ABSTRACT
Postvaccination serologic testing (PVST) is utilized to monitor the success or failure of vaccination against hepatitis B virus (HBV) infection in infants of hepatitis B surface antigen (HBsAg) positive mothers. This secondary analysis of 1255 infants of HBsAg-positive mothers at 7–14 months age included in two prospective studies aimed to determine the optimal interval for PVST after three hepatitis B vaccine doses. HBsAg and anti-HBs were quantitatively tested with microparticle enzyme immunoassay. The average PVST interval was 3.8 ± 2.2 months. Overall, 1.7% (21/1255) infants had anti-HBs <10 mIU/mL. The non-response rates were 1.6%, 1.1%, 0.9%, 0.7%, 1.1%, 0.7%, and 5.7% when PVST was performed at an interval of 1, 2, 3, 4, 5, 6, and 7–8 months after the third vaccine dose, respectively. Compared with 1 month of PVST interval, the non-response rate in infants who underwent PVST 7–8 months was significantly higher (χ2 = 4.616, P = .032). Anti-HBs titers were significantly declined in infants with medium responses when PVST was performed with longer intervals (χ2 = 27.592, P < .001), actually declined from interval of 6, and 7–8 months (Z = −3.177, P = .001 and Z = −3.715, P < .001), respectively. These results indicate that PVST may be performed at the age of 7–12 months for infants vaccinated on 0, 1, and 6-month schedule. To identify non-responders as early as possible, we suggest that PVST is performed at 7 months age or 1 month after the final vaccine dose.
Introduction
Chronic hepatitis B virus (HBV) infection is a serious global health problem because of its severe sequelae such as cirrhosis and hepatocellular carcinoma. Mother-to-child transmission (MTCT) is the major cause of chronic HBV infection. Since the administration of hepatitis B immunoglobulin (HBIG) and/or hepatitis B vaccination in infants of HBV-infected mothers, MTCT of HBV has been reduced from 70–90% to 5–10% in infants born to carrier mothers with positive hepatitis B e antigen (HBeAg) and from 10–30% to nearly zero in infants born to carrier mothers with negative HBeAg.Citation1–3
Postvaccination serologic testing (PVST) for hepatitis B surface antigen (HBsAg) and antibodies against HBsAg (anti-HBs), proposed by Centers for Disease Control and Prevention (CDC) of USA,Citation4 is critical to monitor the success or failure of the immunoprophylaxis against hepatitis B in infants born to HBsAg-positive mothers. The timing for PVST has been evolved from at age 12–15 months in 1990Citation4 to age 9–18 months in 2005,Citation5 and further to age 9–12 months (or 1–2 months after the final dose of the vaccine series, if the vaccination schedule is delayed) in 2015,Citation6 because of the discontinuation of Hib/HepB vaccine (Comvax) and new data from the Enhanced Perinatal Hepatitis B Prevention Program.Citation7 In addition, to avoid detection of passive anti-HBs from HBIG administered at birth, PVST is not recommended before age 9 months.Citation5,Citation6
Generally, anti-HBs levels ≥10 mIU/mL after completion of 3-dose hepatitis B vaccine series are considered to be seroprotective, and infants with anti-HBs levels ≥10 mIU/mL are defined as vaccine responders.Citation8 Infants who have anti-HBs levels <10 mIU/mL after a 3-dose vaccine series are defined as non-responders and they require an additional 3-dose vaccine series.Citation6 Anti-HBs levels usually reach a peak value at 1 month after completion of primary vaccination course and then anti-HBs levels tend to decline.Citation9–11 Studies have also demonstrated that intervals between the final dose of hepatitis B vaccine and PVST are associated with waning of anti-HBs titers.Citation7,Citation11–14 Thus, we questioned, for infants born to HBsAg-positive mothers who receive vaccination on the 0, 1, and 6 months schedule, whether PVST at the age of 9–12 months may increase the proportion of vaccine non-responders, since the interval between the completion of vaccine series and PVST is 3–6 months, during which anti-HBs levels may be waning. In the present study, we compared the anti-HBs titers in infants who were born to HBsAg-positive mothers and who received hepatitis B vaccine on the 0, 1, 6 months schedule, but who received PVST at different time points to further study an optimal interval for PVST.
Methods
Study subjects
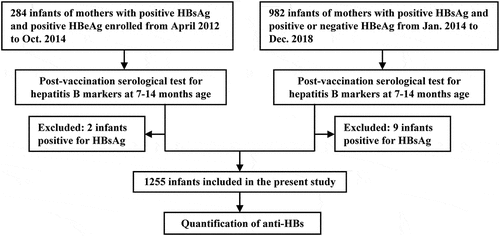
We performed a secondary analysis of the data on two groups of infants participated in two multicenter prospective cohort studies (). Group 1 was composed of 284 infants who participated in the study to evaluate the safety and efficacy of telbivudine used in the third trimester of pregnant women in preventing MTCT of HBV.Citation15 Group 2 consisted of 982 infants who participated in the study to evaluate the protective efficacy of earlier use (within 1 hour after birth) of passive-active immunoprophylaxis against MTCT of HBV.Citation16 A total of 1266 infants at 7–14 months of age were initially enrolled, and 11 infants who were infected with HBV (tested HBsAg positive) were excluded. Thus, in total, 1255 infants were included in the present investigation.
This study was approved by the institutional review boards (IRB) of the Nanjing Drum Tower Hospital. As serum samples used in this study were collected in the previous investigations, in which all mothers gave the written informed consent for their infants, the exemption of written informed consent was approved by the IRB of Nanjing Drum Tower Hospital in this study.
Laboratory tests and outcome assessment
HBsAg and anti-HBs were quantitatively tested with microparticle enzyme immunoassay (Architect, Abbott, North Chicago) in the Nanjing Drum Tower Hospital. Infants who were positive for HBsAg and negative for anti-HBs were considered as immunoprophylaxis failure and HBV infection. The protective anti-HBs level in serum was ≥10 mIU/ml. Infants with anti-HBs levels ≥1000, 100–999.9, 10–99.9, and <10 mIU/ml were, respectively, defined as high-, medium-, low-, and non-responders as described elsewhere.Citation17
Statistical analysis
Normally distributed continuous variables were presented as mean ± SD. The anti-HBs were presented as geometric mean concentration (GMC) followed by minimum and maximum values and compared by Mann-Whitney U tests. Categorical variables were compared by Chi-square or Fisher’s exact tests. All statistical analyses were conducted using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P < .05 indicated statistically significant difference.
Results
Demographic characteristics of enrolled infants
Baseline characteristics of 1255 infants are described in . The average age of infants was 9.8 ± 2.2 months at follow-up. The average PVST interval was 3.8 ± 2.2 months.
Table 1. Baseline characteristics of enrolled infants (N = 1255)
Anti-HBs response to hepatitis B vaccination in different PVST intervals
Overall, 21 (1.7%) of 1255 infants had anti-HBs <10 mIU/ml (ranged 0.5–9.5) and they were defined as non-responders. The non-response rates were 1.6%, 1.1%, 0.9%, 0.7%, 1.1%, 0.7%, and 5.7% in 1 month, and 2, 3, 4, 5, 6, and 7–8 months of PVST intervals after the third vaccine dose, respectively. Compared with 1 month of PVST interval, the non-response rate in infants who underwent PVST at an interval of 7–8 months was significantly higher (χ2 = 4.616, P = .032) ().
Table 2. Non-response rates of infants with different PVST intervals
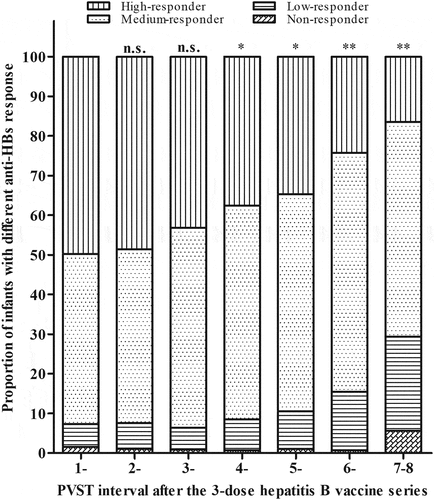
The distribution of anti-HBs responses in infants with different PVST intervals is shown in . Compared with 1 month of PVST interval, the changes of the proportions of high, medium, low, and non-responders showed statistically significant, when PVST was performed at an interval of ≥4 months after the third-dose vaccine (≥10 months age), with the proportions of low- and median-responders increased and high-responders decreased (). In addition, the anti-HBs GMCs in infants between different PVST intervals are presented in . Anti-HBs titers were significantly declined in infants with medium responses when PVST was performed with longer intervals, actually declined from interval of 6, and 7–8 months (Z = −3.177, P = .001 and Z = −3.715, P < .001), respectively.
Table 3. Anti-HBs titers of infants with different PVST intervals
Figure 2. Distribution of anti-HBs responses in infants with different PVST intervals. The proportions of high, medium, low, and non-responders in each interval were compared with 1 month of PVST interval. P values were determined using Mann–Whitney U tests. n.s., not statistically significant; *, P < .05; **, P < .001.
Discussion
Our present study showed that the proportions of non-responders to the 3-dose serial hepatitis B vaccination in infants born to HBsAg-positive mothers were comparable when PVST was performed at each time point during a 6-month period after the third vaccine dose (at 7–12 months age), and the proportion of the non-responders was significantly increased when PVST was performed 7–8 months after final vaccine dose (13–14 months age). Our data support the PVST for hepatitis B serological markers should be performed no later than 12 months age or 6 months after the third dose of hepatitis B vaccine.Citation6
Currently, PVST for HBsAg and anti-HBs after the completion of serial hepatitis B vaccination is recommended to perform at the age of 9–12 months, and not to perform before age 9 months.Citation6 However, in our present study, 1.6% (3/191) and 1.1% (3/278) of infants had anti-HBs levels <10 mIU/ml at the age of 7 and 8 months, respectively, and they were non-responders to hepatitis B vaccine. Based on the seroprotective levels of anti-HBs ≥10 mIU/ml, these infants were susceptible to HBV. The non-responders require revaccination with another serial three-dose hepatitis B vaccine as soon as possible, since they are at great risk for exposure to HBV due to the close contact with their HBV-infected mothers. Although PVST at 9 months age may maximize the likelihood of detecting late HBV infection,Citation6 it may also leave the non-responders at the risk of HBV infection. Currently, it is hard to estimate the transmission rate of HBV in infants who were born to HBsAg-positive mothers and who were non-responders to hepatitis B vaccination, since no such study has been reported. However, HBV transmission may occur in such non-responders. An infant who was negative for both HBsAg and anti-HBs at 7 months age and who was not revaccinated became HBsAg positive at 12 months age,Citation1 although whether the infection in this infant occurred before or after 7 months age remains as an open question, because administration of HBIG and/or hepatitis B vaccine may delay the establishment of HBV infection and increase the incubation period.Citation18,Citation19 Nevertheless, revaccination in non-responders with 3-dose series on the 0, 1, and 6-month schedule, followed by another PVST after the final vaccine dose, is recommended.Citation6Thus, the late HBV infection, if occurred, can be still detected by PVST after the second full vaccination.
Thus, for identification of non-responders to hepatitis B vaccine, PVST may be performed at any time at 7–12 months age or within 1–6 months after the final vaccination. Considering that non-responders are susceptible to HBV infection and require revaccination, we consider that the optimal timing for PVST is at 7 months age or 1 month after the final vaccine dose, because such a practice may identify non-responders as early as possible. The proposed PVST timing here is somewhat different from that at the age of 9–12 months proposed by the CDC of USA;Citation6 the CDC recommends not to perform PVST before 9 months age and the reason is to avoid detection of passive anti-HBs in HBIG administered at birth and to maximize the likelihood of detecting late HBV infection.Citation6 However, in theory, the passive anti-HBs in infants derived from the administration of 100–200 IU HBIG at birth should be almost disappeared at age of 7 months, a time period that covers 12 half-lives (21–24 days) of IgG antibodies. Thus, it is less likely that the passive anti-HBs in infants obtained from HBIG used at birth may influence on the detection of active anti-HBs induced by hepatitis B vaccination. In other words, anti-HBs in infants at 7 months should be actively produced by hepatitis B vaccination, rather than passively obtained from HBIG injected at birth.
Our findings that children at the age of 13–14 months had higher proportion of anti-HBs levels <10 mIU/ml than those at the age of 7–12 months () indicate that anti-HBs considerably decline 6 months after the final vaccine dose. Thus, identification of non-responders to hepatitis B vaccine should be done within 6 months after the final vaccination. Otherwise, those responders who have anti-HBs levels <10 mIU/ml with growing age may be mistakenly considered as non-responders,Citation20 which may draw misleading conclusions.Citation21
One limitation of our study was that the total GMCs of anti-HBs could not be calculated and compared for those with anti-HBs ≥1000 mIU/ml because the upper detection limit of the assay is 1000 mIU/ml and we did not measure anti-HBs after dilution of serum samples. Another limitation was that the non-responders who were suggested to receive another 3-dose series on 0, 1, and 6-month schedule were not further followed up.
In conclusion, our findings indicate that PVST should be taken at the age of 7–12 months for infants who received hepatitis B vaccination on the 0, 1, and 6 months schedule. PVST at 7 months of age or 1 month after the third vaccine dose can identify the non-responders as early as possible so that revaccination with serial three-dose hepatitis B vaccine can be started in a timely manner to maximize the protective efficacy of hepatitis B vaccination in infants born to HBV-infected mothers.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wei KP, Zhu FC, Liu JX, Yan L, Lu Y, Zhai XJ, Chang ZJ, Zeng Y, Li J, Zhuang H. The efficacy of two different dosages of hepatitis B immunoglobulin combined with hepatitis B vaccine in preventing mother-to-child transmission of hepatitis B virus: a prospective cohort study. Vaccine. 2018;36(2):256–63. doi:10.1016/j.vaccine.2017.11.037.
- Cheung KW, Seto MTY, Kan ASY, Wong D, Kou KO, So PL, Lau WL, Jalal K, Chee YY, Wong RMS, et al. Immunoprophylaxis failure of infants born to hepatitis B carrier mothers following routine vaccination. Clin Gastroenterol Hepatol. 2018;16(1):144–45. doi:10.1016/j.cgh.2017.07.013.
- Xu B, Xu C, Feng J, Chen J, Rui Y, Qiu Z, Zhu J, Tang J, Lou H, Chen T, et al. Reduced mother-to-child transmission of hepatitis B after implementation of completely charge-free active-passive immunoprophylaxis: an observational cohort study. Expert Rev Vaccines. 2021;20(7):899–905. doi:10.1080/14760584.2021.1927723.
- Immunization Practices Advisory Committee (ACIP). Protection against viral hepatitis: recommendations of the immunization practices advisory committee (ACIP). MMWR Recomm Rep. 1990;39(RR–2):1–26.
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR–16):1–31.
- Schillie S, Murphy TV, Fenlon N, Ko S, Ward JW. Update: shortened interval for postvaccination serologic testing of infants born to hepatitis B-infected mothers. MMWR Morb Mortal Wkly Rep. 2015;64(39):1118–20. doi:10.15585/mmwr.mm6439a6.
- Ko SC, Schillie SF, Walker T, Veselsky SL, Nelson NP, Lazaroff J, Crowley S, Dusek C, Loggins K, Onye K, et al. Hepatitis B vaccine response among infants born to hepatitis B surface antigen-positive women. Vaccine. 2014;32(18):2127–33. doi:10.1016/j.vaccine.
- Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013;31(21):2506–16. doi:10.1016/j.vaccine.2012.12.012.
- Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13(5):443–47. doi:10.1016/0264-410x(94)00010-k.
- Jafarzadeh A, Montazerifar SJ. Persistence of anti-HBs antibody and immunological memory in children vaccinated with hepatitis B vaccine at birth. J Ayub Med Coll Abbottabad. 2006;18:4–9.
- Xiao M, Qiu Q, Pang X, Liang X, Li L, Cui F, Wang F, Zhang G, Li H, Wang L, et al. Immune response in infants after universal high-dose hepatitis B vaccination: a community-based study in Beijing, China. Vaccine. 2015;33(43):5878–83. doi:10.1016/j.vaccine.2015.06.018.
- Euler GL, Copeland JR, Rangel MC, Williams WW. Antibody response to postexposure prophylaxis in infants born to hepatitis B surface antigen-positive women. Pediatr Infect Dis J. 2003;22(2):123–29. doi:10.1097/01.inf.0000048677.32881.fa.
- Centers for Disease Control and Prevention (CDC). Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2012;61:768–71.
- Nagashima S, Yamamoto C, Ko K, Chuon C, Sugiyama A, Ohisa M, Akita T, Katayama K, Yoshihara M, Tanaka J. Acquisition rate of antibody to hepatitis B surface antigen among medical and dental students in Japan after three-dose hepatitis B vaccination. Vaccine. 2019;37(1):145–51. doi:10.1016/j.vaccine.2018.11.019.
- Hu Y, Xu C, Xu B, Hu L, Liu Q, Chen J, Liu J, Liu L, Yang J, Chen T, et al. Safety and efficacy of telbivudine in late pregnancy to prevent mother-to-child transmission of hepatitis B virus: a multicenter prospective cohort study. J Viral Hepat. 2018;25(4):429–37. doi:10.1111/jvh.12834.
- Huang H, Xu C, Liu L, Chen L, Zhu X, Chen J, Feng J, Chen T, Xu B, Yang J, et al. Increased protection of earlier use of immunoprophylaxis in preventing perinatal transmission of hepatitis B virus. Clin Infect Dis. 2020;ciaa898. doi:10.1093/cid/ciaa898.
- Huang H, Ning M, Liu J, Chen J, Feng J, Dai Y, Hu Y, Zhou YH. Comparison of antibody response to hepatitis B vaccination in infants with positive or negative maternal hepatitis B e antigen (HBeAg) in cord blood: implication for the role of HBeAg as an immunotolerogen. Hum Vaccin Immunother. 2019;15(9):2183–86. doi:10.1080/21645515.2019.1575712.
- Krugman S, Giles JP, Hammond J. Viral hepatitis, type B (MS-2 strain) prevention with specific hepatitis B immune serum globulin. JAMA. 1971;218(11):1665–70. doi:10.1001/jama.1971.03190240019005.
- Kamili S, Sozzi V, Thompson G, Campbell K, Walker CM, Locarnini S, Krawczynski K. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology. 2009;49(5):1483–91. doi:10.1002/hep.22796.
- Ghaziasadi A, Fakhari Z, Aghcheli B, Poortahmasebi V, Farahmand M, Norouzi M, Ghalichi L, Soleimani A, Hedayat Yaghoobi M, Ravanshad M, et al. High prevalence of occult hepatitis B infection (OBI) among healthy children and their parents in Alborz province, Iran; Vertical OBI, myth or truth? Liver Int. 2020;40(1):92–100. doi:10.1111/liv.14252.
- Zhou YH. Occult hepatitis B infection in vaccinated children with negative anti-HBc, true or not? Liver Int. 2020;40(10):2570. doi:10.1111/liv.14504.