ABSTRACT
Diphtheria-tetanus-pertussis (DTP) combination vaccines are a cornerstone of infant vaccinations worldwide. DTP vaccine acceptance could be impacted by sub-optimal relationships between parents and healthcare professionals (HCPs). This survey, conducted in France and India between 14/2/2020 and 26/3/2020, aimed to understand perspectives and expectations of parents and HCPs toward DTP vaccination. Participants were parents (parents/guardians of ≤3-year-old children; France: n = 1002, India: n = 1021) and HCPs (general practitioners/pediatricians initiating DTP vaccination; France: n = 300; India: n = 300) who chose to take part. A representative sample of parents was achieved via quotas and random iterative weighting to match key demographics of the target population. In India, only parents from socio-economic classes A/B/C and private HCPs were included. Whilst DTP vaccine acceptance was high among parents in France (85%) and India (98%), French HCPs overestimated parental acceptance (99% thought parents were very/fairly accepting). The proportions of parents reporting that the HCP is someone they trust versus the proportions of HCPs wanting to be seen as trusted were discrepant in France (76% versus 90%) but not India (83% versus 85%). Some surveyed parents indicated that, ideally, they would like some input in vaccine brand decisions alongside HCPs, an opinion shared by some HCPs. In France, short-term experience post-vaccination was more important to parents than HCPs, for whom long-term protection was more important. In India, these aspects were equally important to both. Increased awareness of parents’ priorities and concerns regarding DTP vaccination can support HCPs in their discussions with parents and help build trust, which may impact vaccine acceptance.
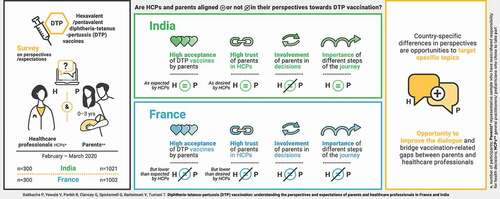
Graphical abstract
Introduction
Multivalent diphtheria-tetanus-pertussis (DTP) combination vaccines are the cornerstone of numerous pediatric immunization programs worldwide.Citation1,Citation2 They contain antigens of up to six major pathogens, including diphtheria, tetanus, pertussis, poliovirus, Haemophilus influenzae type b (Hib) and hepatitis B virus.Citation2 Maintaining a high acceptance and uptake of DTP combination vaccines is paramount, given their benefits in protecting against multiple diseases, reducing the number of injections and simplifying the childhood vaccination calendar.Citation1,Citation2 The main stakeholders in determining vaccine acceptance are parents or guardians of young children and healthcare professionals (HCPs).Citation3,Citation4 It is therefore important to understand the perspectives of both these parties on DTP vaccination. This was explored in the present survey, specifically for pentavalent and hexavalent formulations (containing antigens for five and six pathogens, respectively). So far, most studies have focused on parents’ perspectives on vaccination, with the HCPs’ views reported less frequently and usually in separate surveys.Citation5,Citation6 The World Health Organization (WHO) and the European Center for Disease Prevention and Control highlight the importance of HCPs communicating with caregivers and engaging with local communities to improve vaccine acceptance.Citation7,Citation8 Separate research has shown that HCPs are the most trusted source of information about vaccination in different countries,Citation5 although many HCPs indicate being regularly challenged on vaccine-related issues by their patients.Citation9
To reflect different perspectives based on local contexts, the present survey was conducted in France and India, two countries shown to have different vaccination policies and experience with pertussis-containing vaccines.Citation10–12 Vaccine hesitancy – declared by the WHO as one of the ten greatest threats to global healthCitation13 – has been particularly high in France,Citation11,Citation12,Citation14 leading the French Ministry of Health to extend the number of mandatory childhood vaccines to 11 for children born as of January 1, 2018.Citation15,Citation16 In contrast to France, surveys have shown that parents in India were generally more accepting of childhood vaccines.Citation11,Citation14 Vaccine hesitancy in India was rooted in safety concerns, economic reasons (e.g., household income), religious beliefs and region-specific problems related to access to vaccination services.Citation10,Citation11,Citation17,Citation18 Other vaccination-related differences between France and India relate to the market of available vaccines and reimbursement. Two pentavalent and three hexavalent DTP combination vaccines are currently authorized in France,Citation19 but the hexavalent formulations are included in the mandatory vaccination program and largely reimbursed by health insurance funds (public or private).Citation20–23 The hexavalent DTP vaccines used in France (licensed between 2000 and 2016) contain acellular pertussis (DTPa) antigens (two or more, depending on the formulation) and differ by the need for reconstitution of the Hib component. In India, both DTPa and whole-cell pertussis (DTPw) formulations of pentavalent and hexavalent vaccines are available. The pentavalent vaccines are freely available through the universal immunization program,Citation24 while hexavalent vaccines have only become available within the last 4 years on the private market and need to be paid in full by the parents.Citation25
While vaccination attitudes and policies differ in some respects between France and India, the existing similarities allow for relevant comparisons, which could reveal market-specific complexities of vaccine acceptance. DTP immunization is part of (routine) childhood vaccination programs in both countries and two out of three hexavalent DTP vaccines available in each country are the same. Also, safety-related concerns are causes of vaccine hesitancy in both France and India. Surveying relevant stakeholders in both countries in the same, standardized way could provide detailed and region-specific insights into factors relevant for vaccine acceptance.
The aim of the present survey was to understand the perspectives and expectations of parents versus HCPs towards DTP vaccination. An increased awareness among HCPs of the parents’ main concerns and priorities, and how these differ from their own, may improve dialogue, build trust and help support the child’s vaccination experience.
Methods
Study design
This survey was conducted between February 14 and March 26, 2020 in France and India among parents or guardians of ≤3-year-old children and HCPs initiating DTP vaccination. The sample of parents or guardians (further referred to as “parents”) included individuals who lived with the child for all or most of the time and assumed main or shared responsibility for health-related decisions of the child (including foster parents, adoptive parents and stepparents). Surveyed HCPs who chose to take part in the survey were pediatricians/children’s doctors and general practitioners/family doctors (GPs; in France only) who are involved in the initiation of children onto hexavalent (and pentavalent in India) DTP combination vaccines and are often involved in the decision on which specific vaccine is given. In India, only pediatricians/children’s doctors working in private practices were surveyed.
In France, the survey was conducted in French among HCPs and parents. In India, it was conducted in English among HCPs and in one of seven local languages or English among parents. The local languages used were Hindi, Bengali, Oriya, Marathi, Gujarati, Tamil and Telugu, as appropriate according to the geographic location. For HCPs in both countries and parents in France, the survey was conducted online. In India, interviews with parents were carried out face-to-face.
Details on the questions asked to HCPs and parents are provided in the Supplementary material. Question numbers are included in the tables and figure footnotes.
Parents of more than one child aged ≤3 years were instructed to answer questions referring to their youngest child. Multiple entries by the same participant were prevented using standard research practices. In France, both parents and HCPs were asked to consider hexavalent DTP vaccines only (as they are mandatory), whereas in India HCPs were predominantly asked to consider hexavalent DTP vaccines, but parents were asked to consider DTP vaccines more broadly, encompassing both pentavalent and hexavalent vaccines. While both DTPa and DTPw vaccines are available in India, the survey questions for Indian participants were designed to consider these formulations within the entire DTP vaccine spectrum rather than in separate categories, as parents were unlikely to recall or be aware of these differences in formulations. This approach allowed for the comparison of data collected from Indian parents and HCPs.
The survey was conducted by Ipsos MORI on behalf of GSK, in compliance with guidelines from the Market Research Society (MRS) and the Market Research Society of India (MRSI), the European Pharmaceutical Market Research Association and the European Society for Opinion and Marketing Research. Personal data and responses were protected under the MRS/MRSI Code of Conduct and all applicable laws. The study was granted exemption from ethical approval by the Pearl Independent Institutional Review Board (Protocol #20-IPSO-132). The survey started with a brief introduction including an explanation of the aims, data protection and confidentiality, after which consent to participate was obtained.
Ipsos MORI conducted a pilot study among two parents and three HCPs in France (online) and five parents in India (face-to-face) to test the survey materials. On completion of the pilot study, minor modifications and enhancements were made to the design and content of the questionnaire (see Supplementary material for more information).
Sample selection
In France, a representative quota sample of 1002 parents from proprietary consumer online panels were interviewed online with quotas set on geographic region, age, gender and occupation. In India, a representative quota sample of 1021 parents with an approximately equal mix of parents in socio-economic classes (SEC) A, B and C were interviewed face-to-face across all zones of India (Metro, Tier 1–3 cities and rural areas near each city) with quotas set on geographic region, age and gender. In both France and India, a sample of 300 HCPs chose to take part in the survey. To ensure a good cross-section of HCPs were interviewed online, quotas were set on region.
Data analysis and statistical methods
Only participants who completed the whole survey were included in the analysis. All data were analyzed using quantitative analysis techniques (e.g., frequencies, mean scores). For parents, the final data were weighted to the true population proportions of this target audience. Random iterative method (RIM) weighting was conducted where quotas could not be met and enabled us to weight multiple target characteristics simultaneously. This approach closely matched the sample with the target population on all dimensions by using an algorithm that distorts each variable as little as possible. RIM weighting is useful when the target distributions within interlocking categories of population characteristics are not known. Weighting efficiencies of >90% were observed for all quotas, except for the occupation quota in France for which the weighting efficiency was 66%. Inferential statistical t-tests or z-tests with 95% confidence levels (p < .05) for each test were used to determine if there were notable differences between the parents’ and HCPs’ responses within each country. Such notable differences refer to results from statistical significance testing applied to samples that may not be representative, and although they are likely meaningful, they might not be conclusive.
Results
Sampling and response rates
In France, 25180 consumer online panel members were contacted for the survey, 6295 responded and 1002 parents fit the criteria and completed the survey. In India, 4859 parents were approached, of whom 2351 entered the survey and 1021 fit the criteria and completed the survey. Of the parents who entered, 161 in France partially completed the survey and 1330 in India either did not fit the criteria or partially completed the survey. Of the 505 HCPs contacted in France, 389 responded to the survey and 300 fit the criteria and completed the survey. In India, 757 HCPs were contacted, 477 responded to the survey and 300 fit the criteria and completed the survey. Among the 300 HCPs in France, 165 were GPs and 135 pediatricians. In India, all HCPs were pediatricians working in private practice. A total of 46 HCPs in France and 134 HCPs in India only partially completed the survey.
Characteristics of parents and HCPs are shown in , respectively.
Table 1. Characteristics of parentsa participating in the study
Table 2. Characteristics of HCPs participating in the study
Vaccination acceptance
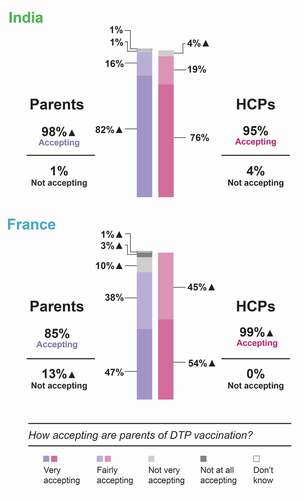
Surveyed HCPs estimated a high DTP combination vaccine acceptance among parents in France and India, with 99% and 95% of HCPs, respectively, considering that parents were very/fairly accepting toward these vaccines (). In India, the parents’ acceptance of DTP combination vaccines that their child had or could have was comparable to how accepting HCPs considered parents to be (98% of parents were very/fairly accepting). In France, however, the surveyed parents’ acceptance was lower than that estimated by HCPs (85% of parents were very/fairly accepting). In total, 13% of surveyed French parents answered that they were not very (10%) or not at all (3%) accepting of DTP vaccination for their children ().
Figure 1. Vaccination acceptance among Indian and French parents and HCPs’ perceptions of vaccination acceptance among parents (general practitioners and pediatricians in France and pediatricians in India).
Most surveyed HCPs (88% in France and 89% in India) described themselves as being strong advocates/advocates of pediatric vaccines who often/sometimes recommend vaccines outside the French national vaccination schedule or Indian universal immunization program.
Trends in trust levels between parents and HCPs
The survey investigated whether parents perceive the HCPs who prescribe/administer DTP combination vaccines as someone they can trust and how important the HCPs regarded these perceptions. In both countries, a high proportion of HCPs reported that, when talking to parents about hexavalent DTP combination vaccination, they want to be seen as a person the parents can trust (90% in France and 85% in India strongly agreed/tended to agree with this statement; ). In France, the proportion of HCPs wanting to be seen as someone parents trust was higher than the proportion of parents agreeing that the HCP who is prescribing/administering the DTP combination vaccine is someone they trust (90% versus 76% strongly agreed/tended to agree). In India, the proportion of HCPs who want to be seen as someone parents trust was comparable to the level of trust in HCPs reported by the parents (85% versus 83% strongly agreed/tended to agree; ). Of note, 5% of French and 12% of Indian parents tended to disagree/strongly disagreed that the HCP prescribing/administering the DTP combination vaccine is a person they trust. Furthermore, 17% of parents in France and 5% of parents in India neither agreed nor disagreed with this statement.
Figure 2. Perception of parental trust in HCPs among parents and HCPs (a) and HCP attitudes toward how they are perceived (b).
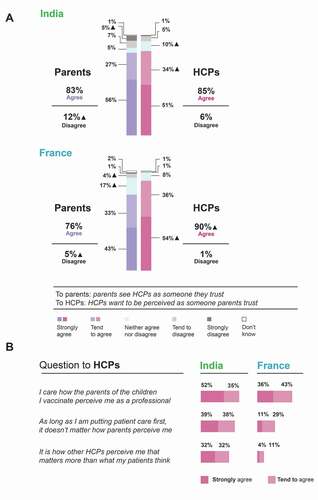
The survey also addressed how much importance the HCPs assigned to how parents and other HCPs perceive them (). In France, a high proportion of HCPs expressed that they care how parents perceive them as a professional (almost 80% strongly agreed/tended to agree) and only a minority (15%) strongly agreed/tended to agree that how other HCPs perceive them matters more than what parents think. A different trend was observed in India, where also many HCPs expressed they care how parents perceive them as a professional (87% strongly agreed/tended to agree) but 64% strongly agreed/tended to agree that other HCPs’ perceptions matter more than what parents think ().
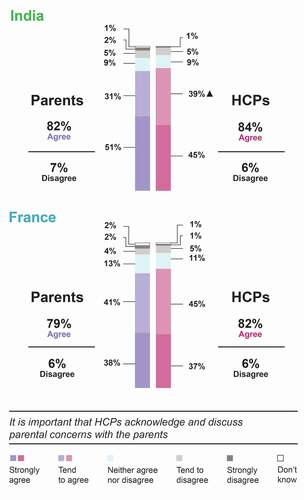
Similar proportions of parents and HCPs in France (79% and 82%, respectively) and India (82% and 84%, respectively), strongly agreed/tended to agree that it is important for HCPs to acknowledge that some parents have concerns about this vaccination and to discuss these concerns with them (). Of note, HCPs were specifically asked to consider hexavalent DTP vaccination whereas parents answered more generally.
Figure 3. Agreement of parents and HCPs on the importance of acknowledging and discussing parental concerns.
Priorities of parents and HCPs in the vaccination “journey”
We listed the different steps taken by parents and HCPs in the vaccination of a child with a DTP combination vaccine in a chronological sequence we called vaccination “journey” (). Of note, the involvement of parents in this journey differs between India and France. In France, the HCP typically prescribes the vaccine during a first visit after which the parent buys and stores the vaccine until a second visit when the vaccine is administered. In India, in most cases, the vaccine is present at the HCP’s office (prescribed/administered during the same visit) and therefore does not have to be bought and stored by the parents. We explored the importance of different steps of the vaccination journey for parents and HCPs.
Figure 4. Importance of different stages of the vaccination journey for parents and HCPs (proportion of participants rating the stages as essential or very important) in India (a) and France (b).
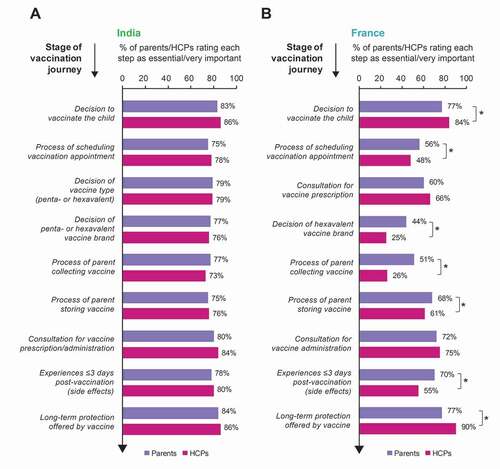
In France, there were discrepancies between parents and HCPs in the relative importance attributed to these different steps (). Moreover, the levels of importance assigned by both parties varied widely between the steps: the proportions of participants rating the different steps as essential/very important ranged from 25% to 90% (). In contrast, the opinions of Indian parents showed overall agreement with those of Indian HCPs, with little divergence between which steps of the journey were rated as more or less important: all steps were ranked as essential/very important by high proportions (73%–86%) of Indian parents and HCPs ().
While both parents and HCPs surveyed in France considered the decision of whether or not to vaccinate and long-term protection throughout the remainder of childhood and beyond as important steps in the vaccination journey, the percentage of HCPs who considered these as essential/very important was higher (84% and 90%) than the percentage of parents with the same opinion (77% for each). By contrast, a higher proportion of parents than HCPs regarded the process by which the parent collects the vaccine prescription (51% parents, 26% HCPs) and the short-term experience (i.e., any crying, irritability, fever, redness) in the first three days when the child returns home after vaccination (70% parents, 55% HCPs) to be essential/very important. Furthermore, in contrast to only 25% of surveyed HCPs, 44% of French parents found the decision of which brand of DTP vaccine the child receives essential/very important (). However, compared to all other steps in the vaccination journey, vaccine brand choice was rated as essential/very important by the lowest proportion of parents and HCPs.
Involvement of parents in vaccine brand choice
Parents were asked if they were aware or not aware that there are different types of DTP combination vaccines (penta- and hexavalent) that protect against different numbers of diseases and different brands made by different companies. HCPs were asked to estimate in what proportion of the cases parents were generally aware that there are different types of DTP combination vaccines and different brands of hexavalent vaccines. HCPs in India underestimated parents’ awareness that there are different DTP vaccine types (HCPs estimated that in 32% cases parents were aware, while 44% of parents claimed to be aware) and correctly estimated awareness of hexavalent brands (HCPs estimated that in 27% of cases parents were aware, while 26% of parents claimed to be aware). By contrast, the French HCPs underestimated parents’ vaccine type and brand awareness by almost two-fold or more (HCPs estimated that only 28% and 15% of parents were aware of types and brands, respectively, while 51% and 46% of parents claimed to be aware; ).
Figure 5. Parent awareness of vaccine types and brands according to parents and HCPs (a) and involvement of parents in vaccine brand discussions (b).
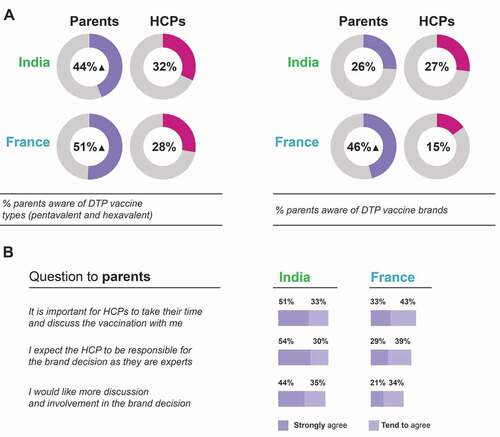
While many surveyed parents (68% in France, 84% in India) strongly agreed/tended to agree that the HCPs should be responsible for the DTP vaccine brand decision, 55% of French and 79% of Indian parents would like more discussion and involvement in this decision (). Furthermore, over 75% of surveyed parents in each country strongly agreed/tended to agree that it is important for HCPs to take their time to discuss DTP combination vaccination (). According to the survey and focusing on those parents whose child received the DTP vaccination, vaccine brands are infrequently discussed in consultations: only 22% of parents in France and 29% of parents in India stated that they recalled DTP vaccine brands being discussed before the vaccine was administered ().
Figure 6. Frequency of vaccine brand discussions (A) and current versus ideal distribution of responsibility for vaccine brand decision between parents and HCPs (B).
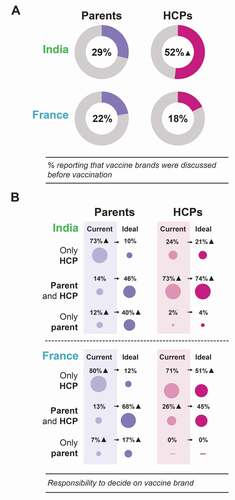
In France and India, parents reported that their involvement in DTP vaccine brand choice was lower than desired. Of the surveyed parents whose child had received the DTP vaccination, 68% (France) and 46% (India) indicated that, in an ideal world, they wish to have input in the DTP vaccine brand decision alongside the HCP, while 17% (France) and 40% (India) would prefer the decision of which brand is administered to their child to be entirely their own. Among French HCPs, only 45% agreed with parents having some input, while in India, 74% of HCPs agreed with this (). None of the surveyed HCPs in France and 4% in India thought that the choice of hexavalent vaccine brand should be the entire responsibility of the parents ().
Priorities of parents versus HCPs when choosing the vaccine brand
An extensive list of vaccine characteristics was tested with participating parents and HCPs, to understand which elements were considered as having priority in the choice of a vaccine for the two groups. The vaccine characteristics were defined as factors that HCPs may or may not consider when choosing a DTP vaccine brand.
For some factors, there was alignment between parents’ and HCPs’ responses. For example, in both countries, ≥66% of parents and HCPs rated vaccine-intrinsic factors related to safety, tolerability and effectiveness as essential/very important for an HCP to consider when choosing a DTP vaccine brand (). Likewise, a vaccine that has been used in many children for a long period of time, going beyond clinical trials with limited numbers of children was considered essential/very important for HCPs to consider in DTP vaccine choice in ≥74% of parents and HCPs in both countries. Lower vaccine cost to the parent was surveyed as a factor in DTP vaccine brand choice in India only, where it was of similarly high importance for both parents (80%) and HCPs (77%) ().
Figure 7. The importance of different aspects that an HCP may or may not consider when choosing a brand of DTP combination vaccine according to parents and HCPs in India and France.
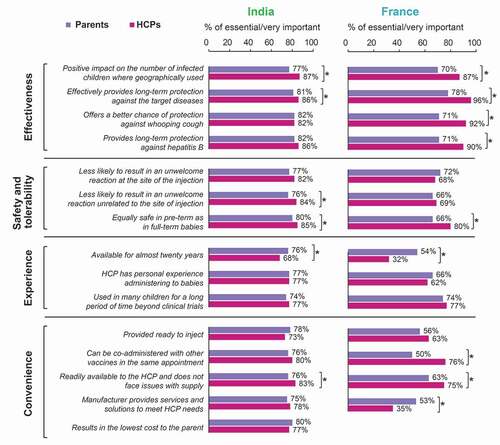
However, discrepancies were also observed between the parents’ and HCPs’ responses. For instance, while effectiveness in providing long-term protection against the diseases for which it is intended and impact on the number of children contracting the diseases were highly relevant for both parents and HCPs, a higher proportion of HCPs than parents valued these factors as important (). This was especially true in France, where a higher proportion of HCPs than parents also rated better chance of protection against whooping cough and long-term protection against hepatitis B as essential/very important when HCPs choose the DTP vaccine brand (). Notably, 74% of HCPs versus 40% of parents in France chose effectiveness in providing long-term protection against the diseases for which it is intended as one of the top 3 things that HCPs should consider when making a DTP vaccine brand choice (Figure S1).
A higher proportion of French parents (34%) reported vaccine use in many children for a long period of time going beyond clinical trials with limited numbers of children as one of the top 3 determinants when HCPs make a DTP vaccine brand choice compared to all other surveyed groups (26% of French HCPs, 17% of Indian parents and 13% of Indian HCPs; Figure S1). In France and in India, higher proportions of parents than HCPs rated availability of the DTP vaccine for almost 20 years as an essential or very important factor ().
Higher proportions of HCPs than parents regarded the vaccine being readily available and not facing issues with supply (France and India) and being given at the same time as other vaccines for other diseases in the same appointment (France) as essential/very important when choosing a DTP vaccine brand ().
Discussion
DTP combination vaccines are among the first vaccines administered to infants. The experience with this vaccination can act as a blueprint for all further vaccinations and may help build trust between the parent and the HCP. The aim of this Ipsos MORI survey was to help understand the perspectives of parents related to DTP vaccination and identify parallels and differences between parents and HCPs. A better awareness of these points may help HCPs invest the limited appointment time in discussing the aspects that are most important for parents. The survey focused on two culturally and economically different countries: France and India. The results showed that the overall parental acceptance of DTP combination vaccines was high in France and India but was overestimated by the French HCPs. Likewise, trust in HCPs was high among parents in both countries but could still be improved in France. While parents and HCPs in India rated each step of the vaccination journey as similarly important, discrepancies were seen between the two parties in France. Some surveyed parents in both countries indicated that, ideally, they wish to have input in the vaccine brand decision and differences were seen between parents and HCPs in the factors they deemed important for HCPs to consider when choosing the vaccine brand.
A separate survey of 1500 mothers of 0–17-month-old children in France showed that the proportion of mothers in favor of vaccination increased during the first 2 years (2018 and 2019) after implementation of the mandatory pediatric vaccination extension in France compared to the previous years.Citation26 This was accompanied by an increase in uptake of vaccines with suboptimal coverage.Citation26 A large-scale retrospective analysis on data from 290 surveys across 149 countries also showed that confidence in the importance, safety and effectiveness of vaccines improved in France between 2015 and 2019.Citation14 These results are in line with the overall high acceptance of DTP combination vaccines among French parents seen in our survey. Acceptance was however lower than in Indian parents. The high DTP vaccine acceptance among Indian parents in the current survey is in agreement with previously published data on high levels of public trust in vaccines as a necessary healthcare measure.Citation14 A possible explanation for the lower vaccine acceptance in French compared to Indian parents could be that the perceived risk of disease is lower in France because parents are no longer (or very rarely) confronted with the infectious diseases these vaccines have successfully prevented. Willingness to vaccinate has indeed been shown to decrease when the risk of infection is lower.Citation27
In previous studies, about a quarter of surveyed French GPs reported they did not find pediatric/adolescent vaccines useful, and their attitudes toward different vaccines varied depending on the amount of information and market experience for each vaccine.Citation28–30 Our survey, in contrast, found that HCP advocacy of pediatric vaccination reached 88% in France and 89% in India, which could reflect a more positive attitude of French HCPs in our survey toward primary series vaccination.
The aforementioned analysis of 290 surveys across 149 countries showed that trusting HCPs more than family, friends or other non-medical sources for health advice was a strong determinant of vaccine uptake.Citation14 Other studies have also shown a positive correlation between trust in HCPs and vaccination of young children,Citation4 underscoring the importance of a trusting relationship. The high proportions of parents who indicated in our survey that they see HCPs as someone they can trust are in line with previous findings, citing physicians as the most reliable and trusted source of vaccine-related information among the general public.Citation5–7,Citation31 However, in France, the proportion of parents agreeing that the HCP prescribing/administering the DTP combination vaccine is someone they trust was lower than the proportion of HCPs wanting to be seen as trusted and nearly one fifth of the parents neither agreed nor disagreed (or had no opinion) that the HCP is someone they trust. There is thus still room for improvement. One possible explanation for the trust gap observed between French parents and HCPs may be that HCPs may not manage to convince the parents of the necessity or benefit of the vaccination. As reported previously, some French HCPs doubt the benefit of vaccination themselves.Citation30 Physicians and nurses from various countries have also already reported that they are not confident in convincing their patients of vaccination if they lack detailed information or consider a vaccine-related topic too controversial.Citation9,Citation30 Less effective communication might erode the trust relationship between patients and HCPs and threaten to decrease vaccine acceptance and uptake.Citation32 These findings highlight the need for intensified and continuous education of vaccine-prescribing and -administering HCPs (both physicians and nurses).
Country-specific differences between France and India were apparent when analyzing how parents and HCPs prioritized different steps of the vaccination journey. In France, a higher proportion of parents than HCPs found short-term experience after vaccination (i.e., the potential occurrence of side effects such as irritability or fever) and the process by which the parent collects and stores the vaccine important, while a higher proportion of HCPs than parents found long-term protection important (although more than three quarters of the parents also considered this as an important part of the vaccination journey). In India, all these aspects were similarly important to parents and HCPs. In accordance with the present Ipsos MORI survey, previous studies have shown that some parents have doubts about vaccine safety and may hesitate to vaccinate their children due to safety concerns.Citation10,Citation11 The fact that parents in France need to buy and store vaccines for their children may explain why a higher proportion of French parents versus HCPs considered vaccine collection and storage as important.
Although rated as important by a low proportion of HCPs and parents, the decision of which vaccine brand the child receives was considered an essential/very important step in the DTP vaccination journey by a higher proportion of French parents than HCPs. This was in line with nearly half of the surveyed French parents claiming to be aware of hexavalent DTP vaccine brands (higher than what HCPs expected) and with the parents’ desire for more involvement in the vaccine brand decision. Some parents and HCPs in India and France seemed to share the opinion that, in an ideal world, parents should be responsible together with the HCP for deciding which DTP vaccine brand is given to the child. By contrast, almost none of the HCPs thought this decision should be entirely the parents’ compared to 17% of French and 40% of Indian parents thinking it should. The voiced wish of some parents to be involved in vaccine brand decisions is a continuation of the trend seen in the last 20 years, with the general public depending on access to reliable and transparent vaccine-related information for high vaccination acceptance.Citation6,Citation7,Citation9,Citation32 However, it may be difficult to accommodate time for discussion and shared decision making in vaccine consultation appointments. Vaccine-administering physicians and nurses from different countries have already reported being pressured to meet the vaccine administration quota for their patients.Citation9,Citation32
As for the vaccine features that HCPs should consider when choosing a DTP combination vaccine brand, the reported parental priorities are in line with the previously observed preference of the general public for using vaccines they are familiar with.Citation10,Citation12,Citation18 In India only, vaccine price was included in the survey as a criterion for vaccine brand choice (because parents have to pay for the vaccine if they opt for a hexavalent instead of pentavalent vaccine) and a similar proportion of Indian parents and HCPs rated it as important, in line with previous findings.Citation9,Citation10 The higher focus of HCPs compared to parents (in France and India) on convenience factors (such as availability/supply and possibility to co-administer) was expected, given the usually high vaccination-related workload of HCPs.Citation9 The higher proportions of HCPs than parents considering factors related to protection as important in DTP vaccine brand choice, especially in France, may be explained by the fact that most diseases these vaccines protect against have become very rare in France and parents, contrary to HCPs, may no longer be aware of their potential severity. HCPs, more than parents, may base their choice on scientific evidence and knowledge, while parents may be more influenced by confidence in the products due to long-term use.
A major strength of the present Ipsos MORI survey is that it looked into similarities and discrepancies between parents and HCPs and explored vaccination-related aspects that may be improved in the future. Furthermore, the participating parents and HCPs were surveyed at the same point in time, using a comparable set of questions. Additionally, the present analysis offers valuable insight into under-examined attitudes specifically toward DTP vaccination.
There were several limitations to this survey. Individual questioning was applied and as social forces are known to substantially impact individual healthcare behaviors,Citation7,Citation8,Citation32 exploring the community context may have added valuable information. Furthermore, the survey did not address the reasons for the lack of trust toward HCPs where this existed. It would have been relevant to know which, if any, sources of healthcare information the parents found they can trust more and why. Finally, response differences between Indian and French parents may be partially attributed to the survey format (face-to-face in India versus online in France) and the focus on the three higher socio-economic classes in India (as their children are the likely recipients of non-reimbursed hexavalent DTP vaccines). Some of the contacted parents and HCPs did not complete the survey. Since the surveys were formulated using inclusive and reassuring wording, the incomplete surveys were likely caused by participants’ lack of time or commitment. The omission of partially completed surveys from the final data set is not expected to bias the survey outcomes, since the sample size was large enough to ensure proper data weighting (for parents) and statistical analysis.
Conclusions
The results of this survey indicate that, while many French and Indian parents and HCPs agree on several vaccine-related issues, there are some discrepancies in their attitudes that highlight possible areas for improvement. Overall, the discrepancies were more prominent in France than in India. While the introduction of mandatory vaccination in France seems to have improved vaccine acceptance compared to what was previously published, this measure should ideally be accompanied by a dialogue between parents and HCPs to help ensure optimal effects. The results of this survey suggest that it may be important to first bridge the trust gap between parents and HCPs in France before a change in attitudes may be observed. The country-specific differences provide opportunities to target specific topics which may improve the dialogue between parents and HCPs.
Disclosure of potential conflicts of interest
ET, RP and VB are employees of the GSK group of companies and declare financial and non-financial relationships and activities. ET, RP and VB hold shares in the company. GS’s and GC’s company received funding from the GSK group of companies to perform the study. VY declares having received personal fees from the GSK group of companies. PB declares no conflicts of interest.
Authors’ contributions
ET, RP and VB: conceptualization, writing – original draft, writing – review and editing; GC and GS: conceptualization, formal analysis, methodology, writing – review and editing; VY: conceptualization, writing – review and editing; PB: writing – review and editing. All authors attest they meet the ICMJE criteria for authorship.
Supplemental Material
Download TIFF Image (2.7 MB)Acknowledgments
The authors thank Rafael Lage for his continued input in the ideation of the project and in the design of the survey and the study participants for their contribution to the study and Lauriane Harrington for her input in the conception of the survey. The authors thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Irena Zurnic Bönisch and Natalie Denef provided medical writing support, Gil Costa designed the figures and Camille Turlure coordinated the manuscript development and editorial support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1961468.
Additional information
Funding
References
- Wang S, Tafalla M, Hanssens L, Dolhain J. A review of Haemophilus influenzae disease in Europe from 2000-2014: challenges, successes and the contribution of hexavalent combination vaccines. Expert Rev Vaccines. 2017;16(11):1095–13. doi:10.1080/14760584.2017.1383157.
- Maman K, Zöllner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother. 2015;11(9):2132–41. doi:10.1080/21645515.2015.1044180.
- Brown KF, Kroll JS, Hudson MJ, Ramsay M, Green J, Long SJ, Vincent CA, Fraser G, Sevdalis N. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine. 2010;28(26):4235–48. doi:10.1016/j.vaccine.2010.04.052.
- Smith LE, Amlot R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35(45):6059–69. doi:10.1016/j.vaccine.2017.09.046.
- Ames HM, Glenton C, Lewin S. Parents’ and informal caregivers’ views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence. Cochrane Database Syst Rev. 2017;2:Cd011787.
- Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014;112:1–11. doi:10.1016/j.socscimed.2014.04.018.
- European Centre for Disease Prevention and Control. Let’s talk about hesitancy. Stockholm (Sweden): ECDC; 2016.
- World Health Organization. Improving vaccination demand and addressing hesitancy. [Internet]; 2020 [accessed 2020 July 7]. https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/ .
- Wiot F, Shirley J, Prugnola A, Di Pasquale A, Philip R. Challenges facing vaccinators in the 21(st) century: results from a focus group qualitative study. Hum Vaccin Immunother. 2019;15(12):2806–15. doi:10.1080/21645515.2019.1621147.
- Agrawal A, Kolhapure S, Di Pasquale A, Rai J, Mathur A. Vaccine hesitancy as a challenge or vaccine confidence as an opportunity for childhood immunisation in India. Infect Dis Ther. 2020;9(3):421–432.
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Rey D, Fressard L, Cortaredona S, Bocquier A, Gautier A, Peretti-Watel P, Verger P; On Behalf Of The Barometre Sante G. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk-benefit balance. Euro Surveill. 2018;23(17):17–00816. doi:10.2807/1560-7917.ES.2018.23.17.17-00816.
- World Health Organization. Ten threats to global health in 2019. [Internet]; [accessed 2020 10 December]. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 .
- de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396(10255):898–908. doi:10.1016/S0140-6736(20)31558-0.
- French Ministry of Solidarity and Health. Décret n° 2018-42 du 25 janvier 2018 relatif à la vaccination obligatoire. 2018.
- Ministère des Solidarités et de la Santé. 11 vaccinations indispensables, obligatoires au 1er janvier 2018. 2018.
- Priya PK, Pathak VK, Giri AK. Vaccination coverage and vaccine hesitancy among vulnerable population of India. Hum Vaccin Immunother. 2020;16(7):1502–1507.
- Sharma S, Akhtar F, Singh RK, Mehra S. Understanding the three As (Awareness, Access, and Acceptability) dimensions of vaccine hesitancy in Odisha, India. Clin Epidemiol Global Health. 2020;8(2):399–403. doi:10.1016/j.cegh.2019.09.010.
- Vaccination Info Service France. Table of existing vaccines in France. [Internet]; [accessed 2020 August 3]. https://vaccination-info-service.fr/Les-vaccins-existants-en-France/Tableau-des-vaccins-existants-en-France .
- Base donnees publique medicaments. [Internet]; 2020 [accessed 2020 October 6]. http://base-donnees-publique.medicaments.gouv.fr/ .
- l’ Assurance maladie. Consultations in metropolitan France: your reimbursements. [Internet]; 2019 [accessed 2020 October 6]. https://www.ameli.fr/yvelines/assure/remboursements/rembourse/consultations/metropole#text_649 .
- Ministère des Solidarités et de la Santé. Calendrier des vaccinations et recommandations vaccinales 2020. 2020.
- Vaccination Info Service France. Reimbursement of vaccines. [Internet]; [accessed 2020 August 3]. https://vaccination-info-service.fr/Questions-frequentes/Questions-pratiques/Remboursement-des-vaccins .
- National Health Portal I. Universal immunisation programme. [Internet]; 2018 [accessed 2020 10 December]. https://www.nhp.gov.in/universal-immunisation-programme_pg .
- Chitkara AJ, Parikh R, Mihalyi A, Kolhapure S. Hexavalent vaccines in India: current status. Indian Pediatr. 2019;56(11):939–50. doi:10.1007/s13312-019-1651-y.
- Cohen R, Martinot A, Gaudelus J, Subtil D, Stahl JP, Pujol P, Picquet V, Lepetit H, Longfier L, Leboucher B. Infant mandatory vaccinations: confirmation of a positive impact. Med Mal Infect. 2020;50(1):74–77. doi:10.1016/j.medmal.2019.11.007.
- Baumgaertner B, Ridenhour BJ, Justwan F, Carlisle JE, Miller CR. Risk of disease and willingness to vaccinate in the United States: a population-based survey. PLoS Med. 2020;17(10):e1003354. doi:10.1371/journal.pmed.1003354.
- Collange F, Fressard L, Pulcini C, Sebbah R, Peretti-Watel P, Verger P. General practitioners’ attitudes and behaviors toward HPV vaccination: a French national survey. Vaccine. 2016;34(6):762–68. doi:10.1016/j.vaccine.2015.12.054.
- Raude J, Fressard L, Gautier A, Pulcini C, Peretti-Watel P, Verger P. Opening the ‘Vaccine Hesitancy’ black box: how trust in institutions affects French GPs’ vaccination practices. Expert Rev Vaccines. 2016;15(7):937–48. doi:10.1080/14760584.2016.1184092.
- Verger P, Fressard L, Collange F, Gautier A, Jestin C, Launay O, Raude J, Pulcini C, Peretti-Watel P. Vaccine hesitancy among general practitioners and its determinants during controversies: a National cross-sectional survey in France. EBioMedicine. 2015;2(8):891–97. doi:10.1016/j.ebiom.2015.06.018.
- Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vaccin Immunother. 2013;9(8):1755–62. doi:10.4161/hv.25085.
- SAGE Working Group. Report of the SAGE Working Group on vaccine hesitancy. 2014.
