ABSTRACT
Currently, many companies around the world are actively developing COVID-19 vaccines. Fourteen vaccines with reliable safety and effectiveness are being successfully distributed to the public. However, there is no specific clinical trial data of the vaccines currently on the market on cancer patients at various stages, so the safety and effectiveness on cancer patients is unknown. This mini-review aims to discuss the impact of COVID-19 on cancer patients, and the urgent need of COVID-19 vaccines for cancer patients. In this review, we described the current status of the COVID-19 vaccine usages in cancer patients, as well as discussed potential problems in the use of vaccine. In addition, we included an original survey of the acceptance of the COVID-19 vaccines in 209 cancer patients and their family members. COVID-19 vaccine can provide cancer patients with social and medical benefits; therefore, clinical trials of vaccines on cancer patients are in great need.
Introduction
COVID-19 is one of the most devastating pandemics in history, to which all people are generally susceptible. Cancer patients are more susceptible to infection than healthy people due to the compromised immune status caused by malignant tumors and the treatments they receive.Citation1 Therefore, in the era of economic globalization, we cannot respond to the COVID-19 pandemic by simply reducing going out, avoiding gatherings, wearing masks and other control measures. The Advisory Committee on Immunization Practices (ACIP) has found through studies of multiple groups that cancer patients are at higher risk of severe COVID-19, and it is recommended that cancer patients should be given priority to be vaccinated.Citation2 According to the current global situation of COVID-19, the development of safe and effective COVID-19 vaccine is essential to reduce morbidity and mortality. Although clinical trials of various vaccines have proven their safety and effectiveness, there are no specific data on cancer patients under various treatment stages. Therefore, the aim of this review is to update oncologists with information about COVID-19 in cancer patients, describe the potential problems and current usage of SARS-CoV-2 vaccine for cancer patients, discuss the necessity of increasing clinical trials for cancer patients, and investigate the acceptance of COVID-19 vaccine in cancer patients and their families.
The impact of COVID-19 on cancer patients
According to the latest global cancer data released by the World Health Organization’s International Agency for Research on Cancer (IARC), it is estimated that there were about 19.3 million new cancer cases in 2020 and about 10 million deaths in the world.Citation3 The number of people still alive after 5 y of being diagnosed with cancer was 50.6 million. Due to the fact that COVID-19 is a new type of disease, inevitably the population lacks specific immunity to the virus. Everyone has the possibility of being infected, especially patients with malignant tumors with a compromised immune status.Citation4 In addition to the threats it gave to the health of the infected people, the pandemic also caused delay of treatment to thousands of cancer patients, making them take the risk of incurability or even death.
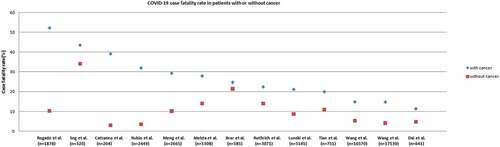
In order to find out the impact of COVID-19 infection on the mortality of cancer patients, we conducted an extensive literature search through PUBMED and summarized 13 comparative studiesCitation5–17 with more than 200 subjects, which compared the mortality of cancer patients versus non-cancer patients infected with COVID-19. Among all studies, 12 of them showed that the death rate of cancer patients with COVID-19 was higher than that of patients without cancer infected with COVID-19, as shown in . These studies came from various countries including the United States, Europe, China, Cuba, and India. The sample size of the two studies in the United States exceeded 10,000.Citation10,Citation11 Wang et al. studied on 17,130 patients from the United States, and the case fatality rate (CFR) of cancer patients was 14.8%, while for non-cancer patients it was only 4.1%.Citation11 A study of 1308 patients from New York showed that the mortality rate of cancer patients was 28%, and that of non-cancer patients was 14%.Citation7 The same result also appeared in Wuhan, China, with a mortality rate of 29.4% for cancer patients and 10.2% for non-cancer patients.Citation9
Figure 1. Scatter plot of COVID-19–related CFRs from series comparing rates from patients with cancer (blue dots) with patients without cancer (red dots)
Therefore, the most important thing is to minimize the risk of infection for cancer patients, especially those who are receiving cancer therapy. It is necessary to prevent and control COVID-19 for cancer patients by vaccination, and it is urgent to actively develop vaccines suitable for cancer patients.Citation18
At present, there are a large number of cancer patients in the world, and the age of cancer incidence is younger. With various treatment modalities, the 5-y survival rate is significantly improved, and many cancer patients were able to return to work after treatment. Studies have shown that the COVID-19 vaccine can reduce the viral load in nasopharyngeal and bronchoalveolar lavage samples, in addition to the ability to prevent infection of SARS-CoV-2.Citation19 Therefore, the vaccination not only can enable active immunity to SARS-CoV-2 in cancer patients, but can also significantly reduce the social impact of COVID-19 on cancer patients. The huge number of cancer patients may put tremendous pressure on socio-economic and medical resources if the cancer patients cannot be vaccinated.
Vaccine development during the COVID-19 pandemic
There are currently more than 200 COVID-19 vaccines under development in the world, of which more than 60 vaccines are undergoing clinical trials.Citation20 As of this writing, distribution of 14 COVID-19 vaccines have been launched through the Emergency Use Authorization. At present, the main types and strategies adopted in COVID-19 vaccines include inactivated vaccines, viral vector vaccines (including adenovirus vectors, attenuated influenza virus vectors), mRNA vaccines, and recombinant vaccine virus, etc.Citation19,Citation21–25 All these vaccines focus on the immune response to the SARS-CoV-2 Spike (S) protein.Citation26,Citation27 summarizes the detailed analysis of the vaccines. Published data have shown that the COVID-19 vaccines have short-term side effects, including local symptoms (such as pain, redness, swelling and induration) and systemic responses (such as fever, chills, myalgia, and headache).Citation28
Table 1. Currently approved COVID-19 vaccines
Although the safety and immunogenicity data of Pfizer’s COVID-19 vaccine reported in people with chronic diseases have shown effectiveness, there are no detailed clinical trial data in cancer patients at various stages of treatment and after treatment. Pfizer suggested that people who receive the mRNA COVID-19 vaccine during chemotherapy or other immunosuppressive therapy are not recommended to be vaccinated again until their immunity is restored.Citation29 If implemented clinically, it is hard to guarantee that this special group can be effectively protected.
In view of the direct and indirect benefits of COVID-19 vaccination for cancer patients, more detailed clinical trials on cancer patients in various treatment stages are urgently needed. The key features of these trials will include the dosage, safety, reactogenicity and immunogenicity of the vaccine in cancer patients, which may be different from the general population with chronic disease. Cancer patients are undergoing surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, etc. which will have varying degrees of impact on the immune function of the human body.Citation30 These factors may affect the immune response to the vaccine.Citation31 It is suggested that clinical trials start with low-dose vaccines on cancer patients at various treatment stages and after treatment. With the establishment of safety and reaction data, the vaccine dose can gradually increase until stabilized.
Current status of COVID-19 vaccination in cancer patients
At present, cancer patients are classified as unsuitable for vaccination in some marketed vaccines. The main reason is the lack of clear clinical trial data, which makes it impossible to determine the safety and side effects of the COVID-19 vaccine for cancer patients. Most vaccines currently on the market do not have clinical trial data on cancer patients or recovered patients, so their safety and side effects on cancer patients are unknown. In addition, the COVID-19 vaccine also has certain requirements for the physiological functions of human body. The most common side effects after immunization of the COVID-19 vaccine are fever, redness, swelling, and pain at the injection site, which usually go away within two to 3 d. Generally, no special treatment is required. However, if the symptoms are severe or persistent, one should be seen by a doctor.Citation28,Citation32 Due to the lack of clinical data, the adverse reactions of cancer patients after vaccination cannot be accurately assessed. Therefore, to ensure the safety of and the effectiveness of the vaccine, some vaccines are not recommended for patients with malignant tumors. In order to solve these problems, it is necessary to carry out clinical trials of COVID-19 vaccines for cancer patients and recovered patients to determine the dosage, safety, reactogenicity and immunogenicity of the vaccine in such populations.
Potential safety and immunogenicity issues of COVID-19 vaccine in cancer patients
During the Phase III clinical trial of Pfizer’s COVID-19 vaccine, cancer patients with immunodeficiency, patients receiving chemotherapy, radiotherapy, bone marrow or organ transplants, and patients with HIV were not allowed to participate in the trial due to potential immunosuppression.Citation33 To date, there is no published research related to the impact of the COVID-19 vaccine on cancer treatment. Moreover, there are many COVID-19 vaccines under development, with many different strategies. Therefore, further research is needed on the safety, reactogenicity and immunogenicity of vaccines in cancer patients and the impact of vaccines on cancer treatment.
Vaccines not recommended for cancer patients
Not all vaccines are recommended for cancer patients. Live attenuated vaccines are designed to achieve propagation of pathogenic microorganisms through multiple generations to reduce their toxicity but maintain immunogenicity. The vaccination is similar to mild natural infection. Because of its certain biological activity and pathogenicity, it can reproduce in the body after injection. If it cannot be controlled, it will cause infectious diseases. By then the vaccine will become the cause of the disease. The immune function of cancer patients is weakened during the cancer treatment; thus, the immunity to the virus is reduced.Citation34,Citation35 Therefore, vaccination of live attenuated vaccines is not recommended for cancer patients.
Time of vaccination for cancer patients
Ideally, cancer patients should be vaccinated prior to cancer treatment,Citation36,Citation37 at least 2 weeks in advance. Since patients will frequent high-risk places such as hospitals after starting cancer treatment, and their immune functions will decline due to the treatment, cancer patients are prone to infections of COVID-19. The vaccination should also be avoided during chemotherapy and immunotherapy. Vaccines are biological products, and side effects such as fever may occur after vaccination. If the vaccine is administered during surgery, radiotherapy or chemotherapy, it may affect the judgment of the cause of side effects such as fever, and may also impair the efficacy of the vaccine.
The impact of various cancer treatments on vaccines
The impact of immunosuppression on vaccination is twofold: it may cause derivative diseases, and secondly it may weaken the immune response to the vaccine. Vaccine-derived diseases only occur when individuals are vaccinated with live attenuated vaccines. Vaccination of inactivated vaccines and genetically engineered vaccines is safe and will not cause vaccine-derived diseases. Secondly, due to the impaired immune function of cancer patients, the immune response to the vaccine is compromised, and the amount of antibodies that may be produced is insufficient, which reduces the protection of the vaccine. But this does not mean that people with weakened immune systems cannot be vaccinated. Currently, we are in urgent need of vaccine data on the type of cancer treatment, the timing of treatment, past immune dysfunction, and health conditions related to vaccine-induced immunity.
Taking influenza vaccine as an example, a study of 51 lung cancer patients showed that after vaccination with trivalent inactivated influenza vaccine, the seroprotection rate of lung cancer patients against B strain was significantly lower than that of chronic obstructive pulmonary disease (COPD) patients (64% vs 92%). Among lung cancer patients, patients receiving double-platinum chemotherapy showed lower seroprotection rates than patients receiving single-drug therapy.Citation35 This study showed that the immune response of the vaccine was weakened in an immunosuppressed individual.
More and more cancer patients are receiving immunotherapy now.Citation38 The number of cancer patients receiving immune checkpoint inhibitor (ICI) treatment has been increasing, with a majority of patients with lung cancer, head and neck cancer, kidney cancer and melanoma. ICI tends to increase the patient’s immunity rather than reduce the patient’s immune response. But considering that patients still have the risk of potential infection, they should also be vaccinated appropriately. In patients receiving ICI (PD-1, PD-L1 or CTLA-4 inhibitors, etc.) immunotherapy, due to the activation of immunity in the body,Citation39 the vaccine may cause overactive immunity and trigger an “inflammatory storm,” which may cause severe inflammation and damage to important organs. Therefore, for such patients, we need more clinical trials to prove the safety of the vaccine.
Although we do not have the data on the clinical use of the COVID-19 vaccine on cancer patients for the time being, referring to the relevant data of the flu vaccine, it is reasonable to presume that COVID-19 vaccine will have clear long-term data on safety in cancer patients. When cancer patients received PD-1 immunotherapy, vaccination of inactivated influenza vaccine did not increase immune-related adverse events.Citation40
Similarly, in the study of influenza vaccination in targeted therapy for cancer patients, trastuzumab targeted therapy during breast cancer surgery was beneficial to influenza vaccination without increasing the risk of adverse events. This result supported patients underwent breast cancer surgery in receiving flu vaccine during adjuvant trastuzumab treatment.Citation41
Vaccine dosage for cancer patients
The dose of the vaccine may also be different between cancer patients and healthy adults. For example, Sodhi et al. reported hepatitis B vaccination on 967 uninfected cancer patients between 5 months and 85 y old who were going to receive chemotherapy. The results showed that cancer patients who received double doses of hepatitis B vaccine had a higher seroprotection rate than those who received regular doses of hepatitis B vaccine (49% vs 75.9%).Citation42 Therefore, the immune response and the duration of immunity of the conventional dose of the COVID-19 vaccine in cancer patients at various stages of treatment still need to be further studied. Moreover, studies have shown that TMPRSS2 is one of the pathways for SARS-CoV-2 to enter cells.Citation43 But in some prostate cancer patients, the TMPRSS2 gene is highly variable. Therefore, it is needed to be considered whether patients with such gene mutations and patients with orally administered serine protease inhibitor like camostatinmesylate can be exempted from vaccination.
Acceptance of the COVID-19 vaccine in cancer patients
115 cancer patients and 94 family members of Hubei Cancer Hospital were recruited in our study to investigate the acceptance of COVID-19 vaccine. The staff conducted face-to-face question-and-answer surveys by convenient sampling with self-designed questionnaires. The demographics of all subjects are listed in . Out of a total of 209 subjects, 40.7% were male and 59.3% were female. Among cancer patients, 13.4% received surgery, 26.8% received chemotherapy, 5.7% received radiotherapy, and 9.1% received targeted drug therapy, immunotherapy or other treatments. 24.4% of the subjects were younger than 40 y old, and the rest 75.6% were older than 40. The education level of most subjects is high school or below, accounting for 58.9%.
Table 2. Demographics of subjects
The survey results are listed in . The data has not been published. The results showed that with the assumption of free vaccination, about 35% of cancer patients would accept the vaccination, and 65% of them refused. 67% of the family members of the patients would accept the vaccination, and 33% of them refused. Compared with the patient population, family members were more willing to receive COVID-19 vaccination. More than 90% of those who would like to receive the vaccine, including patients and their families, implied their intention to protect their family members from infection.
Table 3. Reasons for subjects’ acceptance or rejection of COVID-19 vaccination [N (%)]
According to the analysis of the reasons for refusing vaccination in the questionnaire, the main reason for cancer patients to refuse vaccination was fear of the side effects, followed by doubts about the effectiveness of the vaccine. The main reason for the patient’s family members to refuse the vaccine is doubts about its effectiveness.
Therefore, it is necessary to carry out clinical trials for cancer patients and obtain test data to answer the concerns of cancer patients.
Conclusions
Cancer patients should be vaccinated reasonably under the guidance of their doctors taking their own treatment plans into account, so as to effectively prevent and control the corresponding infections and improve the quality of life of the patients. In theory, cancer patients can be vaccinated, but there are exceptions. For cancer patients who are receiving or have just finished radiotherapy and chemotherapy, targeted therapy, stem cell transplantation or immunosuppressive therapy, or are in an acute response, it is best for them not to get the COVID-19 vaccine. For patients who have recovered and do not need treatment in the short term, and the cancer is not active, they should consider vaccinating with an approved vaccine with corresponding indications.
If cancer patients do have contraindications to vaccination, we can use the “cocoon” protection strategy.Citation44 The “cocoon” protection strategy means that people around the susceptible (such as family members, friends, colleagues, healthcare providers, etc.) should be vaccinated to gain immunity to diseases. Susceptible people have no access to the source of infection and are protected from pathogen infection like in a cocoon. In addition, during the pandemic, cancer patients still need to adopt physical protective measures after being vaccinated, such as wearing masks, hand disinfection and other hygiene measures.
After cancer patients are vaccinated, because of the particularity of cancer, the side effects of vaccination may be more serious than healthy people. It is still unknown whether there are potential adverse reactions after vaccination, what is the impact on the long-term clinical outcome of tumors, and if there is impact between vaccines and cancer treatments. Long-term close observation and follow-ups are still in need.
There were some limitations of this study. The survey adopted convenience sampling instead of random sampling, which may affect the representativeness of research samples. Only vaccines announced by the time of writing were listed, as potentially more vaccines are being developed. With the changes in the global COVID-19 pandemic and the continuous development of vaccines, the population awareness and demand for vaccines will continue to change. The survey here cannot represent the acceptance of vaccines in different periods of the pandemic.
Since cancer patient has a higher risk of being infected with SARS-CoV-2 and a worse prognosis, the COVID-19 vaccine can provide cancer patients with social and medical benefits. In view of the safety and immunogenicity results of recent multi-population COVID-19 vaccine clinical trials, it is believed that it is necessary and urgent to carry out more detailed phase III clinical trials for cancer patients at various treatment stages.
Disclosure of potential conflicts of interest
All other authors declare no competing interests.
References
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–37. doi:10.1016/S1470-2045(20)30096-6.
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, Lee GM, Talbot HK, Romero JR, Oliver SE, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857–59. doi:10.15585/mmwr.mm6949e1.
- International Agency for Research on Cancer. Global Cancer Observatory.
- Nabioullin R, Sone S, Mizuno K, Yano S, Nishioka Y, Haku T, Ogura T. Interleukin-10 is a potent inhibitor of tumor cytotoxicity by human monocytes and alveolar macrophages. J Leukoc Biol. 1994;55(4):437–42. doi:10.1002/jlb.55.4.437.
- Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–91. doi:10.1158/2159-8290.CD-20-0422.
- Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Perez-Perez M, Lopez-Alfonso A, Gullón P, Lara MÁ. COVID-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22. doi:10.1016/j.lungcan.2020.05.034.
- Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Disc. 2020;10(7):935–41. doi:10.1158/2159-8290.CD-20-0516.
- Rubio MC, Sanchez L, Abreu-Ruíz G, Bermejo-Bencomo W, Crombet T, Lage A. COVID-19 and cancer in Cuba. Sem Oncol. 2020;47:328–29. doi:10.1053/j.seminoncol.2020.07.009.
- Meng Y, Lu W, Guo E, Liu J, Yang B, Wu P, Lin S, Peng T, Fu Y, Li F, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75. doi:10.1186/s13045-020-00907-0.
- Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2020 Dec 10. doi:10.1001/jamaoncol.2019.5367.
- Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev. 2020;100775. doi:10.1016/j.blre.2020.100775.
- Rüthrich MM, Giessen-Jung C, Borgmann S, Classen AY, Dolff S, Grüner B, Hanses F, Isberner N, Köhler P, Lanznaster J, et al. COVID-19 in cancer patients: clinical characteristics and outcome—an analysis of the LEOSS registry. Ann Hematol. 2021;100:383–93. doi:10.1007/s00277-020-04328-4.
- Lunski MJ, Burton J, Tawagi K, Maslov D, Simenson V, Barr D, Yuan H, Johnson D, Matrana M, Cole J, et al. Multivariate mortality analyses in COVID‐19: comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2020;127:266–74. doi:10.1002/cncr.33243.
- Brar G, Pinheiro LC, Shusterman M, Swed B, Reshetnyak E, Soroka O. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. JCO. 2020;38(33):3914–24. doi:10.1200/JCO.20.01580.
- Cattaneo C, Daffini R, Pagani C, Salvetti M, Mancini V, Borlenghi E, D’Adda M, Oberti M, Paini A, De Ciuceis C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126(23):5069–76. doi:10.1002/cncr.33160.
- Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi:10.1016/S1470-2045(20)30309-0.
- Sng CCT, Wong YNS, Wu A, Ottaviani D, Chopra N, Galazi M, Benafif S, Soosaipillai G, Roylance R, Lee AJX, et al. Cancer history and systemic anti-cancer therapy independently predict COVID-19 mortality: a UK tertiary hospital experience. Front Oncol. 2020;10:595804. doi:10.3389/fonc.2020.595804.
- Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S. COVID-19 and cancer: from basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol. 2021 Jan;90:107247. doi:10.1016/j.intimp.2020.107247.
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–88. doi:10.1038/s41586-020-2607-z.
- Draft landscape of COVID-19 candidate vaccines. [accessed 2021 Jan 15]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:583077. doi:10.3389/fimmu.2020.583077.
- Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi:10.3389/fimmu.2019.00594.
- Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–21. doi:10.1038/sj.gt.3302213.
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization. Silver Spring (MD): US Department of Health and Human Services, Food and Drug Administration; 2020.
- Beyer WEP, Palache AM, de Jong JC, Osterhaus ADME. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002 Jan 31;20(9–10):1340–53. doi:10.1016/S0264-410X(01)00471-6.
- Marian AJ. Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries. Cardiovasc Pathol. 2021 Jan-Feb;50:107278. doi:10.1016/j.carpath.2020.107278.
- Brest P, Refae S, Mograbi B, Hofman P, Milano G. Host polymorphisms may impact SARS-CoV-2 infectivity. Trends Genet. 2020;36:813–15. doi:10.1016/j.tig.2020.08.003.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of aphase 1/2, single-blind, randomised controlled trial. Lancet (London, England). 2020;396:467–78. doi:10.1016/S0140-6736(20)31604-4.
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html .
- Passwell J, Levanon M, Davidsohn J, Ramot B. Monocyte PGE2 secretion in Hodgkin’s disease and its relation to decreased cellular immunity. Clin Exp Immunol. 1983 Jan;51(1):61–68.
- Poland GA. Influenza vaccine failure: failure to protect or failure to understand? Expert Rev Vaccines. 2018;17(6):495–502. doi:10.1080/14760584.2018.1484284.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2: preliminary report. N Engl J Med. 2020;383:1920–31. First data for the mRNA-1273 vaccine demonstrating safety, reactogenicity and immunogenicity of this platform in 18–55 year old adults.. doi:10.1056/NEJMoa2022483.
- Vaccines and Related Biological Products Advisory Committee. Vaccines and Related Biological Products Advisory Committee December 10, 2020, meeting: sponsor briefing document. Silver Spring (MD): US Department of Health and Human Services, Food and Drug Administration; 2020.
- Hosoda T, Yokoyama A, Yoneda M, Yamamoto R, Ohashi K, Kagoo T, Ueno H, Boku S, Yano T. Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54(6):1327–28. doi:10.3109/10428194.2012.739285.
- Nakashima K, Aoshima M, Ohfuji S, Suzuki K, Katsurada M, Katsurada N, Misawa M, Otsuka Y, Kondo K, Hirota Y. Immunogenicity of trivalent influenza vaccine in patients with lung cancer undergoing anticancer chemotherapy. Hum VaccinImmunother. 2017;13(3):543–50. doi:10.1080/21645515.2016.1246094.
- Anderson H, Petrie K, Berrisford C, Charlett A, Thatcher N, Zambon M. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999;80:219–20. doi:10.1038/sj.bjc.6690342.
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100.
- Wolchok JD, Rollin L, Larkin J. Nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:2503–04. doi:10.1056/NEJMoa1709684.
- Postow MA, Hellmann MD. Adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:1165. doi:10.1056/NEJMra1703481.
- Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70(2):193‐199. doi:10.1093/cid/ciz202.
- Joona TB, Digkas E, Wennstig AK, Nyström K, Nearchou A, Nilsson C, Pauksens K, Valachis A. Influenza vaccination in breast cancer patients during subcutaneous trastuzumab in adjuvant setting. Breast Cancer Res Treat. 2020 Nov;184(1):45–52. doi:10.1007/s10549-020-05815-y.
- Sodhi JS, Raja W, Zargar SA, Showkat A, Parveen S, Nisar S, Wani MA, Javid G, Khan M, Aejaz S, et al. The efficacy of accelerated, multiple, double-dose hepatitis B vaccine against hepatitis B virus infection in cancer patients receiving chemotherapy. Indian J Gastroenterol. 2015 Sep;34(5):372–79. doi:10.1007/s12664-015-0595-y.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8. doi:10.1016/j.cell.2020.02.052.
- Bitsor M, Galanakis E. Vaccine-preventable infection morbidity of patients with chronic kidney disease and cocoon vaccination strategies. Expert Rev Vaccines. 2015;14(10):1385–95. doi:10.1586/14760584.2015.1075397.
