ABSTRACT
To control seasonal influenza epidemics in elders, a quadrivalent, inactivated, split-virion influenza vaccine (IIV4) comprising A and B lineages is produced for young individuals and adults aged ≥60 years. In this phase III, randomized, double-blind, active-controlled trial, we compared safety and immunogenicity of IIV4 with a licensed quadrivalent inactivated vaccine (IIV4-HL) produced by Hualan Biological Engineering during the 2019 influenza season. Participants were randomly assigned to receive IIV4 (n = 959) or IIV4-HL (n = 959). Compared to IIV4-HL, geometric mean titers (GMT) of hemagglutination inhibition (HAI) titers and seroconversion rate (SCR) of IIV4 demonstrated better antibody responses in A lineages (H1N1 and H3N2) (P < .01) and equivalent antibody responses in B lineages (B/Yamagata and B/Victoria) (P > .01) in both age groups. After immunization, IIV4 provided a satisfactory SCR and seroprotection rate (SPR) in elders. No discernible variation in immunogenicity was observed between the two age cohorts. In both age groups, IIV4 and IIV4-HL recipients experienced similar levels of solicited and unsolicited adverse events (AEs), and the incidence of AEs was low in both vaccine groups. Most AEs were of mild-to-moderate severity and no grade 3 AEs in IIV4 group, but AEs in adults aged 60–65 were little higher than in adults over 65 years in IIV4 and IIV4-HL groups (IIV4: 14.66% vs. 10.36%; IIV4-HL:14.67% vs. 11.43%). Totally, IIV4 was generally well tolerated and induced high antibody titers against all four influenza strains in elderly, making it a compelling alternative for the elderly aged ≥60 years.
Trial registration: Clinical Trials.gov: 2015L00649-2.
Introduction
If influenza epidemic strains mismatch to the recommended vaccine strains, vaccine protection is reduced, especially for B-lineage.Citation1 Compared with a trivalent vaccine, if influenza vaccines include B lineage strains (Yamagata and Victoria), it is expected to reduce the vaccine’s availability by approximately 25% and reduce the probability of B lineage mismatch, indicating that quadrivalent influenza vaccines are necessary to prevent influenza and reduce vaccine use.Citation2 Each year, World Health Organization (WHO) determines the exact strains of the two influenza A strains (H1N1 and H3N2) and B lineage strains (Yamagata and Victoria) present in inactivated or recombined quadrivalent influenza vaccines based on influenza disease surveillance data from the previous year.Citation3 Several quadrivalent influenza vaccines, including adjuvanted influenza vaccine used in elderly or children, have completed phase III clinical trials and licensed for use or are awaiting approval; these vaccines demonstrated higher tolerability and efficacy.Citation4–6 As the elderly population grows, the demand for suitable influenza vaccines increases.
Older adults are particularly susceptible to severe influenza outcomes, and more than 90% of influenza-related deaths occur in adults over 65 years.Citation7 Meanwhile, influenza accounts for nearly 30% of all disability-adjusted life years lost to infectious disease.Citation8 Influenza vaccines provided substantial protection against influenza A/H1N1 and type B but reduced protection against A/H3N2.Citation9 This is especially true during seasons when A/H3N2 is the predominant circulating strain when dramatic increases in hospitalization rates occur in the population aged 65 and older. The reduced vaccine effectiveness may be associated with an aging immune system,Citation10 ensuring that the protection of quadrivalent influenza vaccines is essential. We have completed phase III trial to evaluate the tolerability and efficacy of IIV4 in adults aged 3–60 years in 2017, and the results demonstrated better safety and immunogenicity. In 2020, IIV4 became available in China for seasonal immunization for adults aged 3–60 years (issuance numbers: 20205325, 20205326, 20205327, and 20205329). In 2019, IIV4-HL produced by Hualan Biological Engineering was the only licensed quadrivalent influenza split vaccine in China (China Drug Approval No.: S20083016),Citation11 with a phase IV clinical Trials.gov Identifier of NCT01511744.Citation12 Meanwhile, IIV4 and IIV4-HL are influenza split vaccines containing two influenza A strains (H1N1 and H3N2) and B lineage strains (Yamagata and Victoria) comparable to each other. We then selected IIV4-HL for a phase III trial to investigate its safety and immunogenicity in the elderly as a candidate influenza vaccine for young adults and the elderly population.
Materials and methods
Subjects and study design
This a phase III, randomized, double-blind, controlled trial comparing IIV4 (lot: 201808004) and licensed IIV4-HL (lot: 201809B033) in the elderly was performed at two centers in China from April 28, 2019 to November 21, 2019 (ClinicalTrials.gov identifier: 2015L00649-2). All participants provided a written informed consent form (ICF) before any trial procedures. Before this study, IIV4 had already completed phase III clinical trial in young adults and proved to have good safety and immunogenicity in 2017 (ClinicalTrials.gov identifier: 2015L00649).
The primary objective was to describe the treatment-emergent adverse event (TEAE) (injection-site and systemic adverse events) of each vaccine during 28 days following vaccination and serious adverse events (SAEs) for six months in all participants. Secondary objectives included evaluating SCRs and geometric mean titers (GMTs) of IIV4 on 28th day after inoculation.
The trial was approved by National Medical Products Administration (NMPA) and monitored by Guangdong Provincial Centers for Disease Control and Prevention (CDC). It was conducted following principles of the Declaration of Helsinki, Good Clinical Practice (GCP) of China, and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
Participants
Healthy subjects aged 60 years or above without influenza vaccination in the previous three years were recruited and stratified by age (60–65 years vs. ≥65 years) and randomly assigned to receive a single dose of either IIV4 or IIV4-HL. They were excluded from study if they: had influenza or flu-like symptoms within three months (fever <armpit temperature ≥38 °C, accompanied by cough or sore throat); had systemic hypersensitivity to any of vaccine components; blood pressure ≥150/100 mmHg after drug control; currently suffer from allergic diseases, such as urticaria and angioedema; suffer from autoimmune disease or immune function deficiency; received immunosuppressive therapy.
Vaccination
IIV4 (quadrivalent, split-virion influenza vaccine) contained 60 μg (15 μg of each strain) of HA antigen produced using standard techniques for inactivating and purifying influenza vaccine strains grown in embryonated chicken eggs. The four influenza vaccine strains were recommended by WHO and approved by NMPA for 2019/2020 season (H1N1 lineage: influenza infectious virus IVR-190 from A/Brisbane/02/2018; H3N2 lineage: NYMC X-327 reassortant derived from A/Kansas/14/2017; B/Victoria lineage: NYMC BX-69A reassortant derived from B/Maryland/15/2016; and B/Yamagata lineage: BVR-1B reassortant derived from B/Phuket/3073/2013). The licensed IIV4-HL (Influenza Vaccine, Split Virion, Inactivated) contains some seasonally circulating influenza A and B strains. IIV4 was provided in a prefilled 0.5-mL vial, and IIV4-HL was provided in syringe according to Pharmacopoeia of the People’s Republic of China 2015 (Volume III) and was administered intramuscularly.
Participants (60–65 years vs. ≥65 years) were randomized 1:1 to receive IIV4 and IIV4-HL. Randomization was performed using the permuted block method with stratification according to random codes (1–1920) generated by SAS statistical software. In addition, vaccines were assigned using random codes (1–1920) generated by SAS statistical software, confirming that investigators and participants were blinded to the vaccine administered. All participants received one vaccination.
Immunogenicity
Hemagglutination inhibition (HAI) titers were measured at baseline (day 0) and day 28 after vaccination. The lower limit of quantitation was set at the reciprocal of the lowest dilution used in the assay (5) and the upper limit of quantitation at the highest dilution (20,480). Seroprotection was defined as an HAI titer ≥40. Seroconversion was defined as having HAI titer <10 on day 0 and HAI titer ≥40 measured 28 days after vaccination, or having HAI titer ≥10 on day 0 and 4-fold increase from baseline in HAI titer 28 days after vaccination.
HAI assays were performed following Standard Operating Procedures of National Influenza Center published by Chinese Center for Disease Control and Prevention. Chicken red blood cells were provided by Hubei Yukou Poultry Industry Co., Ltd, China, and HA antigens of egg origin were bought from National Institute for Biological Standards and Control, London, United Kingdom. Serum samples were diluted 1:5 with receptor destroying enzyme (Cholera filtrate, Sigma-Aldrich, USA) and diluted twofold serially. HAI titers are defined as the inverse of the maximum serum dilution that totally suppresses hemagglutination.
Safety
After vaccination, participants were kept under observation for 30 min. Reports of local (pain, redness, and swelling) and systemic reactogenicity (fever, fatigue, headache, gastrointestinal symptoms, etc.) were solicited using memory aids (e.g., diary cards) during the week after vaccination. The unsolicited adverse events were also reported by subjects automatically up to 28 days with contact cards, and serious or medically attended adverse events were recorded via a telephone interview from day 29 to 6 months after vaccination.
The intensity of AEs was graded on a standard scale (0–4): grade 1 and 2 symptoms were defined as those that did not interfere with normal activities, whereas grade 3 and 4 symptoms were defined as those that prevented normal activities (grade 3 redness and swelling: diameter >100 mm; grade 3 fever: temperature >39°C).
Statistical analysis
The target sample size was estimated using a step-down strategy to control the total type I error, based on the power to evaluate non-inferior immunogenicity of IIV4 versus IIV4-HL for shared strains, considering the drop-off rate of about 20%. Finally, 1920 subjects were determined using PASS15 software.
Non-inferior immunogenicity was tested by comparing GMTs, SCRs, and SPRs, according to the “Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines” supporting the Licensure of Seasonal Inactivated Influenza Vaccines in the elderly, which was issued by the Center for Biologics Evaluation and Research (CBER) of Food and Drug Administration (FDA) in 2007. A strain was considered non-inferior if the lower limit of the two-sided 95% confidence interval (CI) around GMT ratio of IIV4 group to IIV4-HL group (new vaccine/registered vaccine) exceeds 0.67; the lower limit of 2-sided 95% CI is around the difference of SCR ≥ −10%. Meanwhile, the regulatory criterion for IIV4 acceptability for accelerated approval was a lower limit of 95% CI for SCR ≥ 30% and SPR ≥ 60% in people aged 60 years and above.
Superiority immunogenicity was determined by evaluating whether the lower limit of the two-sided 95% CI on GMT ratio (IIV4/IIV4-HL) should meet or exceed 1.5. Meanwhile, the lower limit of the two-sided 95% CI of the difference between SCR ratios should meet or exceed 10%.
Fisher exact probability test was used for statistical analysis of AEs incidence in all participants who received a study vaccine. Analysis of covariance (ANCOVA) model was employed to analyze the adjusted GMT ratio fitted on log10 transformed post-vaccination HAI titer. The 95% CI was calculated using the Clopper-Pearson method, and the difference was analyzed using the chi-square or Fisher exact probability tests using SAS software.
Results
Participants
Between April 28, 2019 and November 21, 2019, 1920 participants were enrolled and randomized. Among them, 959 were vaccinated with IIV4 (457 adults aged 60–65 years and 502 over 65 years) and 959 with IIV4-HL (443 adults aged 60–65 years and 516 over 65 years). After 6-month post-vaccination follow-up was completed, and due to the lack of main efficacy indicators of small number of subjects and the overdue blood sampling affecting the immunogenicity assessment, 1918 safety indicators (959 IIV4 and 959 IIV4-HL) and 1884 serological indicators (938 IIV4 and 946 IIV4-HL) were obtained ().
Figure 1. Study participant disposition.
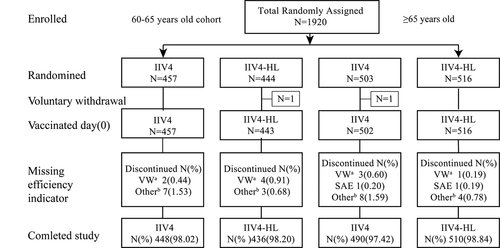
The height, weight, and ethnic group of study participants were well balanced between the two vaccine groups (). Sex ratios were nearly equivalent in IIV4 and IIV4-HL groups of 60–65 years old; however, the adults in IIV4 group contained fewer women (44.84%) than men, and those in IIV4-HL group contained 40.45% over 65 years old. The baseline characteristics and coexisting conditions of participants in the two vaccine groups were similar ().
Table 1. Baseline demographic and coexisting conditions of IIV4 and IIV4-HL vaccine groups
Immunogenicity
Within each age group, pre-vaccination HAI antibody titers were similar for participants receiving IIV4 and IIV4-HL, and both groups increased HAI antibody titer by vaccination day 28 ().
Table 2. Immunogenicity GMTs stratified by age cohort: day 28s post-vaccination
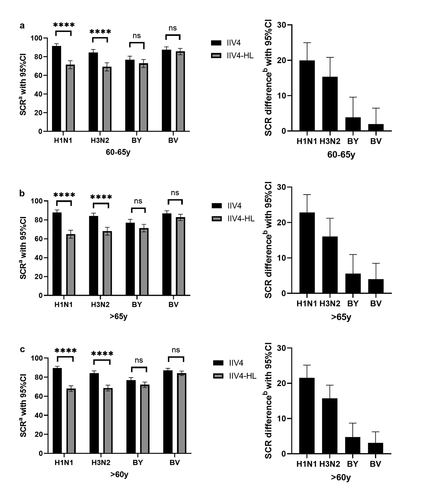
For the four strains (A/H1N1, A/H3N2, B/Brisbane, and B/Yamagata), post-vaccination HAI antibody responses induced within each age group by IIV4 were comparable to IIV4-HL-induced responses. Adults over 65 years old were less likely to generate higher antibody titers of A/H1N1, B/Yamagata, and B/Victoria compared to those of 60–65 years, but not of H3N2 strain. GMTs for these four strains increased by 5.57–22.03 fold with IIV4 and 4.90–11.76 fold with IIV4-HL in the two age groups. As expected for H1N1 and H3N2, GMTs increased to a greater extent with IIV4 than with IIV4-HL, both in 60–65 age group (29.25 and 14.45 fold for IIV4 vs. 10.04 and 7.76 fold for IIV4-HL) and in age group over 65 (26.21 and 14.51 fold for IIV4 vs. 9.10 and 7.24 fold for IIV4-HL). GMT ratios (95% CI) of IIV4/IIV4-HL were 2.83 (2.50, 3.20), 1.80 (1.63, 1.99), 1.06 (0.99, 1.14) and 1.07 (0.99, 1.16), respectively, and were similar for both age group. The lower limit of two-sided 95% CI of GMTs ratios was >2 for H1N1 and H3N2 strain in each age group, and > 0.67 for influenza B strains, which meet superiority standards for A strains and noninferiority standards for B strains, indicating that, through GMT comparison, IIV4 immunogenicity was non-inferior to that of IIV4-HL.
IIV4 met all CBER criteria for older vaccine recipients. Comparing SCR of IIV4 and IIV4-HL, SCR of IIV4 was from 76.84% to 89.55%, and the lower limit of the two-sided 95% CI of SCR was >30% for the two trial vaccine groups in two age stages. SCR difference (95% CI) (IIV4-IIV4-HL) of A/H1N1, A/H3N2, B/Yamagata, and B/Victoria strains was 21.58 (18.02, 25.15), 15.72 (11.95, 19.48), 4.77 (0.84, 8.70) and 3.06 (−0.10, 6.24), and the lower limit of the two-sided 95% CI of SCR difference of influenza A(H1N1 and H3N2) was >10% and of B(Yamagata and Victoria) was >-10% for all the participants, indicating that IIV4 being superior for influenza A(H1N1 and H3N2) and non superior for influenza B(Yamagata and Victoria). In addition, SCR difference was similar in both age cohorts ().
Figure 2. SCR by age cohort in the two vaccine groups.
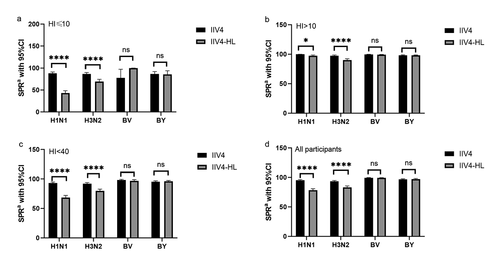
In the participants, post-vaccination SPRs (pre-vaccination (day 0) HAI titer <10) were ≥ 75% (88.18 for H1N1, 86.85 for H3N2, 77.78 for B/Yamagata, and 86.72 for B/Victoria) (), while post-vaccination SPRs (pre-vaccination (day 0) HAI titer ≥10) were 98.18, 89.70, 99.06, and 97.36) (). The lower limit of the two-sided 95% CI of SRPs in IIV4 group was >-60% (). Post-vaccination SPRs of H1N1 and H3N2 in IIV4 were higher than in IIV4-HL, especially when pre-vaccination (day 0) HAI titers <10 or 40 were used, but no statistically significant difference was observed in B/Yamagata and B/Victoria ().
Figure 3. SPR by vaccine group.
Reactogenicity and safety
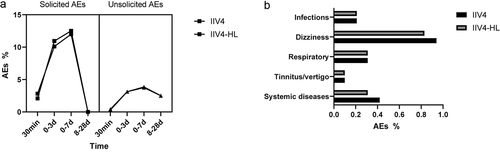
The most commonly reported AEs and vaccine-related AEs occurred between 30 min and 7 days after vaccination, with similar frequency in both vaccine groups (). No difference was observed between the two groups in unrelated AEs between 30 min and 28 days (). Most AEs were mild or moderate in severity between the vaccine groups (). As for the solicited AEs, almost all injection-site and systemic reactions were transient and self-limiting without a clinically meaningful difference (). Myalgia was the most common solicited injection-site reaction, whereas fever and headache were the most common solicited systemic reactions. No severe AEs were found among study participants, and no types of grade 3 reactions were reported in IIV4 recipients, but grade 3 AEs (one nausea, two diarrhea, one fatigue, and one erythema) of no more than 0.52% of IIV4-HL recipients were reported. The incidence of AEs in the elderly aged 60–65 years old was slightly higher than in those aged over 65 years old (14.66 vs. 10.36 for IIV4, 14.67 vs. 11.43 for IIV4-HL).
Table 3. Reactogenicity: solicited injection-site and systemic reactions in the two vaccine groups
Figure 4. The incidence of AEs.
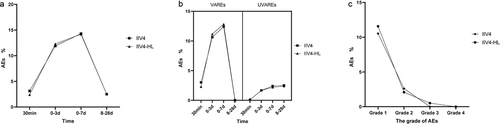
The frequencies of solicited AEs and unsolicited AEs were also similar for both vaccines (). The most frequently reported unsolicited AEs related to vaccination were dizziness (0.94% for IIV4 and 0.83% for IIV4-HL) and respiratory disease (0.31%) in both IIV4 and IIV4-HL groups.
Figure 5. The incidence of unsolicited AEs.
As for SAEs, 16 SAEs cases (0.83%) were observed six months post-vaccination, including two deaths, one for IIV4 group and one for IIV4-HL group because of tumor and cerebral infarction. All SAEs were unrelated to the studied vaccines.
Discussion
Comparing HAI antibody responses of a candidate IIV4 versus a standard dose, licensed quadrivalent inactivated vaccine IIV4-HL demonstrated that IIV4 was non-inferior for influenza B (B/Victoria and B/Yamagata) strains and superior for influenza A (H1N1 and H3N2) strains than IIV4-HL in both age cohorts. IIV4 had an acceptable reactogenicity and safety profile compared with IIV4-HL in two age cohorts.
Because of age-related declines in immune function, older adults exhibited lower antibody response to influenza vaccination, especially to B strains.Citation13,Citation14 The correlation between HAI titer and protection is weaker in elders, requiring high HAI titers to achieve the protective effect.Citation15 In phase III clinical trials for the elderly, all the immunogenicity indicators of IIV4 reached the standard of protective effect. Meanwhile, reactogenicity and tolerability of IIV4 with a standard dose of 15 μg of HA were non-inferior to IIV4-HL and fulfilled CBER immunogenicity acceptance criteria in elderly strata.Citation16 In this research, A strains outperformed B strains in terms of immunogenicity (GMT, SCRs, and SPRs). The lower limit of the two-sided 95% CI of GMT ratio for IIV4 vs. IIV4-HL was>0.9, beyond the required value of 0.66. According to SCRs and SPRs, IIV4 demonstrated positive results even compared with the high dose (60 μg) of quadrivalent influenza vaccine in elders.Citation17 For the two age subgroups, the immunogenicity studies for the two trial vaccines are similar, providing more detailed and robust data to use IIV4 in elders.
Both age groups well tolerated both vaccines. Most solicited local and systemic reactions with either vaccine were mild to moderate in severity and resolved within one week. No grade 3 AEs related to vaccines were reported for IIV4. Compared with other seasonal split-virion influenza clinical results, the quadrivalent, inactivated, split-virion influenza is well tolerated and could improve protection against influenza .Citation13,Citation18
In the subgroup analysis by age, AEs were generally higher for subjects aged 60–65 years than those aged ≥65 years, mainly due to the decline in the function of inflammatory cells with age.Citation19 HAI titers induced by A/H1N1and B lineages were higher in individuals aged 60–65 years than in elders over 65 years old, while those induced by A/H3N2 were lower, making it challenging to evaluate the level of antibody titers induced by IIV4 in the two age subgroups. Another study found that producing influenza antibodies decreases with age,Citation20 which contradicts our results. This could be due to the small age gap between the two age subgroups in our study.
IIV4 contains both B lineages influenza strain antigens, which could be employed to prevent unpredictable B lineages circulation or co-circulation. In addition, IIV4 contains viral surface protein (HA and NA) and internal proteins (matrix protein and nucleoprotein) to increase immunogenicity.Citation21 In China, the influenza vaccine coverage remains low, mainly because of vaccine supply shortage.Citation20,Citation22,Citation23 IIV4 provides better immunogenicity and safety, so it can be used to improve influenza vaccine coverage in China.
The advantages of this study, including the trial design, randomized controlled, and prospective phase III trial, are sufficient to meet the primary endpoint. We thoroughly considered participants’ complexity and excluded the factors affecting vaccination, making the participant population more representative. We divided the participants into two age groups and evaluated safety and immunogenicity of IIV4 and IIV4-HL to make the results more detailed and reliable. However, our study had some potential limitations. First, vaccine immunogenicity is not equivalent to “clinical protection.” Second, no epidemiologic surveillance is found to infer absolute efficacy, though some case-control study or test-negative design of influenza vaccines have estimated the effectiveness in elderly in the real world.Citation24,Citation25 Third, vaccination of a single influenza season makes it challenging to evaluate the efficacy against various influenza types and subtypes.
In conclusion, IIV4 demonstrated non-inferior immunogenicity and tolerability compared to licensed IIV4-HL in adults aged 60 years and over. The results indicate that IIV4 can be used as a candidate vaccine against seasonal influenza in elderly adults aged 60 years or older.
Abbreviations
| AE | = | Adverse event |
| ANCOVA | = | Analysis of Covariance |
| CBER | = | The Center for Biologics Evaluation and Research |
| CI | = | Confidence Interval |
| FDA | = | Food and drug administration |
| GCP | = | Good Clinical Practice |
| GMT | = | Geometric Mean Titer |
| HI | = | Hemagglutination Inhibition |
| ICF | = | Informed Consent Form |
| ICH | = | International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| NMPA | = | National Medical Products Administration |
| PPS | = | Per Protocol Set |
| QIV | = | Inactivated quadrivalent influenza vaccine |
| SAEs | = | Serious Adverse Events |
| SCR | = | Seroconversion Rate |
| SPR | = | Seroprotection Rate |
| SS | = | Safety Set |
| TEAE | = | Treatment emergent adverse event |
Authors’ contributions
The study staff assisted in phase III randomized controlled study implementation, including the study design, data collection, analysis, and interpretation. Xiaoming Yang, Xiaoyuan Huang, Xuanxaun Nian, and Jikai Zhang were responsible for study concept, design, and manuscript writing. Renfeng Fan, Kai Duan, Yingshi Chen, and Xinguo Li provided laboratory support. Peiyu Zeng, Jiayou Zhang, Jian Zhou, Wei Zhao, Wei Chen, Zhiqiang Ou, Jinglong Deng, and Shaomin Chen were responsible for the clinical study at the investigator site.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Guangdong Provincial Center for Diseases Control and Prevention for the help on trial implementation; Beijing Key-Tech Statistical Consulting Co, Ltd conducted the data analysis and received fees from Wuhan Institute of Biological Products Co. Ltd.
Additional information
Funding
References
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK, Marshfield G. Influenza Study, Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199(2):159–67. doi:10.1086/595861.
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010;28(Suppl 4):D45–53. doi:10.1016/j.vaccine.2010.08.028.
- Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, Cox MMJ, Team PSCS. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N Engl J Med. 2017;376(25):2427–36. doi:10.1056/NEJMoa1608862.
- Statler VA, Albano FR, Airey J, Sawlwin DC, Graves Jones A, Matassa V, Heijnen E, Edelman J, Marshall GS. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in children 6-59months of age: a phase 3, randomized, noninferiority study. Vaccine. 2019;37(2):343–51. doi:10.1016/j.vaccine.2018.07.036.
- Airey J, Albano FR, Sawlwin DC, Jones AG, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza virus vaccine compared with a comparator quadrivalent inactivated influenza vaccine in a pediatric population: a phase 3, randomized noninferiority study. Vaccine. 2017;35(20):2745–52. doi:10.1016/j.vaccine.2017.03.028.
- Treanor JT, Albano FR, Sawlwin DC, Graves Jones A, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: a phase 3, randomized noninferiority study. Vaccine. 2017;35(15):1856–64. doi:10.1016/j.vaccine.2017.02.066.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–72.
- Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, Maringhini G, van Lier A, Haagsma JA, Havelaar AH, et al., B.C. On Behalf Of The. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23(16). doi:10.2807/1560-7917.ES.2018.23.16.17-00454.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. doi:10.1016/S1473-3099(16)00129-8.
- Ng TWY, Cowling BJ, Gao HZ, Thompson MG. Comparative Immunogenicity of Enhanced Seasonal Influenza Vaccines in Older Adults: a Systematic Review and Meta-analysis. J Infect Dis. 2019;219:1525–35.
- N.M.P. Administration, Influenza Vaccine (Split Virion),Inactivated. http://app1.nmpa.gov.cn/data_nmpa/face3/base.jsp?tableId=25&tableName=TABLE25&title=%E5%9B%BD%E4%BA%A7%E8%8D%AF%E5%93%81&bcId=152904713761213296322795806604&CbSlDlH0=qAk7racmf3Kmf3KmfKJzH8SG.zGAeIHwe4nC9ZxjqRZqqEV. (Accessed July 7 2021).
- clinicaltrials, Influenza Vaccine (Split Virion) Inactivated, Quadrivalent. Hualan Biological Engineering. https://clinicaltrials.gov/ct2/show/NCT01511744?term=NCT01511744&draw=2&rank=1. ( Accessed July 7 2021).
- Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged >/=18 years. BMC Infect Dis. 2013;13:343. doi:10.1186/1471-2334-13-343.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–39. doi:10.4049/jimmunol.176.10.6333.
- U.S.F.a.D. Administration, Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-data-needed-support-licensure-seasonal-inactivated-influenza-vaccines. ( Accessed July 7 2021).
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–45. doi:10.1056/NEJMoa1315727.
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–78. doi:10.1016/j.vaccine.2013.08.069.
- Bartoszko J, Loeb M. The burden of influenza in older adults: meeting the challenge. Aging Clin Exp Res. 2021;33(3):711–17. doi:10.1007/s40520-019-01279-3.
- Wang Y, Cheng M, Wang S, Wu F, Yan Q, Yang Q, Li Y, Guo X, Fu C, Shi Y, et al. Vaccination coverage with the pneumococcal and influenza vaccine among persons with chronic diseases in Shanghai, China, 2017. BMC Public Health. 2020;20(1):359. doi:10.1186/s12889-020-8388-3.
- Andrew MK, Bowles SK, Pawelec G, Haynes L, Kuchel GA, McNeil SA, McElhaney JE. Influenza Vaccination in Older Adults: recent Innovations and Practical Applications. Drugs Aging. 2019;36(1):29–37. doi:10.1007/s40266-018-0597-4.
- Jiang M, Li P, Yao X, Hayat K, Gong Y, Zhu S, Peng J, Shi X, Pu Z, Huang Y, et al. Preference of influenza vaccination among the elderly population in Shaanxi province, China. Hum Vaccin Immunother. 2021;5:1–7. doi: 10.1080/21645515.2021.1913029.
- Lau JTF, Ng CSM, Wu AMS, Ma YL, Lau MMC. Low coverage of influenza vaccination among Chinese children aged 12-23 months: prevalence and associated factors. PLoS One. 2018;13(10):e0205561. doi:10.1371/journal.pone.0205561.
- Russell K, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018;36(10):1272–78. doi:10.1016/j.vaccine.2018.01.045.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of Influenza Vaccine in the Community-Dwelling Elderly. N Engl J Med 2007;357(14):1373–81. doi: 10.1056/NEJMoa070844.
