ABSTRACT
Globally, measures, such as lockdown, quarantining, and physical distancing, have been implemented to curb the spread of COVID-19. As the vaccines are now available and reintegration into society is beginning, measures such as vaccine certificates are being implemented around the world. We conducted a scoping review to identify the initial digital solutions for COVID-19 vaccine certificates and evaluate them on the basis of purpose and use case, technological architecture, and ethical and legal implications. Articles identified from a Google search and a search of MEDLINE, Ovid and preprint servers were reviewed in duplicate, and data were extracted using a data extraction form. Data were extracted for date, location, type of article, source, companies identified for creating vaccine certificates, technology used, type of evidence provided (article quoting research study or an expert opinion), digital architecture, security and privacy measures, and use cases. Technology emerged as the most dominant theme followed by ethics, travel, legal concerns, public policy, and scientific concerns. Our review identified eight solutions that are working toward COVID-19 vaccine certificates world-wide, all optimizing blockchain technology. COVID-19 vaccine certificates are being considered in 11 countries and are in place in 5 others. Many issues concerning the themes we identified remain to be addressed to facilitate successful implementation.
Introduction
Globally, unprecedented measures have been implemented to reduce the spread of the COVID-19 pandemic and prevent healthcare systems from being overwhelmed. These measures include states of lockdown, travel restrictions, work from home orders and quarantine of citizens. These have had serious psychological and socio-economic consequences.Citation1-3 A key focus to mitigating and recovering from the economic impacts of COVID-19 will be safely reintegrating individuals into the workforce and society.
COVID-19 vaccines were first approved for use in December 2020.Citation4 With vaccination rates increasing around the world, social and travel restrictions are beginning to ease. There are currently international travel requirements in place for the Yellow Fever vaccine under the International Health RegulationsCitation5 and it is possible that similar requirements for COVID-19 vaccines may be added.Citation6,Citation7 Beyond facilitating international travel, digital proof of vaccination is being considered as a mechanism to facilitate return to work and reopening of the economy.Citation8
The use of digital vaccine certificates is evolving rapidly. We have undertaken a scoping review to assemble and map existing digital solutions for vaccine certificates. The secondary objective is to evaluate the initial digital solutions with respect to (1) purpose and use case, (2) technological architecture, and (3) ethical, legal, and policy considerations. A scoping review is a process to map key concepts, main sources, and types of evidence available and also to highlight the gaps in the research area which are complex or have not been previously reviewed in a comprehensive manner.Citation9 Considering that the development of digital vaccination certificate solutions is still in its early stages and changing rapidly, the flexibility and breadth of a scoping review lends particularly well to this topic. We use the findings of this review to consider what potential barriers may exist to implementation of these solutions.
Methods
The scoping review was guided by the methodological framework developed by Arksey and O’MalleyCitation10 and reported by the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (available in supplementary materials).Citation11 The following five steps have been followed in this scoping review: (i) identifying the research question, (ii) identifying relevant studies, (iii) selection of eligible studies, (iv) charting the data, and (v) collating and summarizing the results.
Identifying the research question
The main research question was “What are the existing digital solutions for vaccine certificates available globally?”
The research sub-questions were as follows:
What is the purpose and use case of these initial solutions?
What is the technological architecture for these initial solutions?
What are the ethical, legal, and policy implications for these initial solutions?
Identifying relevant studies
Grey literature search
Digital solutions for vaccine certificates may be described on the websites of the groups who have developed them or in news articles or blogs. Therefore, we undertook a comprehensive Google search of grey literature to identify existing vaccine certificates around the world.
On November 18 and November 19, 2020 we followed the guidelines outlined by Canadian Agency for Drugs and Technology in Health (CADTH) to conduct the Google review.Citation12 The search terms used were “coronavirus,” “COVID-19,” “SARS COV2,” “immunization passport,” “immunization certificate,” “vaccine certificate,” “vaccination certificate” and “digital.” A total of 9 searches were conducted combining the search terms and are listed in supplementary Table 1. All searches were conducted using privacy settings with location settings turned off. The Google search was limited to 150 results per search to maintain the search to a manageable level while also capturing relevant content, unless less than 150 results were obtained. Our Google search yielded a total of 1056 results, 641 after removing the duplicates.
As it is an evolving area, we further augmented our results beyond the time of the initial search with additional solutions that emerged. This was done through a second specific Google search to incorporate the most important and recent announcements by EU and Israel and are presented in the results.
Academic search
To ensure the comprehensiveness of our review, we also conducted a search of Embase, Ovid MEDLINE and preprint servers (MedRXIV, BioRXIV) on November 26, 2020. The database search terms included “coronavirus,” “COVID,” COVID19”, “sars cov-2,” “vaccination,” “immunization,” “certificate,” “passport,” “document” “vaccination pass.” The search strategy is available in supplementary materials.
Selection of eligible studies
The scoping review inclusion criteria were articles in the English language that included any discussion of a digital solution for vaccine certificate/documentation or passport for the COVID-19 vaccine published from December 2019 to the search date. Articles were excluded if they 1) did not have relevant content such as primarily discussed immunity passport (i.e., immunity from disease) or primarily discussed various conspiracy theories circulating at the time (such as the use of vaccines to inject microchips), 2) had limited content defined as less than three lines of relevant text, 3) had broken links or 4) had content presented in video format.
Screening process
Two reviewers (SSM, ABB) independently screened titles and content (SSM, DZ) according to the inclusion and exclusion criteria. Data extraction was conducted in duplicate using an electronic data extraction form. Back searching of identified digital solutions was conducted to fill information gaps. Any disagreements that arose during screening were resolved by consensus.
Charting the data
Following full-text screening, two reviewers (SSM, DZ) charted each article chosen for inclusion using the data extraction form to gather common and comparable information on each study. Data extracted included publication date, location, type of article, source, companies identified, technology used, type of evidence provided (articles quoting a research study or a health expert opinion – which included academic thought leaders, politicians, or the CEOs of the companies developing a vaccine certificate), digital architecture, security and privacy measures, and use cases. We categorized articles based on the following six pre-identified themes: 1) legal, 2) technology, 3) ethics, 4) travel, 5) policy, and 6) science. Based on preliminary research we identified these themes as important areas to review when considering the use of vaccine certificates.Citation13–15
Results
Collating, summarizing, and reporting the results
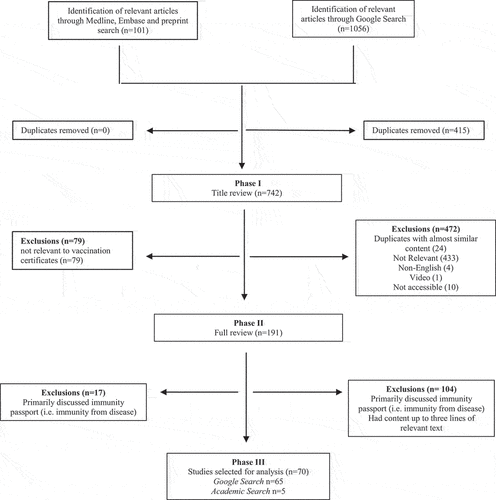
A total of 742 articles were identified for screening (Google n = 641, academic literature n = 101). Based on the title and headline screening, 551 articles were eliminated including 24 duplicates identified with the same heading or near similar content. One hundred and ninety-one articles remained for full text review, of which 70 were included in the final analysis (). Those articles that only discussed immunity passports (i.e., immunity from disease) as opposed to vaccine certificates or passports, had relevant content less than three lines or focused on conspiracy theories that were circulating at the time were excluded from the final analysis.
Characteristics of included articles
Of the 70 relevant articles, nearly half were opinion/editorials (n = 29, 41.4%), followed by news articles (n = 25, 35.7%), research articles (n = 7, 28.7%), company websites (n = 3, 4.3%), and blogs (n = 2, 2.9%). The remaining articles (n = 4, 5.7%) were considered other (event description, guidelines etc.) ().
Table 1. Characteristics of the articles
More than half (n = 54, 77%) of the articles referred to some source of evidence. Sources of evidence included articles quoting “research studies” or an “expert opinion.” Research studies were the source of evidence for 14% (n = 10) of the articles whereas expert opinions included academic thought leaders (n = 12, 17%), politicians (n = 16, 23%), and the CEOs of the companies developing a vaccine certificate (n = 16, 23%). Twenty-three percent (n = 16) did not quote any source of evidence.
Digital solutions for COVID-19 vaccination certificates
Our search identified eight digital vaccine certificate solutions in use or under development (). At the time of the search four solutions (CommonPass, DigiLocker, Covi-pass, and VaccineGuard)Citation18,Citation22,Citation23,Citation32,Citation33 were in the pilot/trial stage or available for use. Three solutions (QDX Health ID, Vaccify, and IBM Digital Health pass)Citation16,Citation17,Citation24,Citation26,Citation34 were in the beta testing and demo stage and no information was available about the product stage of WIShelter SafePass app. Following our initial search, we identified two additional solutions- the EU Digital Covid Certificate (EUDCC),Citation35 formerly known as The Digital Green Certificate for travel among EU countries and the Green PassCitation36 both of which have reached the implementation stage. In June 2021 we reviewed the solutions to update any information on the production stage or use. WIShelter is now available for useCitation27 and Digital Health PassCitation26 now also provides proof of vaccination. No other updates were available for the remaining solutions.
Table 2. Digital solutions for COVID-19 vaccine certificates
Technological architecture
The use of digital certifications was discussed in 78% of articles identified (n = 53). Digital certificate hereby refers to the proof of vaccination in a digital format. Only one article mentioned the use of paper certificates for those who do not own a smartphone and a printout of the Quick Response (QR) code would be given to them.
Implementations
identifies the countries which are considering, plans to implement or have implemented digital vaccine certificates and provides a brief summary of the details. At the time of the search, Denmark, Estonia, UK, and Israel had implemented vaccine certificates.Citation30,Citation31,Citation37,Citation41,Citation57 Estonia is piloting the VaccineGuard app in collaboration with the World Health Organization.Citation30 Finland, Australia, Italy, Sweden, Switzerland, and Spain have plans to implement the vaccine certificate and the details are expected to be released in the upcoming months.Citation37,Citation44,Citation45,Citation47–50,Citation53–56 Australia and Finland are moving forward using the existing health platforms, Express Plus Medicare app and My Kanta, respectively.Citation44,Citation47 Pakistan, Russia, Indonesia, are all considering vaccine certificates but have not shared any confirmed plans yetCitation16,Citation17,Citation24,Citation42,Citation43,Citation46 though Pakistan is considering the implementation of digital platform under development, Vaccify.Citation16,Citation17,Citation24 Canada is currently considering vaccine certificates for international travel only.Citation51,Citation52 Indonesia is also considering the implementation of vaccine certificates and has identified several processes which must be put in place in order for the certificate to be considered halal which will allow it to be accepted by their larger population.Citation46
Table 3. Approach of countries toward a vaccine certificate for COVID-19
Following our initial search, additional solutions were announced and therefore, we incorporated them into . On March 17, 2021, the European Commission introduced a vaccine certificate to ensure free and safe travel within the European Union (EU) countries.Citation35 The EU Digital Covid Certificate (EUDCC), formerly known as The Digital Green Certificate, will be valid for all EU countries as well as some non-EU countries and will be available in both digital and paper formats.Citation35 It works with the help of a QR code issued by a recognized health authority such as a hospital or test center, that embeds a digital signature to protect it against fraud. To check the certificate, the QR code is scanned and the signature verified.Citation58,Citation60,Citation61 Quarantine rules will not apply to those who are fully vaccinated.Citation61 The EUDCC includes information about an individual’s vaccination status as well as COVID-19 test results or recovery status from COVID-19.Citation35 Some EU countries such as France and Germany are developing their own national solutions.Citation60 Israel’s Green pass released on 21st Feb 2021 is also available in both digital and paper format. It displays proof of vaccination or if you had recovered from COVID-19.Citation36
In June 2021, we updated our review of countries implementing digital vaccine solutions. At that time India had also implemented vaccine certificates which users can download from their DigiLocker account or through CoWIN and Aarogya Setu app.Citation23,Citation25 The United States were originally considering implementing a digital vaccine certificate but have since decided against implementing a national solution.Citation39
Data security and privacy
The data privacy and security of the digital platforms identified were based on the fundamentals of blockchain, that is all personal identifiable information is encrypted and cannot be disclosed without the user’s consent.Citation28 All solutions allow the verifier of the vaccine certificate to scan the QR code indicating presence or absence of a vaccine certificate and date and details of the vaccination without disclosing other personal identifiable information.
There are many major standard-setting efforts for data security and privacy underway of which two were mentioned in our articles:
1) Worldwide Web Consortium (W3C): W3C is an open, globally interoperable standard setting body for data security.Citation62 The body is known for such standards as the early versions of HTML.Citation63 Companies such as TrustNet Pakistan, works on W3C standard and looks for instances where Verifiable Credentials (VCs) can be used to address the public health crisis.Citation63,Citation64 The Commons Project also uses W3C standards for their application.Citation32
2) HyperLedger: HyperLedger is another standard-setting body which is an open-source community focused on developing a suite of stable frameworks, tools and libraries for enterprise-grade blockchain deployments with interoperability and tokens as their expertise.Citation62 Quantum Materials Corp’s (QMC) blockchain-based QDX HealthID app, is based on the Hyperledger Sawtooth enterprise blockchain and for smart contracts, it’s using the Digital Asset Modeling Language (DAML).Citation34 Apart from it, WISekey has implemented standards such as OpenID Connect and OAUTH2 to enhance the security of their cloud applications.Citation29
Thematic analysis
We evaluated the articles for six pre-identified themes. Technology emerged as the most dominant theme, appearing in 58.5% (n = 41) articles. Ethics (n = 22, 31%), travel (n = 21, 30%), legal concerns (n = 10, 14%), public policy (n = 9, 13%), and scientific concerns (n = 1, 1.5%) were also among the themes identified. The article identified under scientific concerns largely discussed the science of immunity.Citation65 Public policy concerns identified related to requirements for vaccine certificates for entering malls, gyms, sports matches or other events.Citation66,Citation67 They also focused on the impacts of vaccine certificates at work.Citation68 Articles categorized under travel focused on cross-border travel.Citation69,Citation70
During our review we identified the following four sub themes: 1) reopening of economy, 2) data security, 3) COVID-19 infection prevention, and 4) inequities. Reopening of economy was the most common reason for introducing vaccine certificates (n = 12, 17%). A lot of the discourse identified was around reopening of social events, gyms, and restaurants.
Issues around data security (n = 9, 13%), COVID-19 infection prevention (n = 3, 4.3%), and inequities (n = 3, 4.3%) were also noted. More than half of the articles had no sub themes that were identified (n = 43, 61%).
Purpose and use case
Some airlines are requiring a negative COVID-19 test prior to boarding a flight, and in future this may be adapted to a vaccine certificate for passengers and staff.Citation71 The UK government has sought a proposal for digital health certificates for travel from the company Onfido.Citation72 Health policy expertsCitation73 envision vaccine mandates could be instituted and enforced by local governments or employers – similar to the current vaccine requirements for school-age children, military personnel, and hospital workers.Citation73 CommonPass, a product of the Commons Project is one example which has been trialed out by United Airlines and Cathay Pacific Airways to show COVID-19 test results and has been presented to over 37 governments. In the near future, it will integrate vaccine certificates.Citation19 Germany, Indonesia, Italy, Colombia, Argentina, have considered implementing health passports like CommonPass.Citation18 The company has proposed its use for travel, schools, hotels, and concert venues.Citation20 Guardtime, a KSI blockchain technology company, in collaboration with the Estonian government and the WHO has piloted a digital vaccination certificate program.Citation33 The platform is based on KSI blockchain, an EU-EIDAS certified trust service and X-Road, Estonia’s data sharing platform, and out of its many objectives, one of them is to test the proof of vaccination, similar to the International Certificate of Vaccination or “yellow card.” Covi-pass is another app that is now being considered in 15 countries including France, Canada, and India and is also supporting return to work and society. It is currently available for COVID-19 test results but plans to expand to incorporate vaccine certificates.Citation22 A secure COVID-19 vaccination certificate will play a critical role as economies reopen and international travel resumes.Citation33
Ethical, legal, and policy considerations
More than half of the articles combined (n = 41, 58.5%) discussed legal, ethical, and policy concerns regarding COVID-19 vaccine certificate. The major concerns emerging from the ethical and legal perspective was that of health equity. Technology as much benefit as it brings, can also become a barrier for some, adding to existing inequities.Citation74 Development of vaccine certificates may exclude vulnerable and marginalized populations who do not have access to smart phones.Citation74 In terms of reopening the economy, including access to in-person social events, updating travel guidelines, or going to restaurants, gyms, and salons, one of the important questions will be to ask how society deals with the admission of non-vaccinated people. Alternatively, vaccine certificates may incentivize individuals to obtain vaccination against the virus, which is a social good.Citation75
Discussion
As one of the largest mass immunization campaigns in history is underway, tracking of those who have been vaccinated could become essential as individuals return to work and international travel resumes. This review identified eight early vaccine certificate technologies that were under development. At the time of the initial review four countries had adopted the use of vaccine certificatesCitation30,Citation31,Citation37,Citation41,Citation57 while at least 11 others including the European Union were in the process of planning and implementation.Citation16,Citation17,Citation24,Citation35,Citation37,Citation42–56,Citation58,Citation59 As of June 2021, 1 of these countries had implemented the vaccine certificate while 1 country is now no longer considering the development of national vaccine certificate.Citation25,Citation39
The results of our scoping review point toward the different solutions that are being developed for vaccine certificate globally and the discourse about the challenges it brings. This is a rapidly evolving area, and our study describes the initial exploration of this concept. Since this is a preliminary review, we acknowledge that the use of vaccine certificates will evolve. It is difficult to predict the success or outcomes of the vaccine certificate solutions at this stage as most of the available information about vaccine certificates is from the grey literature and there is limited experience with implementation and evaluation.
All vaccine certificates identified are using blockchain technology which provides a secure system where data control lies with the end-user. Blockchain technology is a distributed ledger technology (DLT) which stores copies of a document on nodes across the entire network. Blockchain is considered essentially secure and cannot be tampered with or changed.Citation62,Citation76 In addition to their use in vaccine certificates, blockchain technology has also recently been used for other healthcare initiatives such as contact tracing among others.Citation77–79 Companies such as IBM, WISekey, and Quantum Material Corp have already been working toward vaccine tracking,Citation21 COVID-19 test results,Citation26,Citation80 and providing access to diagnostics, vaccine education and necessary medical services and dataCitation28 prior to introducing vaccine certificates. At the time of this review, their solutions for vaccine certificates had reached the demo and beta testing trials.Citation26 Though blockchain was considered by all the solutions identified, it lacks uniform data security and privacy standards.
Vaccine certificates come with some ethical challenges. Historically and socially marginalized groups may be less likely to be vaccinated, due to poor access to healthcare but also due to lack of trust in the government as a result of past experiences of medical abuse.Citation81 For those who are unable to be vaccinated because of their religion or health status, or those who have difficulty accessing a vaccine or an antibody testing, a vaccine certificate could impact them unfairly.Citation75,Citation81 To address these ethical challenges, there must be equitable policies for implementing vaccine certificates. To combat the mistrust in government, a community-based approach to promote vaccine uptake executed by the trusted community leaders, organizations, and local health care institutions should be considered.Citation81 Access to technology may also be a barrier to vaccine certificate use. Implementation of vaccine certificates must not marginalize against those who do not have access to or choose not to use technology.Therefore, alternate solutions such as paper-based vaccine certificate with a scannable QR code could be an option for those who cannot access technology.
Although there is much ongoing debate about the medical and ethical issues surrounding vaccine certificates, there has been less inspection of the technical foundations of vaccine certificate solutions.Citation63,Citation82 The majority of the digital solutions we identified have involved a stack of data security and privacy standards, such as Decentralized Identifiers (DIDs) and Verifiable Credentials (VCs) from the World Wide Web Consortium (W3C). The standards can be based on the Semantic Web (an extension of the internet based on standards set by the W3C), with the goal of making data readable by machines.Citation63 This is useful for open public data but when combined with personal data and globally unique identifiers like DIDs, it could be used for other activities.
The leaders of IBM, the World Economic Forum, and International Air Transport Association (IATA) have also voiced the need for a single set of standards that can allow multiple platforms to interoperate.Citation83 To support the interoperability between vaccine certificates, the EU guideline has established criteria which includes 1) the use of minimal personal identifiable data, 2) a unique identifier for vaccination certificates, and 3) basis for a trust framework, i.e. the vaccine certificates should be issued by trusted entities and possible to verify the authenticity.Citation84 The WHO also released their first draft of the guide in March 2021, highlighting the guiding principles of vaccine certificates but the details around uniform technical standards will be shared in their release candidate 2 version.Citation85
Fraud and counterfeit vaccine certificates also posit another challenge and undermine the biosecurity of a COVID-19 vaccine certificate.Citation86 The model based on data integration can be exploited by signature exclusion and replacement attacks. A person can remove the signature of a signed message or a digital document and replace it with another signature, tricking the verifier into believing an invalid message as valid. In this case, it can cause vaccine certificates to be completely fabricated as well.Citation63
While there has been considerable discussion on implementation of digital vaccine certificates, there have been comparatively few implementations. Our review has identified some of the potential obstacles, specifically ethical and legal issues and technology challenges. Facilitating travel appears to be the most compelling use case. Conflating domestic use cases with facilitation of international travel may be creating barriers for implementation in the latter scenario. While, arguably, ethical and legal concerns are less of an issue for international travel use cases compared to domestic uses cases, technology challenges, in particular agreeing to international standards and trust frameworks, would be more problematic. Furthermore, in federal countries, there can be real challenges in harmonizing regional government efforts with federal.Citation87 In many of these countries, the immunization record would be generated at the regional government level and may not adhere to federal standards.
This study has several limitations. We chose to use a Google search for this review as the platforms we were evaluating were in the early development phase and not necessarily searchable in a traditional review platform. Although this allowed us to identify companies and platforms in production, we recognize that this is a rapidly evolving area, and several new solutions will be emerging. Since many of the applications identified were in the beta testing or demo phase, further development will likely still occur. Furthermore, using Google as a search platform may create bias in the articles identified. We attempted to mitigate that by reviewing up to 150 search results, conducting numerous searches and conducting the search in privacy mode. Of the articles identified, the majority were from news articles, opinions/editorials, or research articles (91%) many of which referred to some kind of evidence (i.e., research or expert opinions). Finally, this review was limited to English language content. Since we are reviewing digital solutions around the world, it is likely that other relevant solutions were missed. As the conversations around vaccine certificate evolved, we conducted additional searching to identify countries approach toward vaccine certificates.
Conclusion
In summary, we have identified a number of early digital platforms under development for COVID-19 vaccine certificates around the world. Several countries are moving forward with vaccine certificates in certain settings. The solutions we identified are based upon blockchain technology, which is considered the gold standard for securely storing personal information. It is important to consider limitations to blockchain-related healthcare implementations. Moreover, it is important when considering the implementation of vaccine certificates that it is taken up in an ethical manner that does not discriminate against those who do not have access to the technology as well as access to the vaccines itself. Therefore, it is integral to the success of these blockchain-based solutions for policymakers and government safety and privacy regulators to consider laws that detail appropriate risk management to put in place while still allowing the tool to work as intended.
Contributors
ABB conducted the search. SSM, DZ and ABB conducted screening and data extraction.
SSM and ABB drafted and edited the paper.
KW conceptualized the project and provided expertise.
All authors reviewed and edited the manuscript.
Disclosure of potential conflicts of interest
Kumanan Wilson is the Chief Executive Officer of CANImmunize Inc.
Supplemental Material
Download MS Word (13.8 KB)Supplemental Material
Download MS Word (111.7 KB)Acknowledgments
We would like to acknowledge our librarian, Risa Shorr at The Ottawa Hospital for conducting the academic search.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1969849
Additional information
Funding
References
- Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg [Internet]. 2020 [accessed 2021 Mar 12];78:185–93. doi:10.1016/j.ijsu.2020.04.018.
- Chu IY-H, Alam P, Larson HJ, Lin L. Social consequences of mass quarantine during epidemics: a systematic review with implications for the COVID-19 response. J Travel Med [Internet]. 2020 [accessed 2021 Mar 12];27:7. https://academic.oup.com/jtm/article/doi/10.1093/jtm/taaa192/5922349.
- Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet [Internet]. 2020 [accessed 2021 Mar 3];395:912–20. doi:10.1016/S0140-6736(20)30460-8.
- U.S. Food & Drug Administration. Pfizer-BioNTech COVID-19 Vaccine | FDA [Internet]. 2020 [accessed 2021 May 30]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- World Health Organization. New yellow fever vaccination requirements for travellers [Internet]. Int Travel Health. 2016. [accessed 2021 Mar 14]. https://www.who.int/ith/updates/20160727/en/.
- Kelleher RS. Get ready for needing a negative Covid-19 test to fly within the U.S. [Internet]. Forbes. 2021 [accessed 2021 Mar 14] https://www.forbes.com/sites/suzannerowankelleher/2021/02/09/get-ready-for-needing-a-negative-covid-19-test-to-fly-within-the-us/?sh=297595282eed.
- Government of Canada. Flying to Canada: COVID-19 testing for travellers - Travel restrictions in Canada – travel.gc.ca [Internet]. 2021 [accessed 2021 Mar 14]. https://travel.gc.ca/travel-covid/travel-restrictions/flying-canada-checklist/covid-19-testing-travellers-coming-into-canada?utm_campaign=gac-amc-covid-20-21&utm_source=travel-covid_travel-restrictions_flying_&utm_medium=redirect&utm_content=en.
- Olijnyk Z. COVID-19 vaccine passport development must have legal and ethics expert input: medical journal | Canadian Lawyer [Internet]. Can Lawyer. 2021 [accessed 2021 Mar 15]. https://www.canadianlawyermag.com/practice-areas/privacy-and-data/covid-19-vaccine-passport-development-must-have-legal-and-ethics-expert-input-medical-journal/353900.
- McLuckie A, Landers AL, Curran JA, Cann R, Carrese DH, Nolan A, Corrigan K, Carrey NJ. A scoping review of mental health prevention and intervention initiatives for infants and preschoolers at risk for socio-emotional difficulties. Syst Rev [Internet]. 2019 [accessed 2021 May 30];8:1–19. doi:10.1186/s13643-019-1043-3.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract [Internet]. 2005 [accessed 2021 May 30];8:19–32. https://www.tandfonline.com/doi/abs/10.1080/1364557032000119616.
- PRISMA. TRANSPARENT REPORTING of SYSTEMATIC REVIEWS and META-ANALYSES [Internet]. 2018 [accessed 2021 May 30]. http://www.prisma-statement.org/Extensions/ScopingReviews.
- 1Grey Matters: a practical tool for searching health-related grey literature | CADTH.ca [Internet]. 2019 [accessed 2021 Mar 15]. https://www.cadth.ca/resources/finding-evidence/grey-matters.
- Larson E, Baker S There’s a big problem with coronavirus immunity certificates - Bloomberg [Internet]. 2020 [accessed 2021 Jul 26]. https://www.bloomberg.com/news/articles/2020-04-09/there-s-a-big-problem-with-coronavirus-immunity-certificates.
- Proctor K, Sample I, Oltermann P “Immunity passports” could speed up return to work after Covid-19 | coronavirus | the Guardian [Internet]. 2020 [accessed 2021 Jul 26]. https://www.theguardian.com/world/2020/mar/30/immunity-passports-could-speed-up-return-to-work-after-covid-19.
- Phelan AL. COVID-19 immunity passports and vaccination certificates: scientific, equitable, and legal challenges. Lancet [Internet]. 2020 [accessed 2021 Jul 26];395:1595–98. doi:10.1016/S0140-6736(20)31034-5.
- Vaccify. A vaccination passporting ecosystem. Concept paper [Internet]. 2020 [accessed 2021 Mar 14]. https://vaccify.s3.ap-south-1.amazonaws.com/Vaccify+-+Concept+Paper.pdf.
- Ahmed A. This Pakistani company is working on a digital vaccine passport for COVID-19 [Internet]. 2020 [accessed 2021 Mar 14]. https://propakistani.pk/2020/06/26/this-pakistani-company-is-working-on-a-digital-vaccine-passport-for-covid-19/.
- Martens B Airlines introduce world’s first immunity passport exempting travellers from quarantine | national Post [Internet]. 2020 [accessed 2021 Mar 14]. https://nationalpost.com/travel/covid-19-screening-app-may-be-linchpin-for-travelling-during-the-pandemic.
- Khatib AN, Carvalho A-M, Primavesi R, To K, Poirier V. Navigating the risks of flying during COVID-19: a review for safe air travel. J Travel Med [Internet]. 2020 [accessed 2021 Mar 14];27. https://academic.oup.com/jtm/article/doi/10.1093/jtm/taaa212/5976283.
- Walt V COVID travel: meet CommonPass, the startup trying to restore our faith in safe air travel | fortune [Internet]. 2020 [accessed 2021 Mar 14]. https://fortune.com/2020/10/06/commonpass-covid-air-travel-coronavirus-safety-tech-startup/.
- US firm integrates nanotechnology, blockchain for Covid-19 immunity passports! [Internet]. BLOCKCHAIN Mag.2020 [accessed 2021 Mar 15]. https://blockchainmagazine.net/us-firm-integrates-nanotechnology-blockchain-for-covid-19-immunity-passports/.
- TENTO HEALTH [Internet]. 2021 [accessed 2021 Mar 15]. https://covipass.com/faq/.
- Mumbai AP Covid-19 vaccinated people may get a digital certificate: report - The Hindu BusinessLine [Internet]. 2020 [accessed 2021 Mar 14]. https://www.thehindubusinessline.com/news/covid-19-vaccinated-people-may-get-a-digital-certificate-report/article32652957.ece.
- Shehnaz. COVID19 immunization program: schools can become Vaccine Booth, QR Code Certificate to be obtained after vaccination; Learn and prepare [Internet]. 2020 [accessed 2021 Mar 14]. https://www.businesskhabar.com/business/industries/covid19-immunization-program-schools-can-become-vaccine-booth-qr-code-certificate-to-be-obtained-after-vaccination-learn-and-prepare/.
- Online FE. Covid-19 vaccination certificate: how to download Covid-19 vaccine certificate on Android or iOS via Aarogya Setu App/CoWIN Portal, Download Covid-19 Vaccine Provisional Certificate by Mobile Number or Aadhaar Number | the Financial Express [Internet]. Financ. Express 2021 [accessed 2021 Jun 10]. https://www.financialexpress.com/lifestyle/health/covid-19-vaccine-certificate-heres-how-to-download-it-from-cowin-portal-aarogya-setu-app/2249420/.
- IBM. Digital health pass - overview | IBM [Internet]. 2021 [accessed 2021 Mar 15]. https://www.ibm.com/products/digital-health-pass.
- WIShelter – wISeID [Internet]. [accessed 2021 Jun 17]. https://wiseid.com/wiseid-apps/wishelter/.
- WISeKey international: digital health passport to include a trusted vaccination digital certificate | marketScreener [Internet]. 2020 [accessed 2021 Mar 14]. https://www.marketscreener.com/quote/stock/WISEKEY-INTERNATIONAL-HOL-26786298/news/WISeKey-International-nbsp-Digital-Health-Passport-to-Include-a-Trusted-Vaccination-Digital-Certif-31729113/.
- WISeKey’s Digital Health Passport to Include a Trusted Vaccination Digital Certificate Swiss Stock Exchange: WIHN[Internet]. 2020 [accessed 2021 Mar 14]. https://www.globenewswire.com/news-release/2020/11/09/2123062/0/en/WISeKey-s-Digital-Health-Passport-to-Include-a-Trusted-Vaccination-Digital-Certificate.html.
- guardtime. Estonia, Hungary, and Iceland, together with AstraZeneca Estonia are participating in a pilot of Guardtime’s VaccineGuard | guardtime [Internet]. 2021 [accessed 2021 Mar 14]. https://guardtime.com/blog/estonia-hungary-and-iceland-together-with-astrazeneca-estonia-are-participating-in-a-pilot-of-guardtime-s-vaccineguard.
- guardtime. Products [Internet]. 2021 [accessed 2021 Mar 14]. https://guardtime.com/products.
- CommonPass — the Commons Project [Internet]. [accessed 2021 Jan 27]. https://thecommonsproject.org/commonpass.
- ERR news. Estonia and World Health Organization digitally sign cooperation agreement | news | ERR [Internet]. 2020 [accessed 2021 Mar 14]. https://news.err.ee/1143517/estonia-and-world-health-organization-digitally-sign-cooperation-agreement.
- Ledger Insights. US firm combines nanotechnology, blockchain for COVID-19 immunity passports - Ledger Insights - enterprise blockchain [Internet]. 2020 [accessed 2021 Mar 14]. https://www.ledgerinsights.com/nanotechnology-blockchain-covid-19-immunity-passports/.
- Palmer S Digital green pass, vaccine passport, EUDCC: what is it and who can use it? [Internet]. Euronews; 2021 [accessed 2021 Jun 10]. https://www.euronews.com/travel/2021/06/09/digital-green-pass-vaccine-passport-eudcc-a-guide-to-post-pandemic-travel-documents.
- Ferguson C, Mitnick J. Israel’s “green pass” vaccine passport is an early vision of how we leave lockdown | MIT Technology Review [Internet]. MIT Technol Rev. 2021 [accessed 2021 Jun 10]. https://www.technologyreview.com/2021/03/01/1020154/israels-green-pass-is-an-early-vision-of-how-we-leave-lockdown/.
- COVID Vaccine Passport, the new trend being adopted by world nations | times of India Travel [Internet]. 2021 [accessed 2021 Mar 14]. https://timesofindia.indiatimes.com/travel/travel-news/covid-vaccine-passport-the-new-trend-being-adopted-by-world-nations/as80770025.cms.
- Murray A. Coronapas: the passport helping Denmark open up after Covid - BBC News [Internet]. 2021 [accessed 2021 Jun 10]. https://www.bbc.com/news/world-europe-56812293.
- Kiesnoski K Could vaccine passports be your ticket to quarantine-free vacations? [Internet]. CNBC2021 [accessed 2021 Jun 10]. https://www.cnbc.com/2021/05/04/could-vaccine-passports-be-your-ticket-to-quarantine-free-vacations.html.
- Mzezewa T. Coming Soon: the ‘Vaccine Passport’ - The New York Times [Internet]. The NewYork Times 2021 [accessed 2021 Mar 14]. https://www.nytimes.com/2021/02/04/travel/coronavirus-vaccine-passports.html?.
- BBC News. What is a Covid passport and what are the plans for the NHS app? - BBC News [Internet]. BBC News 2021 [accessed 2021 Jun 10]. https://www.bbc.com/news/explainers-55718553.
- Kramer EA. Russia Mulls “Covid-19 Passports” to Let People with Some Immunity Travel More Easily - The New York Times [Internet]. New York Times. 2021 [accessed 2021 Mar 14]. https://www.nytimes.com/2021/01/19/world/russia-covid-passports.html.
- Reuters. Russia considers COVID-19 vaccination certificates for cross-border travels | Reuters [Internet]. 2021 [accessed 2021 Mar 14]. https://www.reuters.com/article/us-health-coronavirus-russia-cases-idUSKBN2991BI.
- Finland planning to introduce coronavirus vaccine certificate | yle Uutiset | yle.fi [Internet]. 2021 [accessed 2021 Mar 14]. https://yle.fi/uutiset/osasto/news/finland_planning_to_introduce_coronavirus_vaccine_certificate/11775698.
- Finnish institute for health and welfare. COVID-19 vaccination certificate - Infectious diseases and vaccinations - THL [Internet]. 2021 [accessed 2021 Jun 10]. https://thl.fi/en/web/infectious-diseases-and-vaccinations/what-s-new/coronavirus-covid-19-latest-updates/transmission-and-protection-coronavirus/vaccines-and-coronavirus/covid-19-vaccination-certificate.
- Winosa Y Indonesia to approve world’s first halal-certified COVID-19 vaccine | salaam Gateway - Global Islamic Economy Gateway [Internet]. 2021 [accessed 2021 Mar 14]. https://salaamgateway.com/story/indonesia-to-approve-worlds-first-halal-certified-covid-19-vaccine.
- Williams E. COVID-19 vaccine passports on the way via Services Australia | the Canberra Times | Canberra, ACT [Internet]. The Canberra Times. 2021 [accessed 2021 Mar 14]. https://www.canberratimes.com.au/story/7117854/what-could-i-need-a-covid-19-passport-for/.
- The Local. How do you get an Italian Covid vaccination certificate? - The Local [Internet]. 2021 [accessed 2021 Jun 17]. https://www.thelocal.it/20210527/how-do-you-get-an-italian-covid-vaccination-certificate/.
- Euronews. Growing number of Italians hesitant about taking COVID-19 vaccine | euronews [Internet]. euronews 2020 [accessed 2021 Mar 14]. https://www.euronews.com/2020/11/26/growing-number-of-italians-hesitant-about-taking-covid-19-vaccine.
- The Local. Can I use a foreign Covid-19 vaccination certificate to enter Sweden? - The Local [Internet]. 2021 [accessed 2021 Jun 17]. https://www.thelocal.se/20210525/can-i-use-a-foreign-covid-19-vaccination-certificate-to-enter-sweden/.
- Blatchford A World moves to embrace vaccine passports. Trudeau’s not so sure - POLITICO [Internet]. Politico. 2021 [accessed 2021 Mar 14]. https://www.politico.com/news/2021/03/09/trudeau-covid-vaccine-passports-474789.
- MacCharles T. Justin Trudeau floats requiring proof of vaccines for international travellers | the Star [Internet]. Tor. Star 2021 [accessed 2021 Mar 14]. https://www.thestar.com/politics/federal/2021/03/12/justin-trudeau-muses-about-requiring-proof-of-vaccines-for-international-travellers.html.
- Planetski. Will we need a vaccine passport to head to the mountains next winter? - PlanetSKI [Internet]. 2021 [accessed 2021 Mar 14]. https://planetski.eu/2021/02/25/will-we-need-a-vaccine-passport-to-head-to-the-mountains-next-winter/.
- Bullock C Switzerland works on a digital vaccine certificate system | ITIJ [Internet]. ITIJ2021 [accessed 2021 Jun 17]. https://www.itij.com/latest/news/switzerland-works-digital-vaccine-certificate-system.
- The local. What’s the latest on getting a vaccination certificate in Spain? - The Local [Internet]. 2021 [accessed 2021 Jun 17]. https://www.thelocal.es/20210531/whats-the-latest-on-getting-a-vaccination-certificate-in-spain/.
- Mouzo J. Coronavirus: covid passport: the new worlds opening up for the vaccinated | society | EL PAÍS in English [Internet]. 2021 [accessed 2021 Mar 17]. https://english.elpais.com/spanish_news/2021-03-11/covid-passport-the-new-worlds-opening-up-for-the-vaccinated.html.
- Ferguson C, Mitnick J. Israel’s “green pass” vaccine passport is an early vision of how we leave lockdown | MIT Technology Review [Internet]. 2021 [accessed 2021 Mar 17]. https://www.technologyreview.com/2021/03/01/1020154/israels-green-pass-is-an-early-vision-of-how-we-leave-lockdown/.
- Euronews. European commission outlines plans for green pass for freedom of travel within bloc | euronews [Internet]. 2021 [accessed 2021 Mar 17]. https://www.euronews.com/2021/03/17/european-commission-presents-plans-for-green-pass-for-freedom-of-travel-within-bloc.
- European Commission. EU Digital COVID Certificate | European Commission [Internet]. 2021 [accessed 2021 Jun 17]. https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans/eu-digital-covid-certificate_en.
- Imeri D The European Commissions Digital Green Certificate | IHS Markit [Internet]. IHS Markit. 2021 [accessed 2021 May 31]. https://ihsmarkit.com/research-analysis/the-european-commissions-digital-green-certificate.html.
- Hetzner C Europe moves closer to adopting COVID vaccine passports. Here’s who would qualify | fortune [Internet]. Fortune 2021 [accessed 2021 May 31]. https://fortune.com/2021/05/21/europe-moves-closer-to-adopting-covid-vaccine-passports-heres-who-would-qualify/.
- World Economic Forum. Global standards mapping initiative: an overview of blockchain technical standards. White Paper. [Internet]. 2020 [accessed 2021 Mar 14]. http://www3.weforum.org/docs/WEF_GSMI_Technical_Standards_2020.pdf.
- Powers B. Blockchain-based immunity passports don’t end privacy issue - CoinDesk [Internet]. 2020 [accessed 2021 Mar 14]. https://www.coindesk.com/blockchain-immunity-passports-core-privacy-issues.
- Allison I. COVID-19 ‘Immunity Passport’ Unites 60 Firms on Self-Sovereign ID Project - CoinDesk [Internet]. 2020 [accessed 2021 Mar 14]. https://www.coindesk.com/covid-19-immunity-passport-unites-60-firms-on-self-sovereign-id-project.
- Layden ER COUNTERPOINT: idea of immunity passports for COVID-19 has merit | saltWire [Internet]. SALTWIRE2020. [accessed 2021 Jul 26]. https://www.saltwire.com/halifax/opinion/local-perspectives/counterpoint-idea-of-immunity-passports-for-covid-19-has-merit-457210/.
- Football for vaccination? UK could go full Orwell with ‘QR code certificates’ as health authorities pick carrot over stick — RT UK News [Internet]. 2020 [accessed 2021 Jul 26]. https://www.rt.com/uk/507121-vaccination-qr-codes-football/.
- Johnson S Sydney doctor Zac Turner wants anti-vaxxers to be banned with shopping malls, public transport | daily Mail Online [Internet]. Dly. Mail. 2020 [accessed 2021 Jul 26]. https://www.dailymail.co.uk/news/article-8641949/Sydney-doctor-Zac-Turner-wants-anti-vaxxers-banned-shopping-malls-public-transport.html.
- Forrest A Back to normal in 2021? Why the Covid vaccine could create a better society than before | the Independent [Internet]. INDEPENDENT. 2020 [accessed 2021 Jul 26]. https://www.independent.co.uk/news/uk/home-news/coronavirus-vaccine-pfizer-rules-masks-b1722583.html.
- Wyman O. After Coronavirus, E-Passports Could Help Prevent The Next Pandemic [Internet]. Forbes. 2020 [accessed 2021 Jul 26]. https://www.forbes.com/sites/oliverwyman/2020/06/05/after-covid-19-how-e-passports-could-help-prevent-the-next-pandemic/?sh=61853afa1d5f.
- Buckley J What a Covid-19 vaccine means for the travel industry | CNN Travel [Internet]. CNN Travel. 2020 [accessed 2021 Jul 26]. https://www.cnn.com/travel/article/covid-vaccine-travel/index.html.
- Challenges posed with “immunity passports” and vaccination certificates for COVID-19 | healthcare Purchasing News [Internet]. 2020 [accessed 2021 Mar 14]. https://www.hpnonline.com/infection-prevention/screening-surveillance/article/21137049/challenges-posed-with-immunity-passports-and-vaccination-certificates-for-covid19.
- Higham A Coronavirus vaccine: can you be BANNED from work if you refuse vaccine | express.co.uk [Internet]. Express. 2020 [accessed 2021 Mar 14]. https://www.express.co.uk/life-style/health/1360782/coronavirus-vaccine-can-you-be-banned-from-work-refuse-covid-vaccine-EVG
- Kramer J. COVID-19 vaccines could become mandatory. Here’s how it might work. [Internet]. Natl Geogr Mag. 2020 [accessed 2021 Mar 14]. https://www.nationalgeographic.com/science/article/how-coronavirus-covid-vaccine-mandate-would-actually-work-cvd.
- Chandran R Back to work? Not without a check-in app, immunity passport | Reuters [Internet]. 2020 [accessed 2021 Mar 14]. https://www.reuters.com/article/us-health-coronavirus-tech-idUSKBN24701B.
- Phelan AL. COVID-19COVID-19 immunity passports and vaccination certificates: scientific, equitable, and legal challenges. Lancet [Internet]. 2020 [accessed 2021 Mar 14];395:1595–98. doi:10.1016/j.ssci.2020.104791
- Chng E, Atherton J, Hyland. Blockchain and the Digital Passport | cDOTrends [Internet]. 2020 [accessed 2021 Mar 14]. https://www.cdotrends.com/story/14852/blockchain-and-digital-passport.
- Abu-elezz I, Hassan A, Nazeemudeen A, Househ M, Abd-alrazaq A. The benefits and threats of blockchain technology in healthcare: a scoping review. Int J Med Inform. 2020;142:104246. doi:10.1016/j.ijmedinf.2020.104246.
- Abd-alrazaq AA, Alajlani M, Alhuwail D, Erbad A, Giannicchi A, Shah Z, Hamdi M, Househ M. Blockchain technologies to mitigate COVID-19 challenges: a scoping review. Comput Methods Programs Biomed Updat. 2020;1:100001. doi:10.1016/j.cmpbup.2020.100001.
- Mackey TK, Kuo TT, Gummadi B, Clauson KA, Church G, Grishin D, Obbad K, Barkovich R, Palombini M. “Fit-for-purpose?” - Challenges and opportunities for applications of blockchain technology in the future of healthcare. BMC Med [Internet]. 2019 [accessed 2021 Mar 15];17:68. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1296-7.
- D’Amore R No coronavirus vaccine, no entry? Experts say it’s possible in pandemic’s next stage - National | globalnews.ca [Internet]. Glob. News 2020 [accessed 2021 Mar 15]. https://globalnews.ca/news/7457999/ticketmaster-covid19-vaccine-canada/.
- Tanner R, Flood CM. Vaccine passports done equitably. JAMA Heal Forum [Internet]. 2021 [accessed 2021 May 31];2:e210972. doi:10.1001/jamahealthforum.2021.0972.
- Halpin H. Vision: a critique of immunity passports and W3C decentralized identifiers [internet]. In: lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics). Springer Science and Business Media Deutschland GmbH; 2020 [accessed 2021 Mar 14]. 148–68. doi: 10.1007/978-3-030-64357-7_7
- Parker LJ The case for one Covid passport [Internet]. Forbes 2020 [accessed 2021 Mar 14]. https://www.forbes.com/sites/jenniferleighparker/2020/12/22/the-case-for-one-covid-passport/?sh=66ed0b3b301d.
- eHealth Network. Guidelines on verifiable vaccination certificates-basic interoperability elements Release 2 [Internet]. 2021 [accessed 2021 May 30]. https://ec.europa.eu/health/sites/default/files/ehealth/docs/vaccination-proof_interoperability-guidelines_en.pdf.
- World Health Organization. Interim guidance for developing a Smart Vaccination Certificate Interim guidance for developing a Smart Vaccination Certificate ISBN XXX-XX-X-XXXXXX-X (electronic version) Will be available for version 1 release ISBN XXX-XX-X-XX [Internet]. 2021 [accessed 2021 Jun 17]. https://cdn.who.int/media/docs/default-source/documents/interim-guidance-svc_20210319_final.pdf?sfvrsn=b95db77d_11&download=true.
- Liew CH, Flaherty GT. Immunity passports to travel during the COVID-19 pandemic: controversies and public health risks. J Public Health (Bangkok) [Internet]. 2020 [accessed 2021 Mar 14]. Available from https://www.who.int/news-roo.
- Wilson K, Flood CM. Implementing digital passports for SARS-CoV-2 immunization in Canada. CMAJ [Internet]. 2021 [accessed 2021 Jun 10];193:E486–8. doi:10.1503/cmaj.210244.