ABSTRACT
Streptococcus pneumoniae and influenza viruses are associated with significant morbidity and mortality in older adults. Concomitant vaccination against these agents reduces hospitalization and mortality rates. This phase 3 trial evaluated safety, tolerability, and immunogenicity of concomitant and non-concomitant administration of V114, a 15-valent pneumococcal conjugate vaccine containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19F, 19A, 22F, 23F, 33F, and quadrivalent inactivated influenza vaccine (QIV), in healthy adults aged ≥50 years. Participants (N = 1,200) were randomized 1:1 to receive either V114 administered concomitantly with QIV (concomitant group) or QIV plus placebo (non-concomitant group) on Day 1, followed by placebo (concomitant group) or V114 (non-concomitant group) 30 days later. Randomization was stratified by age and history of pneumococcal polysaccharide vaccine receipt. Overall, 426 (71.0%) and 438 (73.5%) participants in the concomitant and non-concomitant groups experienced solicited injection-site adverse events (AEs); 278 (46.3%) and 300 (50.3%) reported solicited systemic AEs. Most solicited AEs were mild or moderate in severity and of short duration. Non-inferiority for pneumococcal- and influenza-specific antibody responses (lower bound 95% confidence interval of opsonophagocytic activity [OPA] and hemagglutination inhibition geometric mean titers [GMTs] ratios ≥0.5) was demonstrated for concomitant versus non-concomitant administration for all 15 pneumococcal serotypes and all four influenza strains. Consistent with previous studies, a trend was observed toward lower pneumococcal OPA GMTs in the concomitant versus the non-concomitant group. V114 administered concomitantly with QIV is generally well tolerated and immunologically non-inferior to non-concomitant administration, supporting coadministration of both vaccines.
Introduction
Streptococcus pneumoniae and influenza virus cause significant burden of disease worldwide in both adult and pediatric populations.Citation1–4 In adults ≥50 years of age, there is an increased risk for pneumococcal disease and influenza-associated morbidity and mortality compared with younger adults.Citation5–7 In addition, pneumococcal pneumonia is a frequent complication of influenza in adults,Citation8,Citation9 leading to more severe clinical outcomes and placing a significant burden on health systems during the influenza season.Citation9,Citation10 Vaccination against both influenza viruses and S. pneumoniae in older adults has been shown to reduce rates of hospitalization and mortality.Citation10–13
Pneumococcal vaccine recommendations for adults vary by country; while some countries recommend vaccination of older adults with the 23-valent pneumococcal polysaccharide vaccine (PPSV23), others recommend administration of a pneumococcal conjugate vaccine (PCV), or a sequential PCV/PPSV23 regimen.Citation14–17 Owing to the emergence of non-PCV serotypes and persistence of some vaccine serotypes included in currently licensed PCVs, such as serotypes 3 and 19A,Citation2,Citation18 there is a need for the development of new PCVs with broader serotype coverage and improved effectiveness against disease caused by serotypes in licensed PCVs. V114 is a recently approved 15-valent PCV that contains serotypes 22F and 33F, in addition to the 13 serotypes included in the 13-valent PCV (PCV13; 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F).Citation19 In 2017, serotypes 22F and 33F were responsible for 9% and 4% of all invasive pneumococcal disease (IPD) cases, respectively, across age groups in the United States, and serotype 22F was associated with 7–8% of IPD cases in adults aged ≥45 years in Europe.Citation2,Citation18 Both serotypes have been associated with invasive disease,Citation20 and serotype 33F is associated with multidrug resistance.Citation21 V114 has been shown to be well tolerated and immunogenic in infants, young adults, and older adults in phase 2 trials.Citation19,Citation22,Citation23
Annual influenza vaccination for older adults is included in routine national vaccination programs in many countries.Citation16 As coverage of both influenza and pneumococcal vaccines in adults is low (45% in adults aged ≥19 years for influenza vaccine in 2016–2017 and 69% in adults aged ≥65 years for pneumococcal vaccine in 2017 in the United States),Citation24 concomitant administration is a desirable strategy to increase vaccine uptake.Citation25 Coadministration of PCV13 or PPSV23 with inactivated influenza vaccines has been shown previously to be well tolerated without adversely affecting immunogenicity (albeit with slightly reduced opsonophagocytic activity [OPA] responses in some studies), when compared with separate administration.Citation25–31 This study was designed to evaluate the safety, tolerability, and immunogenicity of a single dose of V114 when administered either concomitantly (on the same day) or non-concomitantly (30 days later) with quadrivalent inactivated influenza vaccine (QIV).
Methods
Study design
This was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the safety, tolerability, and immunogenicity of V114 administered concomitantly or non-concomitantly with QIV in healthy adults 50 years of age or older. The study is registered with ClinicalTrials.gov as NCT03615482. The trial was conducted from September 24, 2018, to June 24, 2019, at 45 sites across the United States.
Investigators and site staff enrolled participants using central randomization and assignment to vaccination group via an interactive response technology system. The randomization and vaccine identification schedules were created by an unblinded representative of the sponsor. As V114 and placebo differed in appearance, they were prepared, dispensed, and administered by unblinded study personnel who were not involved in any subsequent participant assessments. The participant and the investigator who was involved in the clinical evaluation of the participant remained blinded. QIV was administered open label.
The study was conducted in accordance with the principles of Good Clinical Practice and the ethical principles originating from the Declaration of Helsinki, and was approved by the appropriate institutional review boards, regulatory agencies, and independent ethics committees at participating sites. An external Data Monitoring Committee conducted periodic reviews of safety and tolerability data. Written informed consent was obtained from each participant prior to any study procedure.
The study was designed to enroll approximately 1,200 participants randomized in a 1:1 ratio to receive either V114 with concomitant QIV (concomitant group) or QIV and placebo (non-concomitant group) on Day 1. Approximately 30 days later, participants in the non-concomitant group received V114 and those in the concomitant group received placebo. Randomization was stratified by participant age at enrollment (50–64 years, 65–74 years, and ≥75 years), with at least 50% of participants being ≥65 years of age, and by history of PPSV23 receipt versus non-receipt, with at least 50% of participants being PPSV23-naïve. For participants who had prior vaccination with PPSV23, vaccination was required to have occurred >12 months prior to the first study visit. Blood samples were obtained for immunogenicity assays on Day 1, Day 30, and Day 60. Telephone contacts for safety review occurred on Day 15, Day 45, and Month 7.
Participants
Male or female participants ≥50 years of age in generally good health and/or with stable underlying medical conditions were eligible for the study. Key exclusion criteria included: history of IPD or other culture-positive pneumococcal disease within the previous 3 years, known hypersensitivity to a vaccine component, known or suspected impairment of immunological function, prior administration of any PCV, or prior administration of influenza vaccine during the 2018/2019 influenza season.
Vaccines and administration
V114 (VAXNEUVANCE™; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) is a 15-valent pneumococcal conjugate vaccine. Each 0.5 mL dose contains 2 µg of pneumococcal capsular polysaccharide from serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 23F, 22F, and 33F, and 4 µg of serotype 6B all conjugated to CRM197 carrier protein, adjuvanted with 125 µg of aluminum phosphate.
The Northern Hemisphere 2018–2019 season formulation of QIV (Fluarix® Quadrivalent; GlaxoSmithKline, Research Triangle Park, NC, USA) was used in this study, which included the following influenza strains: A/Michigan/45/2015 (H1N1) pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage), and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage).
V114 was provided as a sterile suspension and QIV was provided as a sterile solution. V114 and QIV were supplied in a prefilled syringe and stored at 2–8°C; placebo (sterile saline) was provided in an ampule and taken up into a syringe by unblinded study personnel who were not involved in any subsequent participant assessments. All vaccines and the placebo were provided in 0.5 mL doses and were administered intramuscularly in separate arms (V114 or placebo in the left arm and QIV in the right arm). Needle gauge size was determined per institutional guidelines for the study site and may have varied based on participant characteristics, such as body habitus.
Safety assessments
Adverse events (AEs) experienced following receipt of study vaccines were recorded by participants using an electronic Vaccination Report Card and subsequently assessed and reported by the investigator. Injection-site reactions (injection-site pain, injection-site swelling, and injection-site erythema) occurring from Days 1–5 after vaccination and systemic AEs (muscle pain/myalgia, joint pain/arthralgia, headache, and fatigue) occurring from Days 1–14 after vaccination were solicited.
Daily body temperature was measured on Days 1–5 after vaccination. In addition, participants were followed for non-solicited injection-site and systemic AEs from Days 1–14 after vaccination. Information for serious adverse events (SAEs) and deaths, regardless of whether the events were considered to be vaccine related by the investigator, were collected from the time of signed consent through to the end of study (approximately 7 months after the first vaccination).
All solicited and non-solicited events were assessed for severity by the investigator. The severity of AEs was categorized as mild (Grade 1), moderate (Grade 2), severe (Grade 3), or potentially life-threatening (Grade 4), adapted from FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials September 2007.Citation32 For solicited injection-site erythema and injection-site swelling, mild events were those measuring >0 to ≤5 cm, moderate events measured >5 to ≤10 cm, and severe events measured >10 cm. For injection-site pain and systemic AEs, the severity was defined by the degree to which the event affected daily activities and the use of medications for pain relief.Citation32
All injection-site AEs were considered to be vaccine related. For systemic AEs, relatedness to study vaccine was assessed by the investigator.
Immunogenicity assessments
Immunogenicity analyses were conducted separately for each of the 15 pneumococcal serotypes in V114 and each of the four influenza strains in QIV. Serum samples were drawn pre-vaccination on Day 1 (baseline) and at 30 and 60 days post-vaccination (Day 30 and Day 60) to assess immune responses. Functional antibodies were measured using serotype-specific opsonophagocytic killing activity using a validated microcolony multiplexed opsonophagocytic assay (MOPA).Citation33 Serotype-specific pneumococcal capsular polysaccharide immunoglobulin G (IgG) antibodies were evaluated using a validated multiplexed electrochemiluminescence assay.Citation34 Both assays were developed by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The validated hemagglutination inhibition (HAI) test was performed by Q2 Solutions (San Juan Capistrano, CA, USA) for the four strains included in the vaccine.
Study endpoints and statistical analysis
Determination of sample size
The planned study sample size of 1,200 participants (600 per intervention group) provided nearly 90% power to demonstrate non-inferiority of V114 administered concomitantly with QIV to V114 administered non-concomitantly with QIV with respect to the serotype-specific OPA geometric mean titers (GMTs) for the 15 serotypes included in V114 and the strain-specific HAI GMT ratios for the four influenza strains in QIV at an overall 1-sided 2.5% alpha level. Assumptions for the sample size calculations were based on an underlying OPA GMT ratio of 0.80 (based on previous studies evaluating concomitant administration of PCV13 with influenza vaccines).Citation26,Citation28
Analysis populations
Safety analyses were conducted on the All Participants as Treated (APaT) population, which consisted of all randomized participants who received at least one dose of study vaccine.
The Per-Protocol (PP) population served as the primary population for the analysis of immunogenicity data. The PP population consisted of all randomized participants without deviations from the protocol that could have substantially affected the results of the immunogenicity endpoints.
Primary safety endpoints and statistical methods
The safety endpoints following any vaccination received on Day 1 or Day 30 included: i) the proportions of participants with solicited injection-site AEs from Day 1 through Day 5 post-vaccination, ii) the proportions of participants with solicited systemic AEs from Day 1 through Day 14 post-vaccination, and iii) the proportions of participants within the broad AE categories of any AE or SAE including vaccine-related SAEs. Point estimates were provided for all safety endpoints and 95% confidence intervals (CIs) were provided for between-treatment differences in the proportions of participants with solicited AEs and the aforementioned broad categories of AEs using the unstratified Miettinen and Nurminen method.Citation35 P-values were also provided for the differences in the proportions of participants with solicited AEs. No multiplicity adjustments were made for the safety comparisons.
Solicited injection-site AEs were also assessed following each individual study vaccine; data are reported for events following V114 only. Systemic AEs could not be assessed following individual vaccines in the concomitant group as events occurring following the vaccinations on Day 1 could not be attributed to a specific vaccine. Systemic AEs following vaccination with V114 in the non-concomitant group are reported.
Subgroup analyses of safety endpoints by age, sex, race, ethnicity, and history of PPSV23 receipt were performed.
Primary immunogenicity endpoints and statistical methods
The primary immunogenicity objectives were to compare V114 administered concomitantly with QIV to V114 administered non-concomitantly with QIV to assess: i) non-inferiority of serotype-specific OPA GMTs at 30 days post-vaccination with V114 for all 15 serotypes included in V114 and ii) non-inferiority of strain-specific HAI GMTs at 30 days post-vaccination with QIV for all four influenza strains included in QIV. The statistical criterion for non-inferiority required the lower bound of the 2-sided 95% CI for the OPA GMT ratio or HAI GMT ratio (concomitant/non-concomitant) to be greater than 0.5.
OPA GMTs and OPA GMT ratios (with corresponding 95% CIs) were estimated using serotype-specific constrained longitudinal data analysis (cLDA) models utilizing data from both vaccination groups.Citation36 The serotype-specific repeated measures models included vaccination group, time, the interaction of time-by vaccination group (with a restriction of the same baseline mean across groups), age stratum, age stratum-by-time interaction, history of PPSV23 receipt stratum, and history of PPSV23 receipt stratum-by-time interaction as fixed effects. Similarly, strain-specific cLDA models were used to calculate the HAI GMTs and HAI GMT ratios (with corresponding 95% CIs).
All hypotheses were tested individually for each pneumococcal serotype and each influenza strain at a 1-sided 0.025 alpha-level. Study success was predicated upon meeting all primary immunogenicity objectives and, thus, no multiplicity adjustments were required to control the 1-sided type-I error rate at 0.025.
To determine whether the intervention effect was consistent across the age strata (50–64 years, 65–74 years, and ≥75 years) and by history of PPSV23 receipt versus non-receipt strata, the estimate of the between-group treatment effect (with a nominal 95% CI) was summarized for the primary immunogenicity endpoints. Subgroup analyses were also performed by sex, race, and ethnicity.
Secondary immunogenicity endpoints and statistical methods
A secondary immunogenicity objective was to compare the serotype-specific IgG geometric mean concentrations (GMCs) for the 15 serotypes included in V114 at 30 days post-vaccination with V114 between vaccination groups. IgG GMCs and IgG GMC ratios (with corresponding 95% CIs) at 30 days post-vaccination with V114 were calculated using serotype-specific cLDA models similar to those described for the primary immunogenicity endpoints.
Additional secondary pneumococcal immunogenicity endpoints included observed serotype-specific geometric mean fold rises (GMFRs) and the proportions of participants with a ≥4-fold rise from pre-vaccination to 30 days post-vaccination with V114 for both OPA and IgG responses. Reverse cumulative distribution curves for OPA titers were generated per serotype.
Secondary influenza immunogenicity endpoints included strain-specific GMFRs from pre-vaccination to 30 days post-vaccination with QIV, the proportions of participants with an HAI titer of ≥1:40 at 30 days post-vaccination with QIV, and the proportions of participants who seroconverted at 30 days post-vaccination with QIV. Seroconversion for HAI responses was defined as either achievement of a 4-fold rise in HAI titer from pre-vaccination to 30 days post-vaccination with QIV among participants who were seropositive (HAI titer ≥1:10) at baseline or achievement of an HAI titer of ≥1:40 at 30 days post-vaccination with QIV among participants who were seronegative (HAI titer <1:10) at baseline.
Descriptive statistics with point estimates and within-group 95% CIs are provided for these endpoints. For the continuous endpoints, the within-group 95% CIs were obtained by exponentiating the CIs of the mean of the natural log values based on the t-distribution. For the dichotomous endpoints, the within-group 95% CIs were based on the exact binomial method proposed by Clopper and Pearson.Citation37
Analysis software
All the analyses were performed using SAS© software, version 9.4. of the SAS System for Unix. Copyright© 2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
Study population
Overall, 1,200 participants were randomized, with 600 in each group (). In the concomitant group, 599 (99.8%) participants were vaccinated with V114 and QIV on Day 1, and 583 (97.2%) received placebo on Day 30 and completed the study. In the non-concomitant group, 598 (99.7%) were vaccinated with QIV and 596 (99.3%) were vaccinated with placebo on Day 1 (two participants received V114 instead of placebo), 586 (97.7%) were vaccinated with V114 on Day 30 (one participant who mistakenly received V114 on Day 1 was given placebo on Day 30), and 583 (97.2%) completed the study. The participant who received two doses of V114 was excluded from the APaT population. The most common reasons for discontinuation were voluntary withdrawal of consent and loss to follow-up (both 1.2% in each group).
Figure 1. Participant disposition.
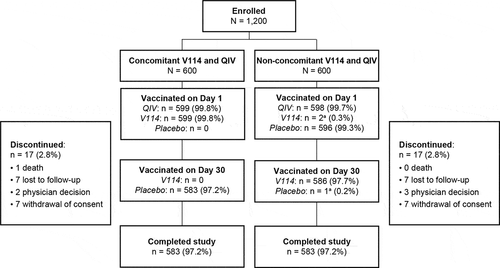
Both groups were generally balanced for baseline characteristics such as age, sex, race, ethnicity, and underlying medical conditions, as well as history of PPSV23 receipt (). The mean age of study participants was 64.2 years (range 50–98 years); 672 (56.1%) were female and 250 (20.9%) had a history of PPSV23 receipt. The most common conditions in the medical history of the participants were hypertension, osteoarthritis, hyperlipidemia, gastroesophageal reflux disease, and seasonal allergy. These and other reported medical conditions were comparable across the two vaccine groups (Supplementary Table 1).
Table 1. Baseline participant characteristics
Safety
Overall, 482 (80.3%) participants in the concomitant group and 488 (81.9%) in the non-concomitant group experienced at least one AE. The most frequently reported AEs were the solicited events of injection-site pain, injection-site swelling, injection-site erythema, fatigue, myalgia, headache, and arthralgia. In total, 430 (71.7%) and 440 (73.8%) of participants in the concomitant and non-concomitant groups, respectively, experienced any injection-site AE following any vaccination (); of these, 426 (71.0%) participants in the concomitant group and 438 (73.5%) in the non-concomitant group experienced solicited injection-site AEs, of which injection-site pain was the most common ( and ; observed differences between the concomitant and non-concomitant group were not statistically significant; injection-site erythema is not shown as the interpretability of results is limited by missing data). Three hundred and forty-one (56.8%) and 345 (57.9%) participants in the concomitant and non-concomitant groups experienced any systemic AE (); of these, 278 (46.3%) and 300 (50.3%) experienced solicited systemic AEs, of which fatigue was the most common ( and ). The majority of participants with solicited AEs had events that were mild or moderate in maximum severity (), and of short duration (≤3 days) (data not shown). The proportion of participants with elevated body temperature (≥100.4°F [38.0°C]) was low (≤1.5% in both groups) (data not shown).
Table 2. Adverse events after any vaccination
Figure 2. Proportion of participants with solicited adverse events after any vaccination by severity.
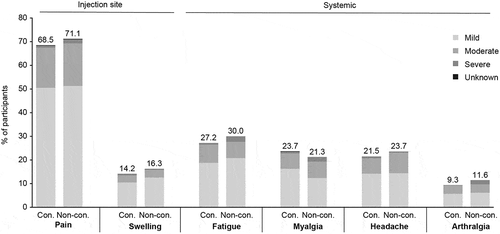
Following V114 vaccination only, the proportions of participants in each group experiencing solicited injection-site AEs were similar (403 [67.2%] in the concomitant group and 408 [69.7%] in the non-concomitant group) (Supplementary Table 2). Solicited systemic AEs after V114 occurred in 202 (34.5%) participants in the non-concomitant group (data not shown).
The proportion of participants experiencing SAEs was low (<4%) across both groups, and no SAEs were considered by the investigator to be related to study vaccine (). One participant in the concomitant group died of myocardial infarction on Day 42. Three participants discontinued study intervention due to AEs; of these, two experienced events considered to be related to study vaccine by the investigator. One participant in the concomitant group experienced sinusitis on Day 4 (lasting for 1.6 months), and one participant in the non-concomitant group experienced upper abdominal pain, fatigue, and nausea on Day 1 (lasting for 4 days, 2 weeks, and 3 days, respectively), rhinorrhea and arthralgia on Day 2 (lasting for 2 days and 3 days, respectively), and myalgia on Day 5 (lasting for 3 days).
Based on subgroup analyses, safety profiles across the two vaccine groups were generally comparable by sex, race, and history of PPSV23 administration (data not shown). Trends were observed toward a lower proportion of participants aged ≥65 years compared with those aged 50–64 years experiencing AEs, and toward lower proportions of Hispanic or Latino participants versus non-Hispanic or non-Latino participants experiencing solicited AEs (Supplementary Tables 3 and 4).
Immunogenicity
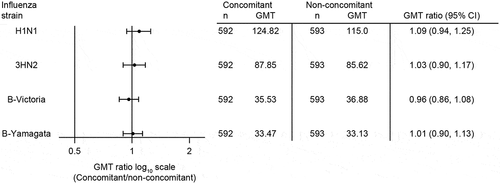
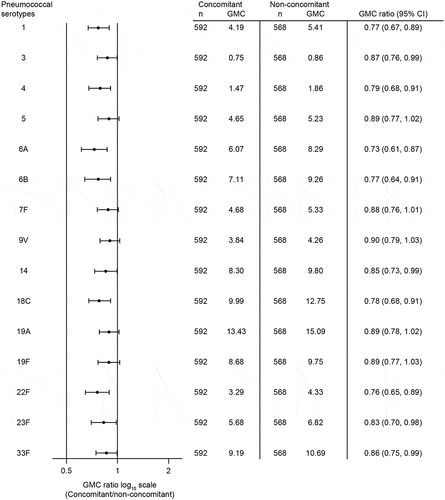
V114 administered concomitantly with QIV was non-inferior to V114 administered non-concomitantly with QIV as assessed by OPA GMTs at 30 days post-vaccination with V114 for all 15 serotypes in the vaccine. The lower bound of the 2-sided 95% CI of the OPA GMT ratio was >0.50 for all serotypes (). A trend toward lower OPA GMTs in the concomitant group compared with the non-concomitant group was observed (). QIV administered concomitantly with V114 was non-inferior to QIV administered non-concomitantly with V114 as assessed by HAI GMTs at 30 days post-vaccination with QIV. The lower bound of the 2-sided 95% CI of the HAI GMT ratio was >0.50 for all strains ().
Figure 3. Estimated serotype-specific OPA GMTs 30 days after vaccination with V114.
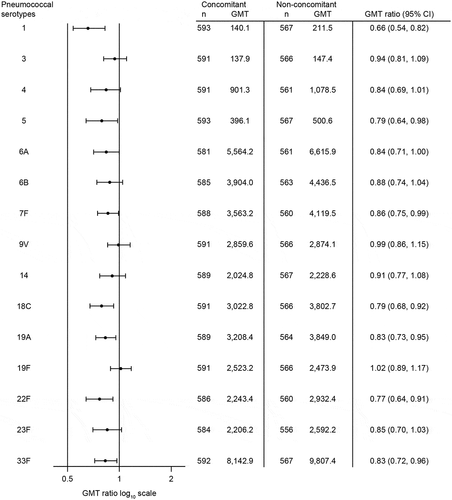
Figure 4. Reverse cumulative distribution curves for OPA GMTs by serotype.
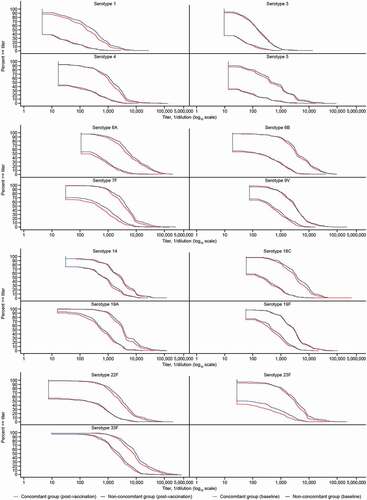
Figure 5. Estimated strain-specific HAI GMTs at 30 days after vaccination with QIV.
IgG GMCs at 30 days post-vaccination with V114 were generally consistent with OPA GMTs (). OPA GMFRs and proportions of participants with a ≥4-fold rise in OPA GMTs and IgG GMCs from baseline to 30 days post-vaccination with V114 were generally comparable between the concomitant and non-concomitant groups; there was a trend toward lower IgG GMFRs in the concomitant group compared with the non-concomitant group (). HAI GMFRs, proportions of participants with HAI titer ≥1:40, and proportions of participants who seroconverted at 30 days post-vaccination with QIV were generally comparable between the concomitant and non-concomitant groups ().
Table 3. GMTs, GMFR, and proportion of participants with ≥4-fold rise in serotype-specific OPA antibodies from pre-vaccination to 30 days after vaccination with V114
Table 4. GMCs, GMFR, and proportion of participants with ≥4-fold rise in serotype-specific IgG antibodies from pre-vaccination to 30 days after vaccination with V114
Table 5. GMT, GMFR, and proportion of participants with seroconversion or HAI titer ≥1:40 from pre-vaccination to 30 days or at 30 days after vaccination with QIV
Figure 6. Estimated serotype-specific IgG GMCs 30 days after vaccination with V114.
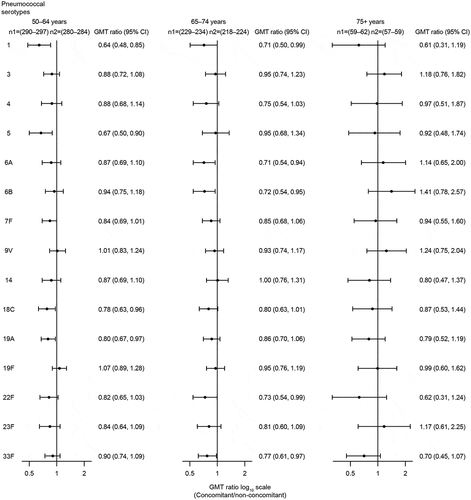
Subgroup analyses of OPA GMT ratios by age, sex, ethnicity, race, and history of PPSV23 administration were generally consistent with results observed in the overall population. Within both the concomitant and non-concomitant groups, there was a trend toward lower serotype-specific OPA GMTs in older (≥65 years) versus younger (50–64 years) participants ().
Figure 7. Estimated serotype-specific OPA GMTs 30 days after vaccination with V114 by age group.
Discussion
This study of adults ≥50 years of age who were in generally good health and/or with stable underlying medical conditions demonstrated that concomitant administration of V114 and QIV on the same day is generally well tolerated, with a safety profile similar to that of non-concomitant administration of QIV and V114 given 30 days apart. The proportions of participants experiencing injection-site and systemic AEs when administered V114 concomitantly or non-concomitantly with QIV are generally comparable with those seen in previous studies of V114 in older adults.Citation22,Citation38 Vaccine-induced immune responses in the concomitant group are non-inferior to the non-concomitant group for the 15 pneumococcal serotypes in V114 and the four influenza strains in QIV, as measured by OPA and HAI GMTs at 30 days post-vaccination. These findings demonstrate that V114 and QIV can be administered concomitantly in healthy older adults without significantly affecting the safety or immunogenicity of these vaccines when given separately.
Consistent with a number of previous studies evaluating the coadministration of PCV13 with trivalent inactivated influenza vaccine or QIV,Citation25–29 there was a trend toward lower pneumococcal serotype-specific OPA GMTs and IgG GMCs 30 days following V114 vaccination in the concomitant group, which resulted in the point estimates of the GMT ratios and GMC ratios being <1.0 for most serotypes. A trend toward lower GMFRs from baseline to 30 days post-vaccination with concomitant administration of V114 and QIV compared with V114 alone was also observed for serotype-specific IgG GMCs. OPA GMFRs and the proportion of participants with a ≥4-fold rise of OPA and IgG responses from baseline to post-vaccination were generally comparable between groups, suggesting that between-group differences in serotype-specific OPA GMTs and IgG GMCs may have limited clinical impact. Notably, in a previous study, small differences in serotype-specific OPA and IgG responses 30 days after concomitant administration of PCV and trivalent influenza vaccine compared with non-concomitant administration were not maintained over time,Citation39 suggesting that the between-group differences observed may be transient. No effectiveness studies are available for the concomitant administration of a PCV and influenza vaccine in adults; however, effectiveness studies of the concomitant use of PPSV23 and trivalent influenza vaccine support the coadministration of both vaccines to prevent pneumonia, influenza hospitalizations, and death.Citation11–13
The coadministration of V114 and QIV did not impact strain-specific influenza responses when compared with non-concomitant administration. These findings are consistent with studies that have evaluated the coadministration of PCV13 and influenza vaccines.Citation25–27,Citation29
Subgroup analyses showed a trend toward lower pneumococcal immune responses in older adults (65–74 years and ≥75 years) compared with younger adults (50–64 years) in both the concomitant and non-concomitant groups. This is consistent with PCV administration in older adults,Citation40 as well as a previous study of PPSV23 and influenza vaccine coadministration in older adults,Citation30 and is likely attributable to immunosenescence.Citation41,Citation42 Within the subgroup of participants with history of PPSV23 receipt, pneumococcal and influenza immune responses were comparable between the concomitant and non-concomitant groups. Similar results were observed in a clinical trial that evaluated concomitant administration of PCV13 and QIV in participants ≥50 years of age who had received PPSV23 at least 1 year prior to enrollment.Citation29
Limitations of this study include the lack of follow-up for immunogenicity beyond 30 days following each vaccination. In addition, the study did not evaluate the efficacy of concomitant versus non-concomitant administration of QIV and V114, given the lack of a well-accepted serotype-specific threshold level of antibody titers or concentrations needed to protect against pneumococcal disease in adults.
In conclusion, in adults ≥50 years of age who are generally in good health, concomitant administration of V114 and QIV is generally well tolerated and is immunologically non-inferior to non-concomitant administration for all 15 pneumococcal serotypes in V114 and all four influenza strains in QIV, supporting coadministration of these two vaccines.
Author contributions
Severance R, Schwartz H, and Davis M: enrollment of participants and/or data collection; review of the manuscript.
Li J and Buchwald UK: study concept and design; preparation of the manuscript.
Connor L, Pedley A, Sterling TM, Nolan KM, and Tamms GM: analysis and interpretation of data; preparation of the manuscript.
Dagan R, Hartzel J, and Musey LK: study concept and design; analysis and interpretation of data; review of the manuscript.
Data sharing
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to [email protected].
Disclosure of potential conflicts of interest
Connor L, Li J, Pedley A, Hartzel J, Sterling TM, Nolan KM, Tamms GM, Musey LK, and Buchwald UK are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Dagan R has received grants/research support from Pfizer, Merck Sharp & Dohme, and Medimmune, has been a scientific consultant for Pfizer, MeMed, Merck Sharp & Dohme Corp, and BiondVax, has served on advisory boards of Pfizer, Merck Sharp & Dohme Corp, and BiondVax, and has been a speaker for Pfizer. Severance R has received research support from Merck Sharp & Dohme Corp.
Supplemental Material
Download MS Word (219.5 KB)Supplemental Material
Download MS Word (67 KB)Acknowledgments
We would like to thank each of the participants, study staff, and investigators in the V114-021 (PNEU-FLU) study group for their invaluable contributions to this study. A full list of investigators for this study can be found in Supplementary Table 5. Medical writing support, including assisting authors with the development of the initial draft and incorporation of comments, was provided by Rachel Wright, PhD of Scion, London, and Jon E. Stek and Karyn Davis of Merck & Co., Inc, Kenilworth, NJ, USA and editorial support was provided by Annabel Ola and Ian Norton of Scion, London, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1976581.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report: Streptococcus pneumoniae, 2018. 2018 [accessed 2020 July 23]. https://www.cdc.gov/abcs/reports-findings/survreports/spneu17.html .
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease. Annual epidemiological report for 2018. 2018 [accessed 2020 Sept 14]. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_IPD.pdf .
- European Centre for Disease Prevention and Control. Seasonal influenza 2018–2019. Annual epidemiological report. 2019 [accessed 2020 Nov 7]. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_seasonal-influenza-corrected.pdf .
- Xu X, Blanton L, Elal AIA, Alabi N, Barnes J, Biggerstaff M, Brammer L, Budd AP, Burns E, Cummings CN, et al. Update: influenza activity in the United States during the 2018-19 season and composition of the 2019-20 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2019;68:544–51. doi:10.15585/mmwr.mm6824a3.
- Kristensen M, van Lier A, Eilers R, McDonald SA, Opstelten W, Van Der Maas N, van der Hoek W, Kretzschmar ME, Nielen MM, de Melker HE. Burden of four vaccine preventable diseases in older adults. Vaccine. 2016;34:942–49. doi:10.1016/j.vaccine.2015.12.052.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi:10.1111/1469-0691.12461.
- Sullivan SG, Price OH, Regan AK. Burden, effectiveness and safety of influenza vaccines in elderly, paediatric and pregnant populations. Ther Adv Vaccines Immunother. 2019;7:2515135519826481. doi:10.1177/2515135519826481.
- Walter ND, Taylor TH, Shay DK, Thompson WW, Brammer L, Dowell SF, Moore MR, Active Bacterial Core Surveillance Team. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–83. doi:10.1086/649208.
- Weinberger DM, Harboe ZB, Viboud C, Krause TG, Miller M, Molbak K, Konradsen HB. Pneumococcal disease seasonality: incidence, severity and the role of influenza activity. Eur Respir J. 2014;43:833–41. doi:10.1183/09031936.00056813.
- Gilchrist SA, Nanni A, Levine O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakers. Am J Public Health. 2012;102:596–605. doi:10.2105/AJPH.2011.300512.
- Dominguez A, Soldevila N, Toledo D, Torner N, Force L, Perez MJ, Martin V, Rodriguez-Rojas L, Astray J, Egurrola M, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospitalization and severe outcomes in the elderly in Spain. PLoS One. 2017;12:e0171943. doi:10.1371/journal.pone.0171943.
- Yin M, Huang L, Zhang Y, Yu N, Xu X, Liang Y, Ni J. Effectiveness and safety of dual influenza and pneumococcal vaccination versus separate administration or no vaccination in older adults: a meta-analysis. Expert Rev Vaccines. 2018;17:653–63. doi:10.1080/14760584.2018.1495077.
- Zhang YY, Tang XF, Du CH, Wang BB, Bi ZW, Dong BR. Comparison of dual influenza and pneumococcal polysaccharide vaccination with influenza vaccination alone for preventing pneumonia and reducing mortality among the elderly: a meta-analysis. Hum Vaccin Immunother. 2016;12:3056–64. doi:10.1080/21645515.2016.1221552.
- Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut– 2017/2018. 2017 accessed 2020 Mar 19]. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2017/Ausgaben/34_17.pdf?__blob=publicationFile .
- Australian Immunisation Handbook. Pneumococcal disease. 2020 [accessed 2020 Aug 3]. https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/pneumococcal-disease .
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2020 global summary. 2020 accessed 2020 Nov 7]. https://apps.who.int/immunization_monitoring/globalsummary/schedules .
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069–75. doi:10.15585/mmwr.mm6846a5.
- Varghese J, Chochua S, Tran T, Walker H, Li Z, Snippes Vagnone PM, Lynfield R, McGee L, Li Y, Metcalf BJ, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26:512e1- e10. doi:10.1016/j.cmi.2019.09.008.
- Rupp R, Hurley D, Grayson S, Li J, Nolan K, McFetridge RD, Hartzel J, Abeygunawardana C, Winters M, Pujar H, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15:549–59. doi:10.1080/21645515.2019.1568159.
- Balsells E, Dagan R, Yildirim I, Gounder PP, Steens A, Munoz-Almagro C, Mameli C, Kandasamy R, Givon Lavi N, Daprai L, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect. 2018;77:368–78. doi:10.1016/j.jinf.2018.06.004.
- Adam HJ, Golden AR, Karlowsky JA, Baxter MR, Nichol KA, Martin I, Demczuk W, Mulvey MR, Gilmour MW, Hoban DJ, et al. Analysis of multidrug resistance in the predominant Streptococcus pneumoniae serotypes in Canada: the SAVE study, 2011–15. J Antimicrob Chemother. 2018;73:vii12–vii9. doi:10.1093/jac/dky158.
- Stacey HL, Rosen J, Peterson JT, Williams-Diaz A, Gakhar V, Sterling TM, Acosta CJ, Nolan KM, Li J, Pedley A, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother. 2019;15:530–39. doi:10.1080/21645515.2018.1532249.
- Ermlich SJ, Andrews CP, Folkerth S, Rupp R, Greenberg D, McFetridge RD, Hartzel J, Marchese RD, Stek JE, Abeygunawardana C, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults ≥50 years of age. Vaccine. 2018;36:6875–82. doi:10.1016/j.vaccine.2018.03.012.
- Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, National Health Interview Survey, 2017. 2018 [accessed 2020 Sept 7]. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.html .
- Song JY, Cheong HJ, Hyun HJ, Seo YB, Lee J, Wie SH, Choi MJ, Choi WS, Noh JY, Yun JW, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine and an MF59-adjuvanted influenza vaccine after concomitant vaccination in 60-year-old adults. Vaccine. 2017;35:313–20. doi:10.1016/j.vaccine.2016.11.047.
- Frenck RW Jr., Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D, Giardina PC, Emini EA, Gruber WC, Scott DA, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19:1296–303. doi:10.1128/CVI.00176-12.
- Schwarz TF, Flamaing J, Rumke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged >/=65 years. Vaccine. 2011;29:5195–202. doi:10.1016/j.vaccine.2011.05.031.
- Schwarz TF, Schmoele-Thoma B. Assessment of functional antibacterial opsonophagocytic antibodies elicited by 13-valent pneumococcal conjugate vaccine administered concomitantly with trivalent influenza vaccine in a randomized clinical trial in adults aged >/=65 years. Vaccine. 2013;31:291–94. doi:10.1016/j.vaccine.2012.10.077.
- Thompson AR, Klein NP, Downey HJ, Patterson S, Sundaraiyer V, Watson W, Clarke K, Jansen KU, Sebastian S, Gruber WC, et al. Coadministration of 13-valent pneumococcal conjugate and quadrivalent inactivated influenza vaccines in adults previously immunized with polysaccharide pneumococcal vaccine 23: a randomized clinical trial. Hum Vaccin Immunother. 2019;15:444–51. doi:10.1080/21645515.2018.1533777.
- Ofori-Anyinam O, Leroux-Roels G, Drame M, Aerssens A, Maes C, Amanullah A, Schuind A, Li P, Jain VK, Innis BL. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine co-administered with a 23-valent pneumococcal polysaccharide vaccine versus separate administration, in adults >/=50 years of age: results from a phase III, randomized, non-inferiority trial. Vaccine. 2017;35:6321–28. doi:10.1016/j.vaccine.2017.09.012.
- Nakashima K, Aoshima M, Ohfuji S, Yamawaki S, Nemoto M, Hasegawa S, Noma S, Misawa M, Hosokawa N, Yaegashi M, et al. Immunogenicity of simultaneous versus sequential administration of a 23-valent pneumococcal polysaccharide vaccine and a quadrivalent influenza vaccine in older individuals: a randomized, open-label, non-inferiority trial. Hum Vaccin Immunother. 2018;14:1923–30. doi:10.1080/21645515.2018.1455476.
- Food and Drug Administration. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007 [accessed 2021 Jan 29]. https://www.fda.gov/media/73679/download .
- Nolan KM, Bonhomme ME, Schier CJ, Green T, Antonello JM, Murphy RD. Optimization and validation of a microcolony multiplexed opsonophagocytic killing assay for 15 pneumococcal serotypes. Bioanalysis. 2020;12:1003–20. doi:10.4155/bio-2020-0024.
- Nolan KM, Zhang Y, Antonello JM, Howlett AH, Bonhomme CJ, Greway R, Green T, Gorguette d’Argoeuves P, Goldblatt D, Murphy RD. Enhanced antipneumococcal antibody electrochemiluminescence assay: validation and bridging to the WHO reference ELISA. Bioanalysis. 2020;12:1363–75. doi:10.4155/bio-2020-0023.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi:10.1002/sim.4780040211.
- Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya: The Indian Journal of Statistics. 2000;62:134–48.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. doi:10.1093/biomet/26.4.404.
- Peterson JT, Stacey HL, MacNair JE, Li J, Hartzel JS, Sterling TM, Benner P, Tamms GM, Musey LK. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine compared to 13-valent pneumococcal conjugate vaccine in adults ≥65 years of age previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Hum Vaccin Immunother. 2019;15:540–48. doi:10.1080/21645515.2018.1532250.
- Frenck RW Jr., Fiquet A, Gurtman A, van Cleeff M, Davis M, Rubino J, Smith W, Sundaraiyer V, Sidhu M, Emini EA, et al. Immunogenicity and safety of a second administration of 13-valent pneumococcal conjugate vaccine 5 years after initial vaccination in adults 50 years and older. Vaccine. 2016;34:3454–62. doi:10.1016/j.vaccine.2016.04.093.
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–602. doi:10.1016/j.vaccine.2013.04.084.
- Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi:10.1186/1742-4933-4-9.
- Krone CL, van de Groep K, Trzcinski K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. Lancet Respir Med. 2014;2:141–53. doi:10.1016/S2213-2600(13)70165-6.
