 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In Sweden, the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in 2009 and replaced by the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) or the 13-valent PCV (PCV13) from late 2009. We assessed the impact of PCVs on rates of antibiotic prescribing, tympanostomy tube placement (TTP), and healthcare resource utilization and direct costs of physician-diagnosed otitis media/acute otitis media (OM) in children ≤2 years of age living in Skåne (PCV7 then PHiD-CV) or Västra Götalandsregionen (VGR; PCV7 then PCV13). Retrospective cohort study using linked patient-level data from national and regional (Skåne and VGR) healthcare databases in Sweden from July 1, 2005, to December 31, 2013 (NCT02742753). Descriptive time-series analyses showed antibiotic prescriptions and TTP incidence declined after PHiD-CV/PCV13 introduction versus the pre-PCV period. The annualized mean frequencies of antibiotic use, primary care visits, outpatient visits, TTP and myringotomy procedures all decreased after PHiD-CV/PCV13 compared with pre-PCV cohorts. Annualized mean total OM-associated healthcare costs decreased in the PCV7 versus pre-PCV cohorts by 20.0% in Skåne and 10.2% in VGR, and further declined in the PHiD-CV and PCV13 cohorts (20.7% and 15.3%, respectively, relative to the PCV7 cohort), although the duration of PCV7 use differed between regions. Decreases in adjusted annualized cost ratios between cohorts per child susceptible to OM were statistically significant after PCV7 introduction and again with either PHiD-CV or PCV13 introduction in both regions. Following sequential PCV introduction, OM-related healthcare utilization and associated costs decreased in the study regions in Sweden.
PLAIN LANGUAGE SUMMARY
What is the context?
Otitis media is one of the most frequent reasons for healthcare visits and antibiotic use among young children. Although it is considered as a mild illness, the overall economic burden is substantial due to its high frequency.
Otitis media can be caused by different bacteria including Streptococcus pneumoniae, which is also responsible for pneumonia and meningitis. Pneumococcal conjugate vaccines Prevenar (Pfizer Inc.), Synflorix (GSK), and Prevenar 13 (Pfizer Inc.) protect against pneumococcal diseases and reduce its occurrence.
However, it is not known how the routine use of these vaccines may affect otitis media-related healthcare resources and costs.
What is new?
In this study, we assessed trends in rates of healthcare utilization and associated costs due to otitis media in young children before (2005–2008) and after (2009–2013) use of pneumococcal conjugate vaccines. The study was conducted in two Swedish regions; one used Prevenar then Synflorix, while the other used Prevenar then Prevenar 13.
We found that compared to the period before pneumococcal conjugate vaccine implementation, the post-pneumococcal conjugate vaccine period was associated with:
A decrease in otitis media-related antibiotic prescriptions and surgical procedures in both regions.
A decrease in the annual frequency of otitis media related antibiotic use, primary care and outpatient visits, and surgical procedures.
A significant reduction in otitis media-related healthcare costs in both regions.
What is the impact on current thinking?
The use of pneumococcal conjugate vaccines effectively reduces healthcare utilization and resources associated with otitis media.
This indirect effect on the reduction of otitis media burden provides further benefit to the implementation of pneumococcal vaccination.
Introduction
Worldwide, childhood otitis media/acute otitis media (OM) is the most common reason for healthcare visits and antibiotic prescriptions.Citation1–4 Before introduction of pneumococcal conjugate vaccines (PCVs), over 80% of children experienced at least one episode of OM by the age of 3 years and over 40% experienced three or more episodes.Citation5 Although the unit cost of treating a single uncomplicated OM episode is relatively low, the overall economic burden to society is high due to its high frequency and because of the high rate of OM-related antibiotic prescribing.Citation6 Furthermore, OM treatment may involve surgical procedures such as myringotomy or tympanostomy tube placement (TTP), which is among the most common surgical procedures in children.Citation7
The most frequent causes of bacterial OM are Streptococcus pneumoniae, non-typeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis.Citation8 PCVs capable of preventing even a small proportion of OM cases could significantly reduce the healthcare burden associated with OM.
13-valent PCV (PCV13; Prevenar 13; Pfizer Inc.) and pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV; Synflorix; GSK) share 10 common serotypes: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F. PCV13 also contains serotypes 3, 6A, and 19A. PHiD-CV induces protection against cross-reactive serotype 19ACitation9 and 8 out of the 10 serotypes are conjugated to the protein D carrier, a surface-expressed protein from NTHi that induces specific antibodies and protects against NTHi OM in an animal model.Citation10 A growing body of evidence from clinical trials and observational studies support an impact of PHiD-CV in reducing NTHi OM.Citation10
The incidence of OM in children aged 0–2 years in Sweden between 2008 and 2010 was 344 per 1,000 person-years.Citation11 In Sweden, the 7-valent PCV (PCV7; Prevenar; Pfizer Inc.) was introduced to the national immunization program in 2009. From late 2009, approximately half of the Swedish regions switched to either PHiD-CV or PCV13. PCVs are administered at 3, 5, and 12 months of age in Sweden, and coverage of three doses is approximately 97%.Citation12 PCVs are reported to be associated with a reduced burden of OM, including a reduction in physician visits, antibiotic prescriptions, and OM-related surgery.Citation13–17
The current study took advantage of the unique epidemiologic environment in Sweden and aimed to assess the impact of PCVs by evaluating trends over time in OM-related outcomes in two Swedish regions: Skåne, where PCV7 was used from 1 January 2009 followed by PHiD-CV from 1 June 2010, and Västra Götalandsregionen (VGR), which used PCV7 from 1 January 2009 followed by PCV13 from 1 January 2010. The primary study objective described trends over time in the incidence rate of OM in Skåne and VGR and has been reported elsewhere.Citation18 In brief, a descriptive time-series analysis showed a decrease in the incidence of OM following introduction of PHiD-CV and PCV13, while predictive models suggested that only PHiD-CV was associated with a statistically significant reduction in OM. Both PHiD-CV and PCV13 appeared to decrease the risk of first OM in young children.Citation18
Here, we report secondary study objectives, which were to assess trends over time in rates of OM-related antibiotic prescriptions, TTP, and OM-related healthcare resource utilization and associated costs before and after sequential use of PCVs in Skåne and VGR.
Methods
Study design and region selection
This observational, cohort study of children ≤5 years of age in two Swedish regions assessed the incidence rates of OM-related antibiotic prescriptions, and TTP, and used an interrupted time-series model to assess the effect of the temporal dynamics on the monthly/annual incidence rates during pre-PCV (from 2005) and sequential introduction of PCVs from 2009 until 2013. We also assessed healthcare resource use and direct healthcare costs associated with OM including antibiotic use, primary healthcare visits, surgical procedures, and in-patient days in ≤2 year-olds.
Approximately 30% of the Swedish population resides in Skåne and VGR, and both offer regional-level health databases that can be linked with national registries at the patient level. The populations in Skåne and VGR were representative of Swedish national averages, as determined by a feasibility study (Supplement).
The study cohort comprised all children born in Skåne and VGR from 1 January 1999 through 31 December 2013 identified from the Swedish Medical Birth Registry. Children were assigned to vaccination cohorts based on their date and region of birth relative to the respective dates of introduction of PCV7, PHiD-CV, and PCV13. A 3-month ‘washout period’ was defined after the implementation of each new vaccine. Children born within the ‘washout periods’ were not included in vaccine cohort comparisons, but were included in the calculation of monthly and annual incidence of OM-related antibiotic prescriptions and TTP, and corresponding time-series analyses. Data were retrieved for all children ≤5 years of age. Here, we focus on children ≤2 years of age, because the incidence of OM begins to decrease from the age of 2 years,Citation5 and the ≤2 year age group was fully represented in all three vaccine cohorts.
The study was conducted in accordance with all applicable regulatory requirements, including all applicable subject privacy requirements and the guiding principles of Good Epidemiological Practice and the Declaration of Helsinki. The study protocol was approved by the Central Ethical Review Board in Sweden. The study is registered at ClinicalTrials.gov (NCT02742753).
Data sources
The National Patient Registry (NPR, Patient Registret) and the Prescribed Drug Registry (PDR) are held by the Swedish National Board of Health and Welfare (NBHW, Socialstyrelsen). The NPR has captured patient characteristics including demography, inpatient and outpatient visits (primary and secondary diagnoses), and procedures since 2001. Data collection is mandatory, and the register is updated annually by the NBHW from all hospitals and outpatient clinics. The PDR was established in 2005 and it records all pharmacy dispensed pharmaceuticals in Sweden and has almost full coverage in ambulatory care, but in-hospital drug use is generally not registered.Citation19
The regional healthcare databases for VGR (Region West Healthcare Database; Vårdatabasen Vega) and Skåne (Patient Administrative System in the region of Skåne; Patientadministrativt system i Skåne) contain patient-level data on diagnoses and procedures from primary healthcare providers (private and public) and hospitals in their regions. Data from the local registries were linked at the patient level with data from the national registries with the support of the NBHW, and de-identified data were transferred with unique identifiers. The analysis was conducted by Parexel International, which is registered with the Swedish Data Protection Authority. Double counting of children with a diagnosis of OM in both the national and regional databases was prevented by implementing analytic rules when the diagnosis occurred in different sources within a two-week interval.
Analyses
Determining trends over time (2005–2013) in the monthly/annual incidence rates of antibiotic prescriptions or TTP for OM
Prescriptions for antibiotics were identified using the Anatomical Therapeutic Chemical Classification system from the PDR. Prescriptions dated within 14 days of the date of a physician appointment/visit related to any diagnosis of OM were included. TTP was identified from the NPR. The monthly/annual incidence of antibiotics dispensed or TTP was defined as the total number of children reported with one or more prescriptions of antibiotics or TTP for OM in a month/year divided by the total exposure (person-years) in that month/year, as estimated from the Medical Birth Registry. As drug prescribing data were only available from mid-2005, the analysis of antibiotic prescriptions was conducted using data from July 1, 2005.
An autoregressive integrated moving average regression analysis was performed using three primary stages according to the Box-Jenkins methodology.Citation20 This model investigated temporal dynamics (cyclical trends, seasonality) in monthly/annual incidence for antibiotic prescriptions or TTP in Skåne and VGR and compared monthly/annual rates of antibiotic prescribing or TTP between the pre-PCV, PCV7, and PHiD-CV/PCV13 cohorts within each region.
The longitudinal dataset was divided into 3 periods: pre-PCV vaccination era, PCV7 era, and the PHiD-CV/PCV13 era. In Skåne, the pre-PCV era was from 1 January 2005 to 31 December 2008, the PCV7 era from 1 April 2009 to 31 May 2010, and the PHiD-CV era from 1 September 2010 to 31 December 2013. In VGR, the pre-PCV era was from 1 January 2005 to 31 December 2008, the PCV7 era from 1 April 2009 to 31 December 2009, and the PCV13 era from 1 April 2010 to 31 December 2013.
Interrupted time-series analyses assessed whether the introduction of PCVs had a significantly greater effect on the rate of antibiotic prescribing or TTP than underlying secular trends. Segmented regression analysis was used to measure the changes in antibiotic prescribing or TTP in level and slope between the different vaccination eras. By allowing for transition periods, each month’s incidence was independently estimated, thereby not introducing any break in the time series, which would make the estimation of autocorrelation and seasonality problematic. The interrupted time-series models were fitted using the following formula:
where t is the month number, PCV is an indicator set to 1 if in the PCV7 or PHiD-CV/PCV13 eras, or 0 otherwise, and PCV13 is an indicator set to 1 if in the PHiD-CV/PCV13 eras or 0 otherwise. Both indicators PCV and PCV13 are delayed for the 3-month transition period to reflect the time taken for the vaccines to be implemented and take effect. t2 and t3 indicate the month numbers when PCV7 and PHiD-CV/PCV13 were introduced, following the 3-month transition period. The initial level of incidence (at time t = 0) is represented by α1, and the change from pre-PCV to PCV7 and from PCV7 to PHiD-CV/PCV13 is represented by α2 and α3, respectively. The initial slope (rate of change of incidence rate) during the pre-PCV period is represented by β1, and changes in slope following implementation of PCV7 and PHiD-CV/PCV13 were estimated by β2 and β3, respectively. The change in slope from the PCV7 to the PHiD-CV/PCV13 period was estimated by β3 – β2.
Children with urinary tract infection diagnosed either in primary care or as an inpatient or outpatient were used as an indicator control disease for the time-series analyses. As reported previously, there was no evidence of seasonality, autocorrelation, or systematic trends in the incidence of urinary tract infection observed in the time-series analysis.Citation18
To allow for the fact that within each calendar year children may have received different vaccinations, or no vaccination according to their age, separate analyses were performed by age category (≤2, 3–5 and ≤5 years).
Estimating healthcare utilization and direct costs
Incidence for healthcare resource utilization outcomes was calculated as annualized mean frequency as the number of events (per person-years) stratified by vaccination period.
Direct costs for OM of any severity were calculated by including healthcare resource utilization for each child based on the count of antibiotic prescriptions filled for OM, use of medical services (primary care and specialist physician visits, hospital stays, and length of hospitalization), procedures associated with TTP, myringotomy, and intravenous medication for OM (Table S1). Costs for all healthcare visits and procedures were calculated using the number of registered events multiplied by a unit cost based on 2017 public price lists or the stated cost of medication adjusted to 2017 values in line with the consumer price index (in Swedish Krona [SEK]). Inpatient stays were calculated by multiplying length of stay by the unit cost for one day of inpatient care treatment at the pediatric clinical department.
For each cost variable, the annualized cost of healthcare per child as determined from the Medical Birth Registry was modeled using a multivariable regression model (gamma model with log link). No interaction terms were included in the model. The regression analysis was adjusted for age, sex, calendar month, observation year, time since PCV7 introduction, and time since PHiD-CV/PCV13 introduction. Cost ratios between the pre-PCV, PCV7, and PHiD-CV/PCV13 cohorts were estimated with 95% confidence interval (CI) for each region.
Statistical analyses were performed using R version 3.4.2. Analyses were performed separately for each region and no direct statistical comparisons between the two regions were performed.
Results
Population
There were 191,596 births in Skåne between 1999 and 2013, of whom 77,962 (41%) had at least one diagnosis of OM ≤5 years of age. There were 60,141 children in Skåne who experienced OM in the pre-PCV cohort, 7,869 in the PCV7 cohort, and 9,952 in the PHiD-CV cohort.
There were 250,327 births in VGR during the study period, and 102,490 (41%) had at least one diagnosis of OM ≤5 years of age. There were 77,966 children who experienced OM in the pre-PCV cohort, 7,272 in the PCV7 cohort, and 17,252 in the PCV13 cohort.
Sex distribution, weight and length at birth, and maternal characteristics and co-morbidities were similar between the cohorts and were reported previously.Citation18
Antibiotic prescriptions for OM
In children aged ≤2 years, the annual incidence per 100,000 person-years of antibiotic prescriptions for OM decreased in Skåne from a peak of 30,688 (95% CI: 30,141; 31,245) in 2007, to 15,516 (95% CI: 15,157; 15,883) in 2013 (Table S2). In VGR, the antibiotic prescribing rate decreased from 29,035 (95% CI: 28,592; 29,486) in 2010, to 17,588 (95% CI: 17,244; 17,938) in 2013 (Table S2).
In Skåne, there was a decrease in the incidence of antibiotic prescribing for OM in ≤2 year-olds from the pre-PCV cohort through the consecutive PCV cohorts that was not observed in 3–5 year-olds or ≤5 year-olds overall (). The annual incidence of antibiotic prescriptions for OM per 100,000 person-years (≤2 years) decreased from 26,171 in the pre-PCV cohort, to 20,932 in the PCV7 cohort, and to 16,133 in the PHiD-CV cohort.
Table 1. Overall incidence rates (per 100,000 person-years) of otitis media-related antibiotic prescriptions and tympanostomy tube placements (TTP) in the pre-PCV, PCV7, and PHiD-CV/PCV13 cohorts*
In VGR, there was no decrease in the incidence of antibiotic prescribing for OM in ≤2 year-olds between the pre-PCV and PCV7 cohorts, but a decrease per 100,000 person-years from 23,619 prescriptions for OM in the PCV7 cohort, to 18,456 in the PCV13 period ().
Antibiotic prescribing for OM showed strong annual seasonality and significant levels of autocorrelation in all ages and in each vaccine era (Figure S1), which mirrored OM incidence rates.Citation18 Before the introduction of PCV, antibiotic prescribing rates for OM in children aged ≤5 years were higher in Skåne than in VGR, particularly among children aged 1 year (Figure S1), also mirroring trends observed in OM incidence rates.Citation18
The interrupted time-series showed a decrease in antibiotic prescribing (events per 100,000 person-years) between the pre-PCV period and the PHiD-CV period that was statistically significant in 1 year-olds (−697, 95% CI: −1138; −255), 2 year-olds (−369, 95% CI: −655; −83), and ≤5 years (−314, 95% CI: −616; −13) (Table S3). In VGR, statistically significant decreases were observed in all age years, with an overall change in slope of −356 (95% CI: −531; −181) from the pre-PCV to the PCV13 period (Table S3).
Trends in TTP for treatment of OM
There was more TTP conducted in Skåne than in VGR over the study period, with a decrease in procedures evident in both regions over time (Table S4). In children aged ≤2 years, the annual incidence per 100,000 person-years of TTP decreased in Skåne from a peak of 1,224 (95% CI: 1,118; 1,339) in 2007 to 889 (95% CI: 806; 981) in 2013. In VGR, the rate of TTP decreased from 893 (95% CI: 817; 977) in 2008, to 483 (95% CI: 429; 544) in 2013.
In both regions, the incidence of TTP decreased from the pre-PCV cohort through the consecutive PCV cohorts in ≤2 year-olds, 3–5 year-olds, and ≤5 year-olds overall (). In children ≤2 years of age, the rate of TTP per 100,000 person-years decreased in Skåne from 1,282 in the pre-PCV cohort to 999 in the PCV7 cohort, and to 632 in the PHiD-CV cohort, and from 790 in the pre-PCV cohort, to 618 in the PCV7 cohort, and then to 370 in the PCV13 cohort in VGR ().
Seasonal trends were less marked for TTP than for antibiotic prescriptions for OM (Figure S2). The interrupted time-series analysis showed no clear change in trend in TTP in Skåne, while there was an apparent reduction in 1–3 year-old children as well as ≤5 year-olds overall in VGR between pre-PCV and PCV13 periods (Table S5).
Healthcare utilization costs
The annualized mean frequencies of antibiotic use, primary care visits, outpatient visits, TTP, and myringotomy all decreased over consecutive cohorts in Skåne (). Similar trends were observed in VGR, except for primary care visits, which increased between the pre-PCV and PCV7 cohorts. The annualized mean number of inpatient days for OM remained relatively constant across the three cohorts (approximately 0.02 days per child in both regions).
Figure 1. Resource use (A) and healthcare utilization costs (B) for otitis media by region in children aged ≤2 years for each vaccine cohort.
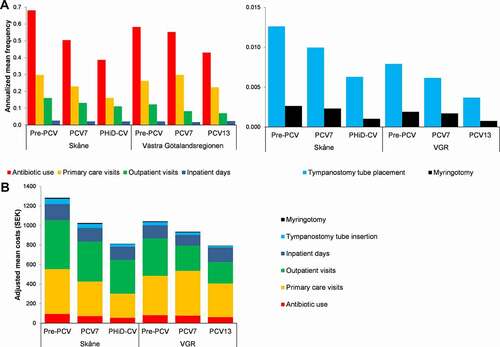
Annualized healthcare utilization in ≤2 year-olds was higher in the pre-PCV cohort in Skåne than in VGR, but the relative reductions over time were higher in Skåne for most outcomes, such that the total healthcare costs per child were similar in the PCV13 and PHiD-CV cohorts, 794 SEK versus 814 SEK per child, respectively (, ).
Table 2. Annualized mean direct cost and healthcare resource use for otitis media in children ≤2 years for the calendar years 2005–2013 for each vaccine cohort
In Skåne, the annualized mean total cost of healthcare utilization for the treatment of OM per child ≤2 years for the calendar years 2005–2013 reduced by 20.0% between the pre-PCV and PCV7 cohorts (1,283 to 1,026 SEK) and by a further 20.7% between the PCV7 and PHiD-CV cohorts (1,026 to 814 SEK) (). The reduction in VGR was less marked, with a 10.2% decrease in the pre-PCV versus the PCV7 cohorts (1,043 to 937 SEK), and a further 15.3% decrease between the PCV7 and PCV13 cohorts (937 to 794 SEK) (). Mean costs of healthcare utilization for the treatment of OM per child decreased between the pre-PCV and PHiD-CV/PCV13 cohorts by 36.6% (1,283 to 814 SEK) in Skåne and by 23.9% (1,043 to 794 SEK) in VGR (). Healthcare utilization and costs per study year are provided in Table S6.
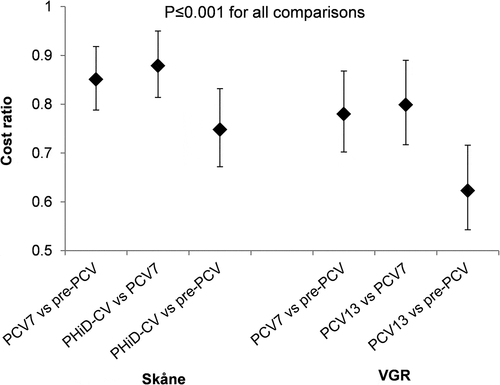
Modeling the overall healthcare costs and adjusting for age, sex, month, and a piecewise linear time trend (based on vaccine periods), we observed marked reductions in cost across Skåne and VGR across the vaccine cohorts. Changes in adjusted annualized total healthcare cost ratios for OM were statistically significant (p ≤ 0.001) after PCV introduction in Skåne and VGR ().
Figure 2. Adjusted* annualized cost ratio between vaccine cohorts for otitis media in children aged ≤2 years.
Discussion
This study used linked region-based and national healthcare databases to estimate the impact of PCV introduction on healthcare utilization due to OM in young children. The analysis captured information from primary care, outpatient, and inpatient records, providing a comprehensive overview of the healthcare utilization and costs associated with OM in two representative regions in Sweden. The data indicate statistically significant reductions in rates of antibiotic prescribing events and TTP procedures, reduced healthcare utilization for the treatment of OM, and direct healthcare costs for OM that were associated with PCV introduction in both study regions. Skåne region switched from PHiD-CV to PCV13 in May 2014, precluding longer follow-up. Nevertheless, the duration of the time-series was sufficient to detect the primary and secondary outcomes.Citation18
OM is one of the most common reasons for the prescription of antibiotics during early childhood, and reduced antibiotic consumption can have positive flow-on effects in terms of reducing antibiotic resistance pressure.Citation21 PCV introduction in other countries has been associated with lower rates of antibiotic prescribing for OM, and a reduction in the proportion of antibiotic-non-susceptible carriage or invasive disease strains.Citation17,Citation22,Citation23 Our observation that PCVs were associated with reduced TTP procedures is consistent with other studies showing similar impacts of PHiD-CV and PCV13.Citation17,Citation24 A study by Gisselsson-Solen in Sweden assessed the impact of higher-valency PCVs on OM cases and OM-related surgical procedures in 0–4 year-olds using national data from the Swedish NBHW from 2005 to 2014.Citation25 Rates for 2013–14 were compared with 2007–08, and comparisons were made between regions that used PHiD-CV and regions that used PCV13. The study showed significant decreases in OM diagnoses (39% reduction in outpatient visits and 42% decrease in hospitalizations) and surgical procedures (18% decrease in TTP) after PCV introduction, and while the impact of PHiD-CV appeared to be greater than PCV13, pre-PCV differences between regions in terms of procedure rates and the number of pediatricians and ear-nose-and-throat surgeons could also explain some of the observed differences.Citation25
Direct healthcare costs associated with the treatment of OM in young children reduced with the introduction of PCV in both regions, with additional reductions observed after changing from PCV7 to PHiD-CV or PCV13. Compared to the pre-PCV period, the adjusted ratio of annualized mean costs per child was 0.748 (95% CI: 0.672; 0.832) for the PHiD-CV cohort in Skåne, and 0.623 (95% CI: 0.543; 0.716) for the PCV13 cohort in VGR.
In Sweden, a cost-effectiveness model that assessed the impact of routine vaccination with PHiD-CV or PCV13 showed that the direct costs of pneumococcal and NTHi-related diseases were driven by costs associated with pneumonia and OM, rather than by invasive pneumococcal disease.Citation26
Although covering a similar time period to that reported by Gisselsson-Solen, our study went beyond national data provided by the NBHW and included region-level data including general practitioner visits and also evaluated the impact of PCV on antibiotic prescribing. Using an age-period-cohort model with multivariable over-distributed Poisson regression models, we previously reported that only PHiD-CV was associated with a significant reduction in the incidence rate of OM, which we postulated could be due to a differential impact of PHiD-CV on OM caused by NTHi, whereas both PHiD-CV and PCV13 appeared to decrease the risk of first OM in young children.Citation18 In the present study, both PHiD-CV and PCV13 appeared to be associated with decreases in healthcare utilization, which might have been more marked for PHiD-CV. However, in the adjusted model, the reductions in direct costs per child were similar for PCV13 and PHiD-CV. It is worth noting that antibiotic-prescribing guidelines for OM in Sweden changed around the time that PHiD-CV/PCV13 was introduced,Citation27 and potential differences in the number of ear-nose-and-throat specialists, pediatricians and hospital beds per capita might have existed between Skåne and VGR. These factors, as well as potentially differing hospital admission policies in each region or changes in the management of OM post-vaccine introduction, might have influenced OM healthcare-seeking behavior and treatment practices.
Another potential limitation of our study is that while the impact on OM-related antibiotic prescribing, procedures, and costs reported in this study may be generalizable to other parts of Sweden, they may not be directly applicable to other countries or settings with different healthcare systems and OM epidemiology. Additionally, the PDR generally does not register pharmaceuticals dispensed in hospital. This is unlikely to have substantially impacted our results given that most OM is treated in outpatient settings. We cannot exclude that children presenting with OM might have had respiratory tract infections or other illnesses that were treated with antibiotics before the OM episode, which might have had an impact on the study outcomes by modifying the severity of the OM episode or influencing further treatment decisions. Finally, national statistics indicate that migration levels are similar in Skåne and VGR, and potential biases are therefore likely to be comparable in the two regions.
Cost-effectiveness modeling was used to support the introduction of higher-valency PCVs into national immunization schedules all over the world; most of these models use invasive pneumococcal disease and pneumonia data from clinical trials, while few used OM data. Moreover, few countries have subsequently measured the economic impact using real-world data.Citation28 As tympanocentesis and middle ear fluid culture to determine OM etiology are not routinely performed in most settings, diagnosis of OM is mainly limited to the use of a combination of diagnostic, procedure, and treatment codes (as done here). Our study is one of the few to use real-world OM data to confirm the cost-effectiveness of higher-valency PCVs. The results of our study were consistent across the two regions and are consistent with trends observed in a real-world analysis conducted in Australia.Citation29 Confirmation of the effectiveness of vaccination programs is necessary to maintain confidence among policymakers, clinicians, and the general public. Our study provides real-world confirmation of the benefit of higher-valency PCVs. Such data can be used to support continuation of PHiD-CV and PCV13 vaccination programs, aid policymakers and governments when choosing a PCV for their national immunization program, and could be used to populate future cost-effectiveness models as next-generation PCVs become available.
In conclusion, our study used real-world data to compare healthcare utilization and associated direct costs for OM in two regions in Sweden that capture approximately 30% of the total population. Following PCV introduction, OM-related healthcare utilization including antibiotic prescriptions, primary care visits, and surgical procedures decreased in the study regions in Sweden, with significant decreases in the associated costs. The data add to evidence supporting an impact of higher-valency PCVs on OM, one with potential activity against NTHi, with decreased antibiotic prescribing, primary care visits and surgical procedures, leading to decreases in direct costs associated with OM treatment.
Abbreviations
CIconfidence interval
NBHWSwedish National Board of Health and Welfare
NPRNational Patient Registry
NTHinon-typeable Haemophilus influenzae
OMotitis media/acute otitis media
PCVpneumococcal conjugate vaccine
PCV7/PCV137-valent/13-valent pneumococcal conjugate vaccine
PDRPrescribed Drug Registry
PHiD-CVpneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine
SEKSwedish Krona
TTPtympanostomy tube placement
VGRVästra Götalandsregionen
Disclosure of potential conflicts of interest
CT was employed by the GSK group of companies. YF was an external consultant of the GSK group of companies during the work under consideration for publication. BA and TD were and MEJ, MH, and EM are employed by Parexel International and received professional fees from the GSK group of companies for the work under consideration for publication.
Author contributions
MEJ, MH, TD, BA, EM, and CT designed the study. MH, TD, BA, and EM acquired the data. MEJ and YF analyzed the data. All authors participated in the interpretation of the data. All authors reviewed and revised the manuscript, and approved the final manuscript as submitted.
Trademark statement
Synflorix is a trademark licensed to the GSK group of companies. Prevenar and Prevenar 13 are trademarks of Pfizer Inc.
Supplemental Material
Download MS Word (526.9 KB)Acknowledgments
The authors would like to thank the infants and children whose data were the bases of these analyses. The authors wish to thank Maria Malmenäs for key input during design, planning, and execution of the study, Jean-Yves Pirçon (GSK) for statistical input during study design, Dorota Borys (GSK) for review of the study results, and Lena Svensson (GSK) and Anna Linnér (GSK) for key input throughout the design and execution of the study. The authors also thank Joanne Wolter (independent on behalf of Modis) for drafting the manuscript and Lotte Mathé (Modis c/o GSK) for manuscript coordination.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1942712.
Additional information
Funding
References
- Auinger P, Lanphear BP, Kalkwarf HJ, Mansour ME. Trends in otitis media among children in the United States. Pediatrics. 2003;112:514–9. doi:10.1542/peds.112.3.514.
- McGrath LJ, Becker-Dreps S, Pate V, Brookhart MA. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000-2011. PLoS One. 2013;8:e81210. doi:10.1371/journal.pone.0081210.
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi:10.1371/journal.pone.0036226.
- Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008-2014. BMC Health Serv Res. 2018;18:318.doi. doi:10.1186/s12913-018-3139-1.
- Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics. 2017;140:e20170181. doi:10.1542/peds.2017-0181.
- Vergison A, Dagan R, Arguedas A, Bonhoeffer J, Cohen R, Dhooge I, Hoberman A, Liese J, Marchisio P, Palmu AA, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi:10.1016/s1473-3099(10)70012-8.
- Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, Grimes AM, Hackell JM, Harrison MF, Haskell H, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013;149:S1–35. doi:10.1177/0194599813487302.
- Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One. 2016;11:e0150949. doi:10.1371/journal.pone.0150949.
- European Medicines Agency. Synflorix product information [internet]. [accessed 2018 Dec 06]. https://www.ema.europa.eu/documents/product-information/synflorix-epar-product-information_en.pdf .
- Clarke C, Bakaletz LO, Ruiz-Guinazu J, Borys D, Mrkvan T. Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage. Expert Rev Vaccines. 2017;16:1–14. doi:10.1080/14760584.2017.1333905.
- Liese JG, Silfverdal SA, Giaquinto C, Carmona A, Larcombe JH, Garcia-Sicilia J, Fuat A, Garces-Sanchez M, Basanta MLA, Hiraldo EM, et al. Incidence and clinical presentation of acute otitis media in children aged <6 years in European medical practices. Epidemiol Infect. 2014;142:1778–88. doi:10.1017/S0950268813002744.
- Public Health Agency of Sweden. Vaccinationsstatistik från Barnavårdcentraler [internet]. [accessed 2017 Dec 18]. www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/vaccinationsstatistik/statistik-fran-barnavardcentraler/ .
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014;59:1724–32. doi:10.1093/cid/ciu683.
- Lau W, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, Sharland M, Wong ICK, Hsia Y. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33:5072–79. doi:10.1016/j.vaccine.2015.08.022.
- Littorin N, Ahl J, Udden F, Resman F, Riesbeck K. Reduction of Streptococcus pneumoniae in upper respiratory tract cultures and a decreased incidence of related acute otitis media following introduction of childhood pneumococcal conjugate vaccines in a Swedish county. BMC Infect Dis. 2016;16:407. doi:10.1186/s12879-016-1750-5.
- Eythorsson E, Hrafnkelsson B, Erlendsdottir H, Gudmundsson SA, Kristinsson KG, Haraldsson A. Decreased acute otitis media with treatment failure after introduction of the ten-valent pneumococcal Haemophilus influenzae Protein D conjugate vaccine. Pediatr Infect Dis J. 2018;37:361–66. doi:10.1097/inf.0000000000001870.
- Palmu AA, Rinta-Kokko H, Nohynek H, Nuorti JP, Jokinen J. Impact of national ten-valent pneumococcal conjugate vaccine program on reducing antimicrobial use and tympanostomy tube placements in Finland. Pediatr Infect Dis J. 2018;37:97–102. doi:10.1097/inf.0000000000001810.
- Edmondson-Jones M, Dibbern T, Hultberg M, Anell B, Medin E, Feng Y, Talarico C. The effect of pneumococcal conjugate vaccines on otitis media from 2005 to 2013 in children aged ≤5 years: a retrospective cohort study in two Swedish regions. Hum Vaccin Immunother. 2021;17:517–26. doi:10.1080/21645515.2020.1775455.
- Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundström A, Westerholm B, Rosén M, et al. The new Swedish prescribed drug register-opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. doi:10.1002/pds.1294.
- Box G, Jenkins G. Time series analysis, forecasting and control. San Francisco (CA): Holden-Day; 1976.
- Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci USA. 2018;115:12896–901. doi:10.1073/pnas.1721095115.
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–48. doi:10.1056/NEJMoa035060.
- Cohen R, Levy C, de La Rocque F, Gelbert N, Wollner A, Fritzell B, Bonnet E, Tetelboum R, Varon E. Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr Infect Dis J. 2006;25:1001–07. doi:10.1097/01.inf.0000243163.85163.a8.
- Wiese AD, Huang X, Yu C, Mitchel EF, Kyaw MH, Griffin MR, Grijalva CG. Changes in otitis media episodes and pressure equalization tube insertions among young children following introduction of the 13-valent pneumococcal conjugate vaccine: a birth-cohort based study. Clin Infect Dis. 2019;69:2162–69. doi:10.1093/cid/ciz142.
- Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36:1027–31. doi:10.1097/inf.0000000000001654.
- By A, Sobocki P, Forsgren A, Silfverdal SA. Comparing health outcomes and costs of general vaccination with pneumococcal conjugate vaccines in Sweden: a Markov model. Clin Ther. 2012;34:177–89. doi:10.1016/j.clinthera.2011.12.007.
- Tyrstrup M, Beckman A, Molstad S, Engstrom S, Lannering C, Melander E, Hedin K. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care- a retrospective study of electronic patient records. BMC Infect Dis. 2016;16:709. doi:10.1186/s12879-016-2018-9.
- Wasserman M, Sings HL, Jones D, Pugh S, Moffatt M, Farkouh R. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17:71–78. doi:10.1080/14760584.2018.1409116.
- Perdrizet J, Lai YS, Williams S, Struwig VA, Wasserman M. Retrospective impact analysis and cost-effectiveness of the pneumococcal conjugate vaccine infant program in Australia. Infect Dis Ther. 2021;10:507–20. doi:10.1007/s40121-021-00409-7.
