ABSTRACT
This article describes a patient who developed Harada disease-like uveitis after quadrivalent human papillomavirus (HPV4) vaccination and experienced resolution without any systemic treatment. To achieve this aim, a case report and a review of related literature on HPV vaccination causing uveitis were conducted. The results of this study show a diagnosis of Harada disease-like uveitis after HPV4 vaccination based on the vaccination history, clinical symptoms, and multimodal imaging. Resolution without any systemic corticosteroid treatment was observed. According to these findings, patients may develop uveitis with Harada-like features, which can be a mild and self-limiting process, after HPV4 vaccination.
Introduction
Harada disease is a bilateral posterior uveitis characterized by optic disc swelling, macular edema, and retinal exudation. When Harada disease is combined with Vogt-Koyanagi disease, a clinical variation that mainly involves anterior segment inflammation, this results in Vogt-Koyanagi-Harada (VKH) disease.Citation1 VKH appears to be more common in Asians and Hispanics.Citation2 Although VKH is an autoimmune uveitis, virusesCitation3 and vaccinesCitation4,Citation5 are reported to be potential triggers of this disease. Previous studies have shown that some patients experience blurred vision after HPV vaccination due to uveitis and later undergo systemic treatment.Citation6
This case report describes a patient who developed Harada disease-like uveitis after HPV4 vaccination and experienced resolution without systemic corticosteroid treatment. Such an incident has never been reported previously.
Case report
A previously healthy 37-year-old woman had a five-day history of acute-onset blurred vision and foreign body sensation without any extraocular symptoms such as headache, dizziness, stiff neck, or tinnitus. The patient had received her third injection of the HPV4 vaccine (Gardasil; Merck, Kenilworth, NJ) ten days prior and denied a history of penetrating ocular trauma or intraocular surgery. Best-corrected visual acuity (BCVA) was 20/25 OU (both eyes), and her intraocular pressure (IOP) was 18 mmHg OU. Conjunctival chemosis and mild hyperemia were observed OU, without anterior chamber cells, flares, or haze. Moreover, there was no keratic precipitate. Dilated fundus examination revealed radial retinal wrinkles surrounding the optic disc and fovea OU (). Optical coherence tomography (OCT) likewise revealed these radial retinal wrinkles (). Fluorescein angiography (FA) demonstrated optic disc swelling and macular edema OU (). B-Scan ultrasonography showed diffuse choroidal thickening and slight choroidal detachment OU. OCT angiography (OCTA) showed diffuse ischemia per se on choriocapillaris OU (). The choroidal thickness of the subfovea increased to 519 μm OD (right eye) and 608 μm OS (left eye) (). HLA-DR4 and cerebrospinal fluid pleocytosis were not tested. Based on the symptoms, multimodal imaging examination, and vaccination history, the patient was diagnosed with Harada disease-like uveitis after HPV4 vaccination.
Figure 1. (a–b) Wide-field fundus photographs showed radial retinal wrinkles (white arrows) surrounding the optic disc and fovea OU at presentation (C-D), which disappeared one month after presentation.
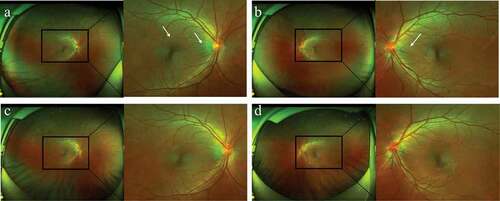
Figure 2. (a–b) OCT also showed radial retinal wrinkles (white arrows) at presentation, (c–d) which then disappeared one month after presentation. (e–f) FA revealed optic disc swelling (white arrows) and macular edema OU at presentation.
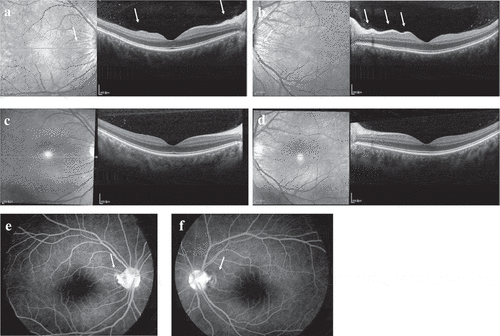
Figure 3. (a–b) OCTA showed diffuse ischemia per se (white arrows) on the choriocapillaris OU at presentation. (c–d) The choroidal thickness of the subfovea increased to 519 μm OD and 608 μm OS at presentation and (e–f) then decreased to 352 μm OD and 365 μm OS three months later.
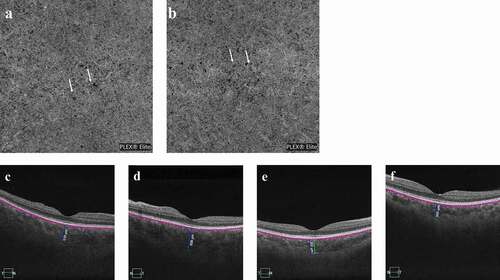
Based on this diagnosis, oral prednisolone and periocular injection of methylprednisolone were recommended to the patient. However, she refused due to concern about the adverse reactions caused by systemic corticosteroids. Prednisolone acetate ophthalmic suspension 1% (Pred Forte® Allergan Pharmacueticals, Ireland) was given three times per day. The patient’s vision remarkably improved three days later; however, she ceased the treatment without authorization. BCVA improved to 20/20 OU one week later. At one month after presentation, the bilateral retinal wrinkles had almost disappeared (). Three months after presentation, the choroidal thickness was reduced to 352 μm OD and 365 μm OS (). All these presentations suggest that the patient recovered without systemic corticosteroids.
Discussion
Human papillomavirus vaccines are administered to an increasing number of women worldwide to prevent infection by high-risk subtypes of human papillomavirus, which are risk factors for cervical cancer and five other cancers.Citation7 Although the effectiveness and safety of these vaccines have been proven,Citation8 the adverse ocular effects related to uveitis due to HPV vaccination that have been recorded in recent decades are a growing concern to ophthalmologists.
Many cases illustrate this risk. A 17-year-old female experienced bilateral vision loss (BCVA: 20/60 OD, 20/100 OS) three weeks after HPV4 vaccination. She was diagnosed with ampiginous choroiditis. Thereafter, oral prednisone 1 mg/kg/day was initiated and taped off within three months. Because of the macular scar that remained, the BCVA only improved to 20/50 OD and 20/60 OS.Citation6 In another instance, a 20-year-old female developed bilateral vision loss (BCVA: 20/60 OU) and panuveitis with exudative retinal detachments after her third HPV4 vaccination. She was placed on oral prednisone 60 mg/day for two weeks and then was tapered off within six weeks. BCVA improved to 20/20 OU and 20/25 OD after five months.Citation9 In addition, a case of posterior uveitis after HPV2 vaccination was reported in a 29-year-old female. This patient was treated with oral prednisone, as well as Ozurdex intravitreal injection.Citation10 These patients developed acute uveitis after HPV vaccination and showed serious symptoms without a tendency toward improvement; thus, they were treated with a relatively long-term administration of oral prednisone.
Although the precise etiology and pathogenesis are ambiguous, researchers have pointed out that the molecular mimicry-induced inflammatory autoimmune response may be a reasonable explanation.Citation1 In this hypothesis, some components of the HPV4 vaccine may turn into antigens that can mimic self-proteins recognized by human leukocyte antigen and later trigger an inflammatory autoimmune response in the choroid that leads to uveitis with Harada-like features. In this case, the temporal correlation of the disease onset and the third injection of the HPV4 vaccine suggests a causal, rather than coincidental, relationship of the factors. Another case of uveitis ten days after yellow fever vaccination also showed a similar timing relationship.Citation4
The present patient experienced acute bilateral visual deterioration ten days after her third injection of the HPV4 vaccine without any prodromal symptoms or extraocular features. Multimodal imaging revealed retinal wrinkles, macular edema, optic disc swelling, and diffuse choroidal thickening in both eyes. The patient was treated with topical prednisolone t.i.d. and recovered within a week without recurrence. Although the possibility of coincidental Harada disease with mild symptoms cannot be excluded because of incomplete uveitis-related laboratory tests in the rapid disease process, a diagnosis of Harada disease-like uveitis after HPV4 vaccination was sustained for several reasons. First, this patient did not show any prodromal symptoms, such as headache, dizziness, stiff neck, or tinnitus, and had relatively mild clinical manifestations. Second, the angiographic findings resembled Harada disease but were not as severe as the typical findings. Third, she showed a tendency toward resolution in the short-term clinical course and recovered without systemic corticosteroid treatment, which is the recommended therapeutic strategy for Harada disease. Despite the possibility that topical corticosteroids were enough to lead to resolution, the researchers thought this was unlikely.
In conclusion, patients who are injected with HPV vaccines may develop uveitis with Harada disease-like features. Thus, patients receiving such vaccination must be informed about the potential ocular adverse effects, including Harada disease-like uveitis. They should immediately consult with and proceed to an ophthalmologic clinic if they experience blurred vision. Moreover, ophthalmologists should directly ask female patients with uveitis about their HPV vaccination history to make a definite diagnosis and optimize the treatment based on the severity of symptoms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Du L, Kijlstra A, Yang P. Vogt-Koyanagi-Harada disease: novel insights into pathophysiology, diagnosis and treatment. Prog Retin Eye Res. 2016;52:84–111.
- Greco A, Fusconi M, Gallo A, Turchetta R, Marinelli C, Macri GF, De Virgilio A, De Vincentiis M. Vogt–Koyanagi–Harada syndrome. Autoimmun Rev. 2013;12(11):1033–38. doi:10.1016/j.autrev.2013.01.004.
- Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, Sugamoto Y, Futagami Y, Itoh K, Mochizuki M, et al. Ocular infiltrating CD4+T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Invest Ophthalmol Vis Sci. 2006;47(6):2547–54. doi:10.1167/iovs.05-1547.
- Campos WR, Cenachi SPF, Soares MS, Gonçalves PF, Vasconcelos-Santos DV. Vogt–Koyanagi–Harada-like disease following yellow fever vaccination. Ocul Immunol Inflamm. 2021;29(1):124–27. doi:10.1080/09273948.2019.1661498.
- Sood AB, O’Keefe G, Bui D, Jain N. Vogt-Koyanagi-Harada disease associated with hepatitis B vaccination. Ocul Immunol Inflamm. 2019;27(4):524–27. doi:10.1080/09273948.2018.1483520.
- Khalifa YM, Monahan PM, Acharya NR. Ampiginous choroiditis following quadrivalent human papilloma virus vaccine. Br J Ophthalmol. 2010;94(1):137–39. doi:10.1136/bjo.2009.159293.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi:10.1128/CMR.16.1.1-17.2003.
- Markowitz LE, Naleway AL, Klein NP, Lewis RM, Crane B, Querec TD, Hsiao A, Aukes L, Timbol J, Weinmann S, et al. Human papillomavirus vaccine effectiveness against HPV infection: evaluation of one, two, and three doses. J Infect Dis. 2020;221(6):910–18. doi:10.1093/infdis/jiz555.
- Dansingani KK, Suzuki M, Naysan J, Samson CM, Spaide RF, Fisher YL. Panuveitis with exudative retinal detachments after vaccination against human papilloma virus. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):967–70. doi:10.3928/23258160-20151008-11.
- Ye H, Feng H, Zhao P, Fei P. Case report: posterior uveitis after divalent human papillomavirus vaccination in an asian female. Optom Vis Sci. 2020;97(6):390–94. doi:10.1097/OPX.0000000000001523.
