Kus JV, Lefebvre B, Haldane D, Garceau R, German G, et al. Increase in ST-11 serogroup W Neisseria meningitidis invasive meningococcal disease in Canada, 2016-2018. Can Commun Dis Rep. 2019;45:164–69. doi:10.14745/ccdr.v45i06a04.
- Tsang RSW, Ahmad T, Tyler S, Lefebvre B, Deeks SL, Gilca R, Hoang L, Tyrrell G, Van Caeseele P, Van Domselaar G, et al. Whole genome typing of the recently emerged Canadian serogroup W Neisseria meningitidis sequence type 11 clonal complex isolates associated with invasive meningococcal disease. Int J Infect Dis. 2018;69:55–62. doi:10.1016/j.ijid.2018.01.019.
- Krone M, Gray S, Abad R, Skoczyńska A, Stefanelli P, van der Ende A, Tzanakaki G, Mölling P, João Simões M, Křížová P, et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019;24. doi:10.2807/1560-7917.ES.2019.24.14.1800245.
- Bennett Decafferkey MT. Consecutive use of two multiplex PCR-based assays for simultaneous identification and determination of capsular status of nine common Neisseria meningitidis serogroups associated with invasive disease. J Clin Microbiol. 2006;44:1127–31. doi:10.1128/JCM.44.3.1127-1131.2006.
- Jolliffe ITCadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci. 2016;374:20150202.
- Blackwell CC, Weir DM, James VS, Todd WT, Banatvala N, Chaudhuri AK, Gray HG, Thomson EJ, Fallon RJ. Secretor status, smoking and carriage of Neisseria meningitidis. Epidemiol Infect. 1990;104:203–09. doi:10.1017/S0950268800059367.
- Breakwell L, Whaley M, Khan UI, Bandy U, Alexander-Scott N, Dupont L, Vanner C, Chang HY, Vuong JT, Martin S, et al. Meningococcal carriage among a university student population - United States, 2015. Vaccine. 2018;36:29–35. doi:10.1016/j.vaccine.2017.11.040.
- Cooper LV, Robson A, Trotter CL, Aseffa A, Collard JM, Daugla DM, Diallo A, Hodgson A, Jusot JF, Omotara B, et al. Risk factors for acquisition of meningococcal carriage in the African meningitis belt. Trop Med Int Health. 2019;24:392–400. doi:10.1111/tmi.13203.
- Stuart JM, Cartwright KA, Robinson PM, Noah ND. Effect of smoking on meningococcal carriage. Lancet. 1989;2:723–25. doi:10.1016/S0140-6736(89)90781-2.
- Miglietta A, Innocenti F, Pezzotti P, Riccobono E, Moriondo M, Pecile P, Nieddu F, Rossolini GM, Azzari C, Balocchini E, et al. Carriage rates and risk factors during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C ST-11 (cc11) in Tuscany, Italy: a cross-sectional study. BMC Infect Dis. 2019;19:29. doi:10.1186/s12879-018-3598-3.
- Sadeghi M, Ahmadrajabi R, Dehesh T, Saffari F. Prevalence of meningococcal carriage among male university students living in dormitories in Kerman, southeast of Iran. Pathog Glob Health. 2018;112:329–33. doi:10.1080/20477724.2018.1514138.
- Dell E, Bedford D, McMahon B, Nicholson A. Meningococcal disease--management of serogroup C clusters in a hyperendemic area. Ir Med J. 2001;94:168–69.
- O’Connor L, Ward M, Bennett D, Mulhall R, O’Lorcain P, Cunney R, McDermott R, Neville E, Heslin J, FitzGerald R, et al. A prolonged outbreak of invasive meningococcal disease in an extended Irish Traveller family across three Health Service Executive (HSE) areas in Ireland, 2010 to 2013. Euro Surveill. 2015;20. doi:10.2807/1560-7917.ES2015.20.21.21139.
- Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi:10.1086/527401.
- Bethea J, Makki S, Gray S, MacGregor V, Ladhani S. Clinical characteristics and public health management of invasive meningococcal group W disease in the East Midlands region of England, United Kingdom, 2011 to 2013. Euro Surveill. 2016;21. doi:10.2807/1560-7917.ES.2016.21.24.30259.
- Campbell H, Edelstein M, Andrews N, Borrow R, Ramsay M, Ladhani S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015-2016. Emerg Infect Dis. 2017;23:1184–87. doi:10.3201/eid2307.170236.
- Oldfield NJ, Cayrou C, AlJannat MAK, Al-Rubaiawi AAA, Green LR, Dada S, Steels OD, Stirrup C, Wanford J, Atwah BAY, et al. Rise in group W meningococcal carriage in University Students, United Kingdom. Emerg Infect Dis. 2017;23:1009–11. doi:10.3201/eid2306.161768.
