ABSTRACT
Convalescent plasma therapy provides a useful therapeutic tool to treat infectious diseases, especially where no specific therapeutic strategies have been identified. The ongoing pandemic puts back the spotlight on this age-old method as a viable treatment option. In this review, we discuss the usage of this therapy in different diseases including COVID-19, and the possible mechanisms of action. The current review also discusses the progress of therapeutic applications of blood-derivatives, from the simple transfer of immunized animal sera, to the more target-specific intravenous administration of human immunoglobulins from a pool of convalescent individuals, in both infectious and non-infectious diseases of various etiologies.
Introduction
The ongoing pandemic of Severe Acute Respiratory Syndrome (SARS) coronavirus-2 (CoV2), with no specific therapeutics available so far, has led to a re-evaluation of the effectiveness of the century-old plasma therapy technique. Plasma therapy has been used throughout history, both in association with other drugs and often as the only option to combat various infectious diseases. Convalescent plasma (CP) is the antibody-rich blood plasma procured from an animal or human who has “convalesced” or recovered from a particular infection. CP is the most basic of all immunotherapeutic strategies, but it can be very effective during a time of emergency. Active immunization by the conventional vaccines containing live-attenuated or inactivated microorganisms, proteins, or toxins derived from pathogens, generates highly specific antibodies that can neutralize the target pathogen in subsequent encounters. In contrast, CP offers passive immunity with the help of donor-derived antibodies to fight invading pathogens immediately. CP potentially contains neutralizing antibodies (NAbs) and immunoglobulin (Ig) G or M that decrease the microbial load and eventually control the symptoms. Transfusion of CP introduces donor-derived antibodies without transferring antibody–producing plasma cells. This strategy does not lead to the generation of long-lived plasma cells or memory B cells in the recipient, unlike in an active immune response post-vaccination. Therefore, in CP therapy, although the protection is not long-lasting, the borrowed antibodies can greatly reduce disease burden and duration, especially when a quick response is necessary, thereby being a potential game-changer in grave situations. Such artificial passive immunity can be secured from convalescent blood products like convalescent whole blood or plasma, pooled intravenous high titer human immunoglobulin, and polyclonal or monoclonal antibodies (mAbs).Citation1 Out of these, the CP is the easiest to obtain and thereby the best alternative in an emergency where well-established therapeutic strategies are lacking and/or quick neutralization of the pathogen itself or pathogen-derived toxins are required.
To effectively neutralize disease-causing pathogens and provide clinical benefit in recipients, blood plasma used in CP therapy must contain disease-specific neutralizing antibodies in sufficient quantities. Also, most data suggest that the CP administration needs to be done at early stages of the disease for positive outcomes.Citation2 A recent exploratory meta-analysis on severe acute respiratory infections reported a statistically significant decrease in mortality following CP therapy, in comparison to placebo-treated or untreated cohorts.Citation3 Additionally, there are several other respiratory diseases where CP has been used both as prophylactic measures and therapeutic interventions. Apart from respiratory infections, CP has also served as an efficient ameliorative agent in hemorrhagic diseases like Argentine Hemorrhagic Fever.Citation4 Importantly, in the ongoing pandemic caused by SARS CoV-2, the effectiveness of CP therapy is once again under consideration, proving the relevance of this age-old technique even in the modern era of molecular medicine. Given its importance, in the current review article, we aim to provide an overview of the therapeutic applications of CP and its derivatives in both communicable and non-communicable diseases and discuss its evolution over the years into more targeted therapeutic approaches like patient-derived mAbs. We also deliberate on the challenges faced in utilizing CP therapy as a treatment modality.
CP therapy: the beginning
CP therapy emerged in the 1890s as an antitoxin-based remedy against diphtheria and tetanus. Emil Adolf von Behring and Kitasato Shibasaburō transferred serum from tetanus toxin-immunized rabbits to mice, leading to the successful prevention of disease development in recipient mice. Subsequently, they administered sheep antiserum in a critical diphtheria patient, who recovered within hours and survived. This work not only earned Kitasato and Von-Behring a Nobel Prize in medicine, in 1901, it also opened the floodgates for CP therapy in various infectious diseases.
The usage of convalescent serum to treat maladies soon evolved into a standard therapeutic strategy in several diseases, which previously had very high mortality rates in specific cohorts like children (diphtheria, tetanus, etc.) or the armed forces (tetanus, gas-gangrene, etc.). Notably, in some cases, serum therapy or an evolved version of that, continued till the modern era, only to give way to the administration of more specific passive immunity.
Diphtheria antitoxin (DAT), a solution of globulin proteins and antibodies obtained from horses immunized with diphtheria toxin, is used in actual or suspected cases of diphtheria or for prophylactic measures under exceptional conditions under an Investigational New Drug (IND) protocol sponsored by CDC (https://www.cdc.gov/diphtheria/dat.html). Diphtheria antitoxin neutralizes the circulating diphtheria toxin molecules, thus negating its toxic effects, and preventing disease development. Similarly, the administration of equine antiserum in tetanus, a disease with an almost 90% mortality rate, helped achieve a drastic reduction of fatalities to almost nil, among wounded soldiers as well as in new mothers and neonates.Citation5 Another disease that was caused by war-time wounds and led to considerable fatalities was Gas Gangrene, a myonecrotic disease caused by the spore-forming anaerobic bacillus Clostridium perfringens. It is still treated prophylactically with an antitoxin that consists of equine serum immunoglobulins against three species of gas gangrene toxin-producing bacilli (C. perfringens type A, C. septicum, and C. oedematiens), along with antibiotics immediately after exposure to neutralize the toxin and reduce disease virulence.Citation6 Eventually, serum therapy was implemented to treat Streptococcal pneumonia and Meningococcal meningitis and was successful in bringing down the high mortality rates in both diseases.Citation7,Citation8
It is worth mentioning here that serum therapy, in addition to treating several infectious diseases, also paved the way for anti-venom therapy to neutralize animal venoms. Antivenoms are typically developed by hyper-immunizing donor animals like horses or sheep with non-lethal doses of one or more venoms to produce a NAb response; these antibodies are then purified from the blood to produce monovalent or polyvalent antivenom.Citation9
Mechanisms of action of CP therapy: the key to success lies in its contents
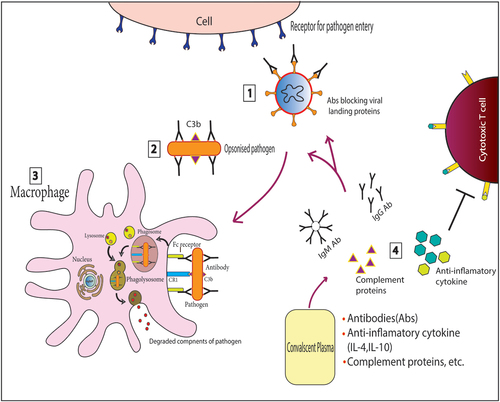
The composition of CP is variable and contains large amounts of albumin, immunoglobulins, complement proteins, cytokines, coagulation, and antithrombotic factors, in addition to different inorganic salts and organic compounds.Citation10 Apart from complement-mediated destruction of pathogens, the NAbs present in CP may be able to neutralize the infecting pathogens by various methods () including blocking the interaction between the pathogen’s membrane proteins and host cell surface proteins that are utilized as ports of entry by the pathogens. For example, viral pathogens like SARS-CoV, SARS-CoV2, and MERS (Middle Eastern Respiratory Syndrome) virus, bind to human ACE2 (hACE2) receptors with the receptor-binding domain (RBD) of the S1 subunit of their spike protein.Citation11 The NAbs in convalescent sera that can bind to the S1-RBD and N-terminal domain of the S1 subunit, inhibit their entry into new host cells. Similarly, antibodies that bind to the S2 subunit of the spike protein, which mediates membrane fusion during viral entry, can limit viral amplification.Citation12 Also, natural IgM present in the serum provides defense against a variety of pathogens by virtue of their flexible antigen-binding sites that enable them to bind to various unrelated antigens,Citation13 while high-affinity IgG probably neutralizes viral pathogens by agglutination as seen with the poliovirus.Citation14
Figure 1. Schematic representation of the mechanism of action of Convalescent plasma (CP).
Application of plasma therapy in different diseases
With time, in medical emergencies or pandemics when vaccines, antivirals, and other specific treatments were unavailable, CP from human patients emerged as a robust therapeutic alternative. While convalescent whole blood was used in early clinical settings, it soon got replaced by CP due to better outcomes with the latter, along with the fact that CP transfusion did not require typing and cross-matching thus further expediting the therapeutic process. Additionally, CP can be administered both via the intravenous as well as intramuscular routes and also can be easily procured without requiring sophisticated techniques for isolation.Citation15 In the following sections, we discuss the pivotal role of CP therapy in the overall management and treatment of various diseases, and also chart the progress of convalescent blood products as a therapeutic modality.
Plasma therapy in viral respiratory diseases
Respiratory infections are among the leading causes of severe illness and death in many countries and are typically known to involve the different parts of the respiratory system. While the common symptoms of respiratory infections include congestion, nasal discharge, cough, fever, and sometimes shortness of breath and fatigue, it may have systemic effects as well.Citation16 Infections of the respiratory tract can be of viral, bacterial, protozoan, or fungal origin, which may cause acute as well as chronic respiratory disorders.
Among respiratory pathogens, viruses cause the largest number of respiratory infections, which can affect both the upper and lower respiratory tracts. The common viruses that establish infection in the respiratory tract are rhinoviruses, respiratory syncytial virus (RSV), influenza and parainfluenza viruses, human metapneumovirus, measles, mumps, adenovirus, and coronaviruses,Citation17 (CDC Reports: https://wwwnc.cdc.gov/travel/yellowbook/2020/posttravel-evaluation/respiratory-infections). Although most viral respiratory infections are transitory and self-limiting, there also exist examples of viruses causing severe pandemics. Vaccines and antiviral agents are available for only some of these viruses. CP has been used successfully both as prophylactic as well as therapeutic in various cases of infectious respiratory diseases.
CP therapy during influenza outbreaks
From time to time, the world has faced several flu outbreaks apart from the standard seasonal ones; the most prominent and arguably the first recorded one being the H1N1 influenza pandemic of 1918. The H1N1 virus infected about one-third of the world population at that time and lasted for 36 months. During this critical period, plasma administration was reported to cause a reduction in the fatality rate, making it the first recorded usage of CP therapy as well. A meta-analysis of eight reports contributed by physicians at that time, demonstrates that patients who received early CP transfusion (after less than 4 days of pneumonia complications) had a statistically significant reduction in mortality and also showed general improvement of symptoms.Citation18 While some adverse effects like chills and aggravation of symptoms were noted in a few seriously ill patients, it could be due to the lack of proper quality control of the transfused CP. Since 1918, three additional pandemics in 1957, 1968, and 2009 were caused by H2N2, H3N2, and H1N1 influenza virus strains, respectively. During the 2009 outbreak, in a study involving 93 seriously ill patients, 20 patients who were administered CP, showed reduced respiratory tract viral load and serum cytokine response, and also a lower mortality rate compared to the control group without any adverse effects.Citation19 Such studies resulted in a better understanding of the role of CP therapy and equipped the global public health community with an executable plan to handle future influenza outbreaks.
Severe respiratory illness in humans due to the avian influenza virus, especially the H5N1 subtype, first appeared in Hong Kong in 1997,Citation20 and has continued to occur over the years. Convalescent H5N1 plasma has been considered as a therapeutic approach to lower the mortality rateCitation18 in a few studies with small sample size. A global approach and large-scale population-based investigations are required to successfully utilize the potential of CP therapy in tackling H5N1 influenza outbreaks.
CP therapy in Severe Acute Respiratory Syndrome (SARS) outbreaks
Coronaviruses are single-stranded RNA viruses of the family Coronaviridae that can infect a range of hosts among mammals and birds.Citation21 There are seven identified coronavirus strains including SARS-CoV, MERS-CoV, and SARS-CoV2 that infect humans (https://www.cdc.gov/coronavirus/types.html). The SARS-CoV outbreak in 2002–2003 in China led to the deployment of CP therapy initiatives to tackle the disease. CP infusion in SARS patients in combination with ribavirin and corticosteroids treatment had successful clinical outcome with no observed side effects, and overall reduction in mortality rates.Citation22
Almost 10 years after the SARS pandemic, another highly pathogenic and transmissible strain of coronavirus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was detected in 2012 in the Middle East. Importantly, the World Health Organization (WHO) has established a protocol for CP therapy in MERS-CoV infection. A recent study at a tertiary care center during the 2015 Korean MERS outbreak suggests that donor plasma with a plaque reduction neutralization test titer equal to or greater than 1:80 is required to obtain a significant serological response after infusion.Citation23 However, larger efficacy trials are required to establish CP as a therapeutic option for MERS. CP therapy in the ongoing pandemic caused by SARS-COV2 is discussed in detail in later sections.
Plasma therapy in other viral diseases
One of the deadliest diseases of modern times is the Ebola virus infection, which is a highly contagious disease with a 40–90% fatality rate. In 2014, a particularly severe outbreak of this disease spread in several east African countries as well as in a few countries in Europe and the USA. Reports of the effectiveness of the passive transfer of antibodies in immunodeficient mice infected with the Ebola virus,Citation24 led to the investigation of the efficacy of CP in humans. While transfusion of CP with unknown antibody concentrations did not show any improvement in survival,Citation25administration of plasma from recovered individuals with high-titer anti-Ebola virus antibodies did achieve a decline in viral load in patients.Citation26
Notably, CP therapy has shown great promise in treating some geographically localized viral diseases like Argentine hemorrhagic fever,Citation4 and Lassa fever.Citation27 Argentine hemorrhagic fever (AHF) is a zoonotic infection caused by the Junin virus of family Arenaviridae, which is endemic to Argentina. While Candid#1, an indigenously developed live-attenuated vaccine against AHF has been able to reduce disease incidence,Citation28,Citation29 disease-specific therapy still involves CP transfusion. Several studies have demonstrated that administration of CP containing specific amounts of disease-specific neutralizing antibodiesCitation30 during early stages of the infection,Citation4 or within 8 days of appearance of symptoms,Citation31 drastically reduces the mortality rate to 1%, which can be as high as 15–30%, if left untreated.Citation4,Citation32 The advantages of CP therapy have also been observed in other severe diseases like Hantavirus disease.Citation33 Furthermore, in animal models of Zika viral disease and Hepatitis E, CP therapy has shown promising results.Citation34,Citation35 The results obtained by the infusion of CP in various viral diseases are summarized in .
Table 1. Outcomes of CP therapy in various viral diseases
From serum therapy to monoclonal antibodies: the passive approach to immediate immunity
Plasma therapy is the instigating foothold that unlocked the era of antibody-based therapeutic approaches against several diseases. With the advent of new technology to separate different blood components, the field evolved toward a more target-specific approach involving intravenous immunoglobulin (IVIG) products as therapeutic agents. IVIG, isolated and pooled from a large number of donors, is composed of polyclonal antibodies and can be used for the management of primary immunodeficiencies, as well as against infectious diseases. Later with the arrival of mAbs, the scope of target-specific immunotherapy was unraveled to a far greater extent than previously possible.
IVIG is a pool of immunoglobulins obtained from several healthy donors who have produced antibodies against different microorganisms and their products. IVIG can also be collected from convalescent donors recently exposed to infectious diseases, vaccines, or ubiquitous microorganisms, in which case, it is known as Hyperimmune polyclonal immune globulin or Hyperimmune IVIG. IVIG is composed mainly of IgG of different subclasses; IgA, traces of other Ig, cytokines, and soluble receptors. IVIG can be further enriched for IgG through cold ethanol precipitation process and some components are added to stabilize the proteins and prevent IgG aggregation.Citation47
IVIG was first used as a therapeutic strategy in a young child with gamma globulin deficiency who suffered from recurrent pneumococcal sepsis, with extraordinary success.Citation48 It is used as a major remedy in several neurological disorders, like Guillain–Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, stiff-person syndrome, etc. In GBS, administration of IVIg leads to enrichment of preexisting regulatory T cell population, with a concomitant decrease in the levels of proinflammatory T cell subsets and cytokines in the cerebrospinal fluid.Citation49 Furthermore, IVIG has a considerable impact on the treatment of hematological diseases like immune cytopenias, hypogammaglobulinemia, and post-bone marrow transplantation.Citation50–52 IVIG treatment also ameliorates a whole gamut of diseases like Kawasaki syndrome, vasculitis, uveitis, skin-related inflammatory diseases, as well as autoimmune disorders like mucous membrane pemphigoid and systemic lupus erythematosus.Citation53 Some of the IVIg products that have been approved by USFDA for therapeutic usage in primary immunodeficiencies as well as neurological, hematological, autoimmune, or other inflammatory diseases are listed in .
Table 2. FDA approved IVIG products in primary immunodeficiencies and non-communicable inflammatory diseases
IVIG treatment was also found to be quite useful in tackling fatal bacterial infections like toxic shock syndrome caused by group A streptococcus, where antibodies against staphylococcal and streptococcal superantigens are administered as a treatment strategy.Citation54 In addition to toxin neutralization, IVIG treatment in these infections dampens the associated strong proinflammatory responses. Likewise, IVIG is found to be significantly better at reducing mortality in Pertussis or whooping cough, an infanthood disease with a high mortality rate during the 1920s,Citation55 and also in Botulism, an otherwise fatal neuro-paralytic syndrome caused by the Clostridium botulinum neurotoxin.Citation56
The only therapeutic modality against HIV/AIDS to date is the administration of antiretroviral drugs, to which the virus has been found to develop resistance in the sub-Saharan African population.Citation57 IVIG treatment in AIDS patients showed a rapid decrease in the HIV structural protein ICD p24 antigen in the recipient’s serum, due to neutralizing anti-p24 antibodies present in the donor-sera. However, no alteration in the plasma HIV RNA copy number was reported after the infusions, even at the highest IVIG dose.Citation58
Disease-specific hyperimmune IVIG is also used as post-exposure prophylaxis in infectious pathologies like Rabies. Since there are no therapeutic medications available for this highly fatal viral encephalitic disease, WHO recommends aggressive and immediate post-exposure prophylactic strategies for disease control, which includes post-exposure administration of rabies immunoglobulins (RIG) of human or equine origin, in addition to vaccination. While the vaccine helps the generation of circulating serum antibodies in the exposed individual after 10–14 days, the immediate administration of the RIG in the wound-bed itself helps protect high-risk rabies-exposed patients in this window period by neutralizing the virus deposited at the bite-wound site.Citation59
After extensive and rigorous clinical testing, some IVIG products have been licensed to be used in clinical settings by USFDA, to combat various infectious diseases. Many of these IVIG-based drugs are used for post-exposure prophylactic interventions to combat pathogens like Rabies (KEDRAB) or Varicella zoster (VariZIG). Similar IVIG products like HepaGamB, are used both as a preventive and a prophylactic drug in Hepatitis B. Moreover, the main line of treatment in cases of inhalation anthrax and infant botulism involves IVIG products like Anthrasil, BabyBiG, etc. The details of the licensed IVIG drugs currently in use are summarized in .
Table 3. List of FDA approvedimmunoglobulin products in infectious diseases
IVIG showed promising trends as cancer therapeutics as well, especially in preclinical mouse models. Administration of IVIG in mice with melanoma or sarcoma induces increased production of IL-12 from mononuclear cells that exert anti-angiogenic function, and also activate Natural Killer cells. IVIG treatment restricts the invasiveness of cancer cells by downregulating matrix metalloproteinases, modulates anti-tumor immune response by inducing M2 to M1 polarization in macrophages, and can also neutralize VEGF to prevent vascularization of tumors.Citation60
Convalescent immunoglobulin therapy has also been used as a therapeutic and preventive approach for IgE-mediated allergic hypersensitivity reactions. Allergen-specific IgG antibodies can block IgE-mediated inflammation by inhibiting the activation of basophils and mast cells, thus eliminating the immediate allergic responses.Citation61
Perhaps, the final step to the ultimate refinement of CP therapy is the development of patient-derived mAb as a therapeutic approach. In the ongoing COVID-19 pandemic, patient-derived investigational mAbs have been considered for emergency use to treat mild to moderately ill patients. Combination therapy with two such mAbs derived from recovered COVID-19 patients, bamlanivimab, and esevimab, with the approval of the FDA-Emergency Use Authorization (EUA) committee, resulted in a significant decrease in viral load and hospitalization in comparison to placebo.Citation62,Citation63 The EUA of the bamlanivimab plus etesevimab combination therapy has been further expanded for post-exposure prophylactic use for certain high-risk cohorts (https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/). Another mAb, sotrovimab, originally isolated from a SARS survivor in 2003, was found to be able to bind to a conserved epitope in the receptor-binding domain of the spike protein of both SARS-COV and SARS-COV2. Thus, this mAb has also been approved by the FDA-EUA committee as an investigational treatment for COVID-19 and is currently being monitored for clinical efficacy (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19). Patient-derived mAbs have been used in Ebola viral disease (EVD) as well. MAb114 is a memory B cell-derived mAb isolated from an EVD survivor, obtained 11 years after clinical infection, which can block the viral entry into host cells. Treatment with MAb114 in EVD patients at early disease onset resulted in lower fatality in specific cohorts.Citation64–66 Presently, Mab114 is being tested in an ongoing clinical trial (https://clinicaltrials.gov/ct2/show/NCT03478891?term=mAb114&cond=Ebola&draw=2&rank=4).
CP therapy in tackling the COVID-19 pandemic: to do or not to do
In times of extreme need, when there is no documented therapeutic modality available, CP therapy often gives a fighting opportunity against the disease. The ongoing COVID-19 pandemic, where no specific treatment has been found to be unequivocally effective against the virus, and standard treatment involves the administration of common antiviral agents with limited efficacy, represents such a dire situation. Therefore, modern medical approaches went back to the basics, and trials with the century-old technique of CP therapy are being widely undertaken. In general, CP infusion has been found to be effective in decreasing the requirement of mechanical ventilation and promoting general improvement of patient condition.Citation36,Citation37 While some studies demonstrated dramatic improvement of critically ill patients on mechanical ventilation when transfused with CP,Citation38 other studies have shown the benefit of CP therapy largely confined to less critical patients.Citation39 Resolution of ground-glass opacities (GGOs) in the lung high-resolution computed-tomography (HRCT) images in patients accompanied by an immediate increase in anti-SARS-CoV-2 antibody titers could be achieved by CP administration, even when done in later stages of the disease.Citation40 In contrast, some records documented positive outcomes of CP therapy only when administered in the early stages of disease development. Typically, transfusion of plasma retrieved from recently recovered patients with high NAb titers showed significant improvement of clinical symptoms in recipients within 3 days of treatmentCitation41 or slowed disease advancement at the very least.Citation42
Notably, few studies have also reported completely different outcomes of CP therapy in COVID-19. In a recent multicentre trial involving 12 clinics in Argentina, CP therapy in severely ill patients did not affect mortality or show any significant improvement in patient condition, when compared to placebo-treated subjects.Citation43 Similarly, multicenter trials in China also hinted at minimal and insignificant effect of CP therapy on disease outcome, in comparison to standard treatment.Citation44 In another randomized clinical trial involving a single academic medical center in Chile, no significant benefit of immediate CP administration versus that upon worsening of symptoms was recorded.Citation45 While these findings point to the perceived inefficacy of CP therapy in COVID-19 management, several points need to be taken into consideration. Most of these studies used CP therapy in conjunction with standard treatment, which itself has oscillated between hydroxychloroquine,Citation67,Citation68 dexamethasone,Citation69 ivermectin,Citation70 etc. over the last few months. The effect of these drugs, which may be synergistic or antagonistic to CP therapy, needs to be considered while interpreting the results of these clinical trials. Additionally, most studies have been done in a single city or country,- which may have affected the outcome, depending on the genetic homogeneity of the population. Therefore, multi-center and multi-country clinical trials need to be undertaken to further prove the efficacy or inefficacy of CP therapy. Furthermore, some of the described studies did not account for the NAb titer of the donor CP, which may affect the outcome either way. Another important point to note while interpreting the clinical trial data is the possibility of antibody-dependent enhancement of the infection, which is currently under investigation in SARS-COV2.Citation71
In general, even in studies that showed positive outcomes of CP therapy in COVID 19, the benefits are most experienced when administered at earlier disease stages, and to younger patients without any comorbidities.Citation46 Thus, timely recognition of critical cases and early transfusion is crucial. Standard guidelines for the use of CP therapy in children, pregnant women, and nursing mothers also need to be established before administration of CP in these cohorts of patients.
In addition to CP therapy, IVIG has also been used in many clinical trials to assess its efficacy in ameliorating SARS-COV2 infection. In a randomized case study, patients who were administered with IVIG displayed reduced in-hospital mortality rate, compared to placebo controls.Citation72 Furthermore, the use of IVIG in conjunction with standard treatment resulted in reduced hospital stay, lessened the requirement of mechanical ventilation, and ensured early recovery.Citation73 Similarly, IVIG used at high doses within 14 days of disease onset increased patient survival and stabilized the ensuing cytokine storm.Citation74
CP therapy: addressing the variables
From the above discussion, it is evident that CP therapy and its more modern derivatives have proven efficacy in treating many life-threatening diseases, over the years. During infectious disease outbreaks like the presently ongoing pandemic, when no known or specific treatment is readily available, CP therapy is the simplest therapeutic modality that can be employed to treat critical cases. Although the advancements in modern medical research have led to the development of highly specific mAbs that can disrupt the infective process of pathogens, it takes time, personnel, and resources to develop them. In a time of global medical emergency, several of these factors may be in shortfall. The economic commitments of treating the global population with such antibodies will be prohibitive as well. Similarly, in managing diseases that are endemic to specific geographical locations, high-end GMP (Good Manufacturing Practice) facilities required for mAb production may not be available. Isolation and transfusion of CP, on the other hand, requires minimal infrastructural support and therefore presents the most economically feasible and practical strategy of disease management in such scenarios. Thus, the success of CP therapy in times of global or local emergencies, even in the modern era of molecular medicine is manifold and well established.
However, several points need to be noted and addressed while dealing with CP as a therapeutic strategy. For example, stringent quality control steps must be followed to minimize the risk of transfusion-associated infections. Also, optimizing the dose of NAb titer in the CP is required prior to transfusion to improve its efficacy. The dosage and timing of administration of CP and tolerability of recipients are also important points for consideration. Donor selection should be done following rigorous validation of recovery from concerned disease. Apart from established criteria pertaining to the general health and age of the donor, the sex of the donor is an important consideration for plasma donations. Donations are mostly accepted from males, and females with no pregnancy history; as multiparous females are more likely to bear anti-HLA antibodies in their plasma that can cause severe complications in the recipient.Citation2 In addition, it is also preferred if the donors and recipients come from the same geographical area to account for any location-specific mutations in the viral pathogen.Citation75 Several other challenges such as lack of NAbs in patient plasma, waning of plasma antibodies, detrimental possibilities like antibody-dependent enhancement of infection, etc., need to be considered, to clearly understand the effects of CP therapy.Citation76 Addressing these issues will further help establish CP therapy as a therapeutic modality and increase its success rate against emerging contagions.
Acknowledgments
We would like to thank our lab mates Dhrubajyoti Mahata, Debangshu Mukherjee, and Nidhi Pandey for their critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transf. 2016;14(2):1. doi:https://doi.org/10.2450/2015.0131-15.
- Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID‐19 convalescent plasma. Vox Sang. 2020;115(6):485–10. doi:https://doi.org/10.1111/vox.12939.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, Makki S, Rooney KD, Group CPS, Nguyen-Van-Tam JS. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi:https://doi.org/10.1093/infdis/jiu396.
- MaizteguiJ, Fernandez N, De Damilano A. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;314(8154):1216–17. doi:https://doi.org/10.1016/S0140-6736(79)92335-3.
- Kaufmann SH. Remembering Emil von behring: from tetanus treatment to antibody cooperation with phagocytes. Am Soc Microbiol. 2017. doi:https://doi.org/10.1128/mBio.00117-17.
- Hifumi T, Yamamoto A, Ato M, Sawabe K, Morokuma K, Morine N, Kondo Y, Noda E, Sakai A, Takahashi J. Clinical serum therapy: benefits, cautions, and potential applications. Keio J Med. 2017:2016–0017-IR. doi:https://doi.org/10.2302/kjm.2016-0017-IR.
- Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38(8):1695. doi:https://doi.org/10.1128/AAC.38.8.1695.
- LukeTC, Casadevall A, Watowich SJ, HoffmanSL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38:e66–e73. doi:https://doi.org/10.1097/CCM.0b013e3181d44c1e.
- Ahmed SM, AhmedM, Nadeem A, Mahajan J, Choudhary A, Pal J. Emergency treatment of a snake bite: pearls from literature. J Emerg Trauma Shock. 2008;1(2):97. doi:https://doi.org/10.4103/0974-2700.43190.
- Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, Rojas-Villarraga A, Ramírez-Santana C, Díaz-Coronado JC, Manrique R. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7):102554. doi:https://doi.org/10.1016/j.autrev.2020.102554.
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–24. doi:https://doi.org/10.1038/s41586-020-2179-y.
- Du L, He Y, ZhouY, Liu S, ZhengB-J, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–36. doi:https://doi.org/10.1038/nrmicro2090.
- Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125(2):79–85. doi:https://doi.org/10.1016/j.imlet.2009.06.003.
- Brioen P, Dekegel D, Boeye A. Neutralization of poliovirus by antibody-mediated polymerization. Virology. 1983;127(2):463–68. doi:https://doi.org/10.1016/0042-6822(83)90159-9.
- Tatum W, Elliott J, Nesset N. The use of plasma as a substitute for whole blood. J Am Soc Anesthesiologists. 1940:363–66. doi:https://doi.org/10.1097/00000542-194011000-00030.
- Dasaraju PV, and Liu C. Infections of the respiratory system. Medical microbiology. 4th ed; Galveston (TX): Univesity of Texas Medical Branch at Galveston;1996. PMID: 21413304.
- Johnston S, and Holgate S. Epidemiology of viral respiratory tract infections. Viral and other infections of the human respiratory tract. London, UK: Springer; 1996. p. 1–38.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi:https://doi.org/10.7326/0003-4819-145-8-200610170-00139.
- Hung IF, To KK, LeeC-K, Lee K-L, ChanK, Yan -W-W, Liu R, Watt C-L, Chan W-M, Lai K-Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–56. doi:https://doi.org/10.1093/cid/ciq106.
- Pollack CV Jr., Kam CW, Mak YK. Update: isolation of avian influenza A(H5N1) viruses from human beings–Hong Kong, 1997-1998. Ann Emerg Med. 1998;31:647–49. PMID: 9581152.
- Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2020. doi:https://doi.org/10.1016/j.jhep.2020.09.031.
- Wong V, Dai D, Wu A, Sung J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. PMID: 12777656.
- Ko J-H, Seok H, Cho SY, Ha YE, Baek JY, KimSH, Kim Y-J, Park JK, Chung CR, KangE-S. Challenges of convalescent plasma infusion therapy in middle east respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–22. doi:https://doi.org/10.3851/IMP3243.
- Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE. Passive transfer of antibodies protects immunocompetent and immunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J Virol. 2001;75(10):4649–54. doi:https://doi.org/10.1128/JVI.75.10.4649-4654.2001.
- Van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, Horby PW, Raoul H, Magassouba NF, Antierens A. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi:https://doi.org/10.1056/NEJMoa1511812.
- Brown JF, Dye JM, Tozay S, Jeh-Mulbah G, Wohl DA, Fischer WA 2nd, Cunningham CK, Rowe K, Zacharias P, van Hasselt J. Anti–Ebola virus antibody levels in convalescent plasma and viral load after plasma infusion in patients with Ebola virus disease. J Infect Dis. 2018;218(4):555–62. doi:https://doi.org/10.1093/infdis/jiy199.
- Jahrling PB, Frame JD, Rhoderick JB, Monson MH. Endemic Lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hyg. 1985;79(3):380–84. doi:https://doi.org/10.1016/0035-9203(85)90388-8.
- Maiztegui JI, McKee KT Jr, Oro JGB, Harrison LH, Gibbs PH, Feuillade MR, Enria DA, Briggiler AM, LevisSC, AmbrosioAM. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J Infect Dis. 1998;177(2):277–83. doi:https://doi.org/10.1086/514211.
- Enria DA, Ambrosio AM, Briggiler AM, Feuillade MR, and Crivelli E. VACUNA CONTRA LA FIEBRE HEMORRAGICA ARGENTINA CANDID# 1 PRODUCIDA EN LA ARGENTINA. INMUNOGENICIDAD Y SEGURIDAD. MEDICINA (Buenos Aires). 2010;70:215–222 . PMID: 20529769.
- Enria D, Fernandez N, Briggiler A, Levis S, MaizteguiJ. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984;324(8397):255–56. doi:https://doi.org/10.1016/s0140-6736(84)90299-x.
- Enria DA, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res. 1994;23(1):23–31. doi:https://doi.org/10.1016/0166-3542(94)90030-2.
- Enria DA, Briggiler AM, SánchezZ. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78(1):132–39. doi:https://doi.org/10.1016/j.antiviral.2007.10.010.
- Vial PA, Valdivieso F, Calvo M, Rioseco ML, Riquelme R, Araneda A, Tomicic V, Graf J, Paredes L, Florenzano M. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome by ANDV. Antivir Ther. 2014;20(4):377–86. doi:https://doi.org/10.3851/IMP2875.
- Wang S, HongS, Deng Y-Q, Ye Q, Zhao L-Z, ZhangF-C, Qin C-F, XuZ. Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring. Cell Res. 2017;27(1):158–60. doi:https://doi.org/10.1038/cr.2016.144.
- Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin J, Purcell RH. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Nat Acad Sci. 1994;91(21):10198–202. doi:https://doi.org/10.1073/pnas.91.21.10198.
- Abolghasemi H, Eshghi P, Cheraghali AM, Fooladi AAI, Moghaddam FB, Imanizadeh S, Maleki MM, Ranjkesh M, Rezapour M, Bahramifar A. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transf Apheresis Sci. 2020;59(5):102875. doi:https://doi.org/10.1016/j.transci.2020.102875.
- Shenoy AG, Hettinger AZ, Fernandez SJ, Blumenthal J, Baez V. Early mortality benefit with COVID‐19 convalescent plasma: a matched control study. Br J Haematol. 2021;192(4):706–13. doi:https://doi.org/10.1111/bjh.17272.
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582–89. doi:https://doi.org/10.1001/jama.2020.4783.
- Erkurt MA, Sarici A, Berber İ, Kuku İ, Kaya E, Özgül M. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transf Apheresis Sci. 2020;59(5):102867. doi:https://doi.org/10.1016/j.transci.2020.102867.
- Ye M, Fu D, RenY, Wang F, Wang D, Zhang F, Xia X, Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–901. doi:https://doi.org/10.1002/jmv.25882.
- Duan K, Liu B, Li C, Zhang H, YuT, Qu J, Zhou M, Chen L, Meng S, HuY. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020;117(17):9490–96. doi:https://doi.org/10.1073/pnas.2004168117.
- Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384(7):610–18. doi:https://doi.org/10.1056/NEJMoa2033700.
- Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez M. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–29. doi:https://doi.org/10.1056/NEJMoa2031304.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama. 2020;324(5):460–70. doi:https://doi.org/10.1001/jama.2020.10044.
- Balcells ME, Rojas L, Le Corre N, Martínez-Valdebenito C, Ceballos ME, Ferrés M, Chang M, Vizcaya C, Mondaca S, Huete Á. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18(3):e1003415. doi:https://doi.org/10.1371/journal.pmed.1003415.
- Zeng Q-L, Yu Z-J, Gou -J-J, Li G-M, MaS-H, Zhang G-F, Xu J-H, LinW-B, CuiG-L, Zhang -M-M. Effect of convalescent plasma therapy on viral shedding and survival in patients with Coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. doi:https://doi.org/10.1093/infdis/jiaa228.
- Arumugham VB, and Rayi A. Intravenous Immunoglobulin (IVIG). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
- Salemi S, Markovic M, Martini G, D’Amelio R. The expanding role of therapeutic antibodies. Int Rev Immunol. 2015;34(3):202–64. doi:https://doi.org/10.3109/08830185.2013.863304.
- Maddur MS, Rabin M, Hegde P, Bolgert F, Guy M, Vallat J-M, Magy L, Bayry J, Kaveri SV. Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain–Barré syndrome patients. Immunol Res. 2014;60(2–3):320–29. doi:https://doi.org/10.1007/s12026-014-8580-6.
- Godeau B, Caulier MT, Decuypere L, Rose C, Schaeffer A, Bierling P, GdÉdTd PTAI. Intravenous immunoglobulin for adults with autoimmune thrombocytopenic purpura: results of a randomized trial comparing 0.5 and 1 g/kg bw. Br J Haematol. 1999;107:716–19. doi:https://doi.org/10.1046/j.1365-2141.1999.01766.x.
- Roifman C, Levison H, Gelfand E. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987;329(8541):1075–77. doi:https://doi.org/10.1016/S0140-6736(87)90494-6.
- Sullivan KM, Kopecky KJ, Jocom J, Fisher L, Buckner CD, Meyers JD, Counts GW, Bowden RA, Petersen FB, Witherspoon RP. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med. 1990;323(11):705–12. doi:https://doi.org/10.1056/NEJM199009133231103.
- Jolles S, Sewell W, Misbah S. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142(1):1. doi:https://doi.org/10.1111/j.1365-2249.2005.02834.x.
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O’Rourke K, Talbot J, Low DE, Group CSS. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. Clin Infect Dis. 1999;28:800–07. doi:https://doi.org/10.1086/515199.
- Bruss JB, Malley R, Halperin S, Dobson S, Dhalla M, Mciver J, Siber GR. Treatment of severe pertussis: a study of the safety and pharmacology of intravenous pertussis immunoglobulin. Pediatr Infect Dis J. 1999;18(6):505–11. doi:https://doi.org/10.1097/00006454-199906000-00006.
- Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13(4):602–14. doi:https://doi.org/10.1128/CMR.13.4.602.
- Phillips AN, Stover J, Cambiano V, Nakagawa F, Jordan MR, Pillay D, Doherty M, Revill P, Bertagnolio S. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in sub–Saharan Africa. J Infect Dis. 2017;215(9):1362–65. doi:https://doi.org/10.1093/infdis/jix089.
- Stiehm ER, Fletcher CV, Mofenson LM, Palumbo PE, Kang M, Fenton T, Sapan CV, Meyer III WA, Shearer WT, Hawkins E. Use of human immunodeficiency virus (HIV) human hyperimmune immunoglobulin in HIV type 1-infected children (Pediatric AIDS clinical trials group protocol 273). J Infect Dis. 2000;181:548–54. doi:https://doi.org/10.1086/315224.
- Bharti OK, Madhusudana SN, Wilde H. Injecting rabies immunoglobulin (RIG) into wounds only: a significant saving of lives and costly RIG. Hum Vaccin Immunother. 2017;13(4):762–65. doi:https://doi.org/10.1080/21645515.2016.1255834.
- Sapir T, Shoenfeld Y. Uncovering the hidden potential of intravenous immunoglobulin as an anticancer therapy. Clin Rev Allergy Immunol. 2005;29(3):307–10. doi:https://doi.org/10.1385/CRIAI:29:3:307.
- Flicker S, Linhart B, Wild C, Wiedermann U, Valenta R. Passive immunization with allergen-specific IgG antibodies for treatment and prevention of allergy. Immunobiology. 2013;218(6):884–91. doi:https://doi.org/10.1016/j.imbio.2012.10.008.
- Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, Koscumb S, Collins K, Schmidhofer M, Shovel JA. Impact of Bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open forum infectious diseases: Oxford University Press US; 2021. ofab254. doi:https://doi.org/10.1093/ofid/ofab254.
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. Jama. 2021;325(7):632–44. doi:https://doi.org/10.1001/jama.2021.0202.
- Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, Ploquin A, Doria-Rose NA, Staupe RP, Bailey M. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351(6279):1339–42. doi:https://doi.org/10.1126/science.aad5224.
- Gaudinski MR, Coates EE, Novik L, Widge A, Houser KV, Burch E, Holman LA, Gordon IJ, Chen GL, Carter C, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet. 2019;393(10174):889–98. doi:https://doi.org/10.1016/S0140-6736(19)30036-4.
- Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–303. doi:https://doi.org/10.1056/NEJMoa1910993.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–39. doi:https://doi.org/10.1093/cid/ciaa237.
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. NEngl J Med. 2020;383(6):517–25. doi:https://doi.org/10.1056/NEJMoa2016638.
- Group RC. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:https://doi.org/10.1056/NEJMoa2021436.
- Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, Solari R, Tinelli MA, Cimino RO, Álvarez L. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMed. 2021;37:100959. doi:https://doi.org/10.1016/j.eclinm.2021.100959.
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–91. doi:https://doi.org/10.1038/s41564-020-00789-5.
- Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi S-R, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:https://doi.org/10.1186/s12879-020-05507-4.
- Raman RS, Bhagwan Barge V, Anil Kumar D, Dandu H, Rakesh Kartha R, Bafna V, Aravinda VT, Raghuram TC. A Phase II safety and efficacy study on prognosis of moderate pneumonia in Coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538–43. doi:https://doi.org/10.1093/infdis/jiab098.
- Cao W, Liu X, Hong K, Ma Z, Zhang Y, Lin L, Han Y, Xiong Y, Liu Z, Ruan L, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12. doi:https://doi.org/10.3389/fimmu.2021.627844.
- Focosi D, Anderson AO, Tang JW, Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev. 2020;33(4):e00072–20. doi:https://doi.org/10.1128/CMR.00072-20.
- Nagoba B, Gavkare A, Jamadar N, Mumbre S, Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19. J Infect Public Health. 2020;13(12):1818–22. doi:https://doi.org/10.1016/j.jiph.2020.08.011.
