ABSTRACT
In recent years, immunotherapy has been widely used to treat patients with malignant tumors. While immune checkpoint inhibitors (ICIs) significantly improve the prognosis of cancer patients, the incidence of immune-related adverse events (irAEs) is increasing. Not only can irAEs accumulate in multiple organ systems throughout the body, but rare adverse reactions may also occur continuously. In severe cases, irAEs can be life-threatening or even lead to death. Therefore, the early identification, diagnosis and treatment of irAEs are very important. Early identification of patients with high-risk irAEs as well as the reduction or avoidance of severe irAEs have important clinical significance. This article will review the research progress of early predictive biomarkers and risk factors for the occurrence of irAEs and propose potential future directions for follow-up research and clinical applications.
Introduction
ICIs mainly kill tumors by enhancing the activity of the immune system, but they also cause a series of adverse reactions called irAEs.Citation1 According to statistics, 94.9% of patients have experienced at least one irAE, and 55.4% of patients have experienced at least one grade 3–4 irAE.Citation2 irAEs can occur at any time after treatment with ICIs, usually within 1–6 months, but also occur within a few days after ICIs treatment or more than 1 year after the end of treatment.Citation3 In recent years, early predictive biomarkers of irAEs have been a research hotspot, and early identification of patients with high-risk irAEs has important clinical significance. The mechanism of irAEs is not yet fully understood. They may be triggered by antigens shared by tumors and inflammatory organsCitation4,Citation5 or may be related to the composition of the intestinal microbiota,Citation6–8 and they may also be related to the existence of an autoimmune toxicity mechanism independent of the antitumor response.Citation9,Citation10 The use of ICIs enhances the activity of T cells against antigens in tumor cells and normal tissues, and increases the levels of autoantibodies and inflammatory factors, resulting in overactivation of the immune system, overrelease of inflammatory factors, and damage to normal tissues that share antigens with tumor cells.Citation11 irAEs can affect multiple organ systems throughout the body, including the gastrointestinal, endocrine, skin, lung, heart, and nerves,Citation12,Citation13 and mortality is the highest when the cardiovascular and nervous system are affected.Citation14 In addition to the currently recognized irAEs, the occurrence of irAEs may vary according to the use of ICIs in different tumor and patient populations, with possible influencing factors including gender, age, hobbies, past history, and physical parameters. The occurrence of irAEs is related to better antitumor efficacy, which suggests that there may be some relationship between efficacy markers and the occurrence of irAEs, such as PD-L1, tumor mutational burden (TMB) and mismatch repair (MMR)/microsatellite instability (MSI). Early identification, diagnosis and treatment are very important. In addition to biomarkers and population characteristics, we should also combine imaging to help diagnosis. Taking “immunotherapy,” “immune checkpoint inhibitor,” “immune related adverse events,” “biomarkers” and “prediction” as the keywords, we searched the articles in PubMed database in recent 10 years, articles related to irAEs prediction indicators were included, and case reports were excluded. We reviewed the predictive indicators and risk factors of irAEs from five aspects: organ-specific biomarkers, nonorgan-specific biomarkers, population characteristic risk factors, immunotherapy efficacy-related biomarkers and imaging parameters.
The organ-specific biomarkers of irAEs
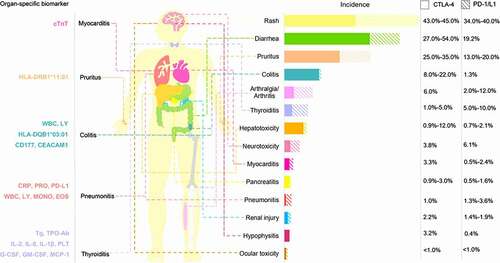
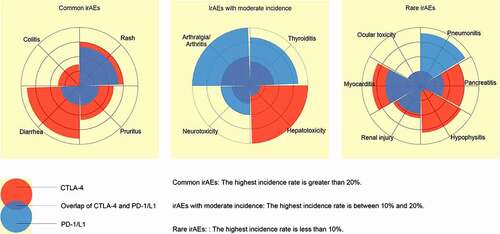
We provide a summary of various irAEs according to their incidences caused by PD-1/L1 inhibitors and CTLA-4 inhibitors, and we divided them into three categories for comparison (). The incidence of each organCitation15–23 and organ-specific biomarker of irAEs is shown in . Several biomarkers that can specifically identify irAEs in specific organs have been found () and are described below.
Table 1. Organ-specific biomarker of irAEs
Figure 1. Comparison of the incidence of irAEs between PD-1/L1 inhibitors and CTLA-4 inhibitors in three categories.
Skin toxicity
Skin toxicity, including rash, pruritus and vitiligo, is the most common adverse event caused by ICIs, but vitiligo is most common in patients with malignant melanoma (MM). Severe skin irAEs include Stevens Johnson syndrome/toxic epidermal necrolysis, drug eruption with eosinophilia and systemic symptoms.Citation1,Citation20,Citation33,Citation34 A prospective single-center clinical trial enrolled 102 patients with advanced tumors treated with ICIs and analyzed the relationship between human leukocyte antigens (HLAs) and irAEs. It was found that those with HLA-DRB1*11:01 were more likely to have pruritus, while HLA-DRB1*01:01 may be a protective factor for pruritus.Citation24 Hasan et al.Citation25 measured the serum levels of BP180, BP230 and type VII collagen in 40 patients with non-small cell lung cancer (NSCLC) treated with PD-1/PD-L1 inhibitors at baseline and 8 weeks after treatment. The results showed that only at baseline, the increase of anti-BP180 IgG was significantly correlated with skin irAEs, but not with BP230 and type VII collagen. It is worth mentioning that BP autoantibody has also been observed in the Japanese.Citation35 We did not identify specific biomarkers for skin rash, vitiligo, or severe skin irAEs. If biomarkers that predict severe skin toxicity can be found, they may be clinically meaningful.
Gastrointestinal toxicity
Gastrointestinal (GI) toxicity mainly manifests as diarrhea and colitis and is one of the most common toxicities associated with ICI treatment. Grade 3–4 immune-related GI toxicity is a common cause of ICI treatment interruption. Shahabi et al.Citation27 reported that in patients with MM treated with ipilimumab, the increased expression of two neutrophil activation markers, CD177 and CEACAM1, during treatment is closely related to GI irAEs, suggesting that neutrophils may play a role in GI irAEs. Hasan et al.Citation24 showed that there is a significant association between the human leukocyte antigen HLA-DQB1*03:01 and colitis in MM patients treated with nivolumab. Fujisawa et al.Citation26 studied MM patients treated with nivolumab and found that the increase in white blood cell count (+27%) and the decrease in relative lymphocyte count (−23%) were associated with grade 3/4 GI irAEs. It is important to take into account the GI symptoms caused by the primary disease when using ICIs in patients with digestive malignancies.
Cardiotoxicity
ICI-related adverse vascular reactions are rare, accounting for approximately 6.3% of all irAEs, but they have a potential risk of death, with a mortality rate of up to 35%. Common adverse cardiovascular reactions include coronary atherosclerosis disease, heart failure, myocarditis, atrial fibrillation and pericardial disease, among which the mortality rate of myocarditis is 39.7–50%, ranking first among all irAEs.Citation14,Citation36–38 Mahmood et al.Citation22 compared the data of patients with and without myocarditis after ICI treatment and found that the risk of major adverse cardiac events with discharge/final troponin T (cTnT) ≥ 1.5 ng/ml increased 4-fold (discharge/final troponin is defined as the troponin measured at the first hospital discharge or the pre-event troponin if the event occurred on the first admission). cTnT is a marker of myocardial injury and is clinically more commonly used in the diagnosis of acute myocardial infarction. When cTnT levels are abnormal in tumor patients treated with ICIs, attention should also be paid to the occurrence of irAEs. Okazaki et al.Citation39 confirmed that the cause of dilated cardiomyopathy in PD-1 deficient mice was the production of high titer autoantibody against heart specific 30 kDa protein, and further identified it as an autoantibody against cardiac troponin I. Muscle antigens desmin and troponin expressed in primary tumor were found in the myocardial tissue of an immune associated myocarditis, and these antigens may trigger the immune response to normal cardiac tissue.Citation4
Pulmonary toxicity
Pneumonia irAEs is a rare but fatal serious adverse event, accounting for 35% of PD-1/PD-L1 inhibitor-related deaths.Citation14 Studies have confirmed that increased white blood cell counts and relative lymphopenia are associated with grade 3/4 lung irAEs in patients with MM and treated with nivolumab;Citation26 high PD-L1 expression, increased eosinophil and monocyte counts, and decreased albumin and C-reactive protein (CRP) are risk factors for lung irAEs in NSCLC patients treated with ICIs.Citation28 Pneumonia irAEs can occur at any stage of treatment. The clinical symptoms mainly include dyspnea (53%), cough (35%) and fever (12%),Citation40 which are similar to respiratory tract infection symptoms and are easy to ignore. Regular patient education material should be provided at the beginning of treatment for early detection and intervention.
Endocrine toxicity
Thyroid dysfunction, hypophysitis and type 1 diabetes (T1D) are both endocrine toxicities. Kurimoto et al.Citation29 prospectively studied the prediction and investigated sensitive biomarkers of thyroid dysfunction during ICI treatment and found that the early increase in thyroglobulin and thyroid autoimmune antibodies and the early decrease in platelets predicted the occurrence of thyroid irAEs. Notably, higher levels of serum IL-1β, IL-2 and GM-CSF at baseline, as well as early reductions in IL-8, G-CSF and MCP-1, were significantly related to the occurrence of thyroid irAEs. Yano et al.Citation30 retrospectively analyzed the HLA genotypes of 11 tumor patients treated with ICIs, and the results suggested that HLA-DR15, B52 and Cw12 may be susceptibility factors for pituitary irAEs. Stamatouli et al.Citation31 described 27 patients with insulin-dependent diabetes mellitus, which differed from classical T1D. The HLA allele HLA-DR4 associated with high risk of spontaneous T1D was dominant, while HLA-DR3, -DQ2, and -DQ8 were less. In addition, 40% of patients were positive for islet autoantibodies (anti-GAD65, anti-IA-2, anti-Znt8, Islet cell antibody). However, in patients with explosive diabetes, there may be no autoantibodies, which were manifested as hyperglycemia, ketosis or ketoacidosis.Citation41 This difference may be that the prevalence of T1D-related autoantibodies varies among different races.Citation42 Unexplainable fatigue, edema, palpitations, hyperhidrosis, dry and consciousness disorder during ICI treatment should be evaluated by an endocrinologist to assist in the diagnosis.
Inflammatory arthritis
Inflammatory arthritis is the most common rheumatic irAEs. Its clinical manifestations include joint pain, swelling, morning stiffness, joint deformity and so on. A large study showed that melanoma, genitourinary cancer, preexisting non-rheumatic autoimmune diseases and receiving combination therapy at baseline were considered to be potential predictors of the development of inflammatory arthritis.Citation43 The results of this study have also been validated in non-U.S. populations.Citation44,Citation45 Cappelli et al.Citation32 evaluated the related HLA type I and type II genes in patients with inflammatory arthritis irAEs, including A* 03:01, B* 08:01, B* 15:01, B* 27.05, B* 52:01, C* 06:02, C*12: 02, DQB1* 03:01, DRB1* 03:01 and DRB1 shared epitope (SE) alleles (HLA-DRB1* 01:01, 01: 02, 04: 01, 04: 04, 04: 05, 04: 01: 08, 10:01, 14:02), they found that 61.5% of inflammatory arthritis irAEs patients had at least one SE allele, HLA DRB1* 04:05 was enriched in inflammatory arthritis irAEs. They also found that the positive rates of HLA A*03: 01, HLA B*52: 01 and HLA C*12: 02 all had an upward trend in inflammatory arthritis irAEs, while DQB1*03: 01 had a downward trend, and HLA B*52: 01 and C*12: 02 may be related to inflammatory arthritis irAEs. Conventional inflammatory arthritis predictive autoantibodies, such as rheumatoid factor and anti-CCP, are poorly predictive of inflammatory arthritis irAEs,Citation46 and rapid clinical assessment remains important in its early detection.Citation47
Neurotoxicity
Neurotoxicity irAEs is uncommon, including gravis myasthenia (GM), Guillain-Barré syndrome, aseptic meningitis, encephalitis, and transverse myelitis. In the case of GM irAEs, activation of subclinical autoimmunity through generalized immune activation. Due to its rarity, current related reports are mainly based on case reports. Huang et al.Citation48 retrieved 45 reports of GM irAEs and analyzed 47 cases of GM irAEs. The positive rates of anti-acetylcholine receptor (AChR) antibody and anti-muscle-specific kinase antibody in GM irAEs group were lower than those in classic GM group. Haugh et al.Citation49 believed that even though the titer of AChR antibodies was very low, it was considered highly specific to GM, preexisting AchR antibodies may reflect underlying genetic or environmental risk factors. The above two studiesCitation48,Citation49 still suggest that conventional autoantibodies are predictive of GM irAEs. Patients with metastatic melanoma developed anti-NMDA encephalitis after treatment with ICIs, and GRIN2A gene encoding NMDA receptor was expressed frequently in melanoma, which may represent a common antigen associated with autoimmune encephalitis.Citation49 Mouse models expressing neo-self antigen showed cerebellar inflammation after using ICIs, whereas this situation was not found in non-neo-self antigen models.Citation50
Nonorgan-specific biomarkers of irAEs
Among the nonorgan-specific biomarkers discovered thus far (), biomarkers in blood cell research are the most, but their predictive value is still controversial.
Table 2. Nonorgan-specific biomarkers of irAEs
Complete blood count
Blood count is a routine clinical test item that includes much information, but the researches mainly focus on white blood cells, neutrophils, lymphocytes and platelets. The study of Isono et al.Citation28 showed that in patients with NSCLC who were treated with PD-1 inhibitors, their white blood cell count and lymphocyte count were risk factors for the occurrence of irAEs. Pavan et al.Citation51 collected baseline data on neutrophils/lymphocytes (NLR) and platelets/lymphocytes (PLR) of patients with aNSCLC treated with ICIs and found that low NLR (<3) and low PLR (<180) were significantly related to the occurrence of irAEs. Khoja et al.Citation52 also analyzed NLR and PLR in patients with MM treated with ipilimumab. In addition to baseline, the detection time also included the second cycle and the end of treatment. However, they found that there was no significant correlation between the occurrence of NLR, PLR and irAEs.
C reactive protein
CRP is also a common clinical indicator. Abolhassani et al.Citation53 measured the CRP levels of MM patients treated with ICIs. When irAEs occurred, 93% of patients had increased CRP from an average baseline of 8.4 mg/L (normal <5 mg/L) to an average of 52.7 mg/L, and 42% of patients had elevated CRP before clinical symptoms. They proposed that elevated CRP could predict the occurrence of irAEs in the absence of infectious diseases. CRP is mostly considered as an infectious marker. When infectious diseases are present, the prediction of irAEs by CRP may be disturbed, so its predictive value may not be high.
Serum sCD163 and CXCL5
Soluble CD163 (sCD163) is a tumor-associated macrophage marker.Citation60 CXCL5 is a biomarker of autoimmune diseases.Citation61 Fujimura et al.Citation54 believed that sCD163 and CXCL5 could predict the occurrence of irAEs. They measured the levels of sCD163 and CXCL5 on days 0 and 42 in patients with MM who were treated with nivolumab, and found that sCD163 increased significantly in patients with irAEs on day 42. Although there was no significant change in CXCL5, it could be a marker of elevated sCD163. IrAEs can occur at any time, and the study only measured sCD163 and CXCL5 levels at baseline and day 42, so it may not be appropriate for patients with tumors that develop irAEs within 40 days. Future studies may need to analyze more time points for these two markers to find the best prediction time, which may have great clinical value.
B cells
B cells play an important role in humoral immunity. At present, there are few studies on B cells to predict the occurrence of irAEs, and the mechanism of action is still unclear. Only one prospective study found that patients with reduced circulating B cells (70% of baseline), CD21lo B cells and plasmablasts that were increased more than 2 times were more likely to develop irAEs, and the severity of the early decline in the number of B cells after treatment was directly related to the timing of the onset of toxicity. Patients with early B-cell changes were at greater risk of irAEs, among patients without grade 3 or higher irAEs, the survival rate of patients with B-cell changes at 6 months was lower than that of patients without changes (0% versus 87%).Citation55
Interferon-γ
In the cellular immunity of Mycobacterium tuberculosis, the PD-1/PD-L1 axis and interferon-γ (IFN-γ) play an important role.Citation62 Hirashima et al.Citation56 speculated that the release of IFN-γ in T lymphocytes would change after ICI treatment. They used QuantiFERON®-TB Gold Plus (QFT-Plus) to measure IFN-γ levels at four time points in 29 patients with NSCLC treated with ICIs and found that severe irAEs were prone to occur when IFN-γ levels were <10 IU/ml at 227 days or 437 days after ICI treatment. The researchers were able to predict irAEs early, but the sample size was small; therefore, a large sample study is needed for verification in the future.
Cytokines
Cytokines can be stimulated by immune cells and may be related to the occurrence of irAEs. Khan et al.Citation57 examined the serum levels of 40 cytokines in 65 patients treated with ICIs and 13 healthy subjects. They found that patients with irAEs had lower baseline levels and higher levels of various cytokines after treatment, suggesting that underlying immune dysregulation may be associated with a higher risk of irAEs. Among them, the inducible CXCL9, 10, 11, and 13 level patterns had the strongest correlation with irAEs.
LCP1 and ADPGK
Jing et al.Citation58 conducted a multiomics analysis on 7 potential predictors of irAEs and found that the combination of CD8 + T cells and TCR diversity achieved maximum predictive efficacy and further comprehensive screening of mRNA, miRNA, lncRNA and protein expression and nonsilent gene mutations across 26 cancer types was performed. It was ultimately found that the combination of T cell-activated lymphocyte cytosolic protein 1 (LCP1) and adenosine diphosphate-dependent glucokinase (ADPGK) had the best prediction accuracy. In the validation cohort, 26 cases of pneumonia were successfully predicted out of 28 cases. These 26 patients all had lung cancer. In other cancer types, the predictive value of LCP1 and ADPGK was not yet clear, so these markers need to be verified in other cancer types.
PD-L1
At present, the clinical detection of PD-L1 expression is mainly used to guide patients’ immunotherapy and efficacy prediction. Sugisaka et al.Citation59 retrospectively analyzed the expression of PD-L1 in 44 patients with NSCLC treated with pembrolizumab and found that those with high PD-L1 expression (≥50%) were more likely to develop irAEs, but these markers were not related to the severity of the occurrence. The higher the expression of PD-L1 was, the better the efficacy of immunotherapy, but its high expression may indicate that they patient are more prone to develop irAEs. PD-L1 expression will guide clinicians to develop treatment plans that maximize benefits for the patients.
Population characteristic risk factors for irAEs
Current studies point out that sex, age, smoking, previous diseases, body mass index (BMI) and Eastern Cooperative Oncology Group performance status (ECOG PS) are related to the occurrence of irAEs (), but there is no unified conclusion yet.
Table 3. Population characteristic risk factors for irAEs
Gender
In general, women have a stronger immune response than men and are more likely to develop autoimmune diseases, which may be related to sex hormones and sex chromosome-related genes.Citation73 Takada et al.Citation63 compared T1D irAEs with non-T1D irAEs using a real database of Japanese populations and found that being female and having melanoma were risk factors for T1D irAEs. Duma et al.Citation64 studied the sex differences in irAEs between melanoma and NSCLC patients treated with PD-1 inhibitors, and the results suggested that premenopausal women with MM and women with NSCLC are prone to irAEs, pneumonia and endocrine toxicity. Kartolo et al.Citation65 studied the predictors of irAEs in patients with MM, NSCLC, and kidney cancer treated with ICIs and ultimately found that being female was a protective factor for irAEs, and males were more prone to skin toxicity, but GI and endocrine toxicity were not sex-related.
Age
In a study of irAEs in patients with NSCLC treated with PD-1 inhibitors, univariate and multivariate analyses of age (≥75 and <75) confirmed that age <75 was a risk factor for irAEs.Citation28 Another pooled analysis from four ICI trials reached the same conclusion and further confirmed that patients <65 years old are more likely to have grade 3 or 4 irAEs than patients ≥75 years old.Citation67 However, a pooled analysis of KEYNOTE-010 (NCT01905657), KEYNOTE-024 (NCT02142738), and KEYNOTE-042 (NCT02220894) studies showed that the incidence of irAEs did not differ between patients aged <75 years and ≥75 years.Citation66 Kartolo et al.Citation65 calculated the average age of patients with and without irAEs and found that there was no significant difference in the average age between the two groups.
Smoking
Kartolo et al.Citation65 also studied the relationship between irAEs and smoking and found that there was no significant association between smoking and irAEs. However, the results of Okada et al.Citation68 do not agree with this conclusion. They found that lung cancer patients treated with PD-1/PD-L1 inhibitors may benefit from heavy smoking but have a higher risk of lung irAEs and pointed out that ≥50 pack-years (Pack-years was calculated as the number of cigarettes smoked per day × smoking year/20) is a risk factor for interstitial lung disease (ILD) of all grades. The former study only analyzed the correlation between smoking and irAEs, while the latter study further analyzed the impact of smoking degree on irAEs. The opposite conclusions reached in these two studies may be caused by the differences in tumor types, drugs, smoking groups and statistical methods. Therefore, studies on smoking as a risk factor should further analyze the degree of smoking.
Past history
Many studies have analyzed the relationship between previous diseases, previous medication history and irAEs. The study by Kartolo et al.Citation65 showed that a history of autoimmune disease and poor kidney function of grade 3 or greater were associated with a higher risk of developing irAEs, while corticosteroid use before immunotherapy was found to have a protective effect against irAEs. Several studies of lung irAEs in patients with small cell lung cancer have suggested that patients with preexisting interstitial lung abnormalities, pneumothorax, pleural effusion, pneumonitis, prior thoracic radiotherapy, combination therapy (anti-PD-1 treatment with chemotherapy, targeted therapy or CTLA-4 blockade) or who take statin medications have a higher risk of irAEs.Citation65,Citation69–71 Whether these diseases and treatment history are only risk factors for lung cancer patients is still unclear, and this needs to be further explored in patients with other cancer types.
Body parameters
The current research on physical parameters mainly includes ECOG PS and BMI. Okada et al. Citation68 evaluated the ECOG PS of patients with lung cancer and analyzed its correlation with lung irAEs. The results suggested that ECOG PS ≥2 was an independent risk factor for ILD irAEs of grade ≥3 and all grades. In the study related to BMI, Kartolo et al.Citation65 compared the mean BMI of patients with and without irAEs and found that there was no difference in mean BMI between the two groups. A multicenter retrospective study by Cortellini et al. analyzed the BMI of 1770 tumor patients who received PD-1/PD-L1 inhibitors, and the results confirmed that overweight (25 ≤ BMI ≤ 29.9) and obesity (BMI ≥ 30) were independent predictors of irAEs for any grade. The incidence of grade 3 and 4 irAEs in obese patients was significantly higher than that in patients with a normal weight.Citation72
Immunotherapy efficacy-related biomarkers
It has been suggested that in the treatment of ICIs, patients who have experienced irAEs benefit more than patients who have not.Citation74 The occurrence of vitiligo usually indicated that patients with MM may benefit from PD-1 inhibitors.Citation75 ILD irAEs correlated with better prognosis in patients with advanced NSCLC.Citation76 This suggests that the occurrence of irAEs may be related to better anti-tumor efficacy. Currently, markers related to the efficacy of ICIs are mainly focused on tumor signatures, including PD-L1 expression, TMB and MSI/MMR. Daud et al.Citation77 showed that the high expression of PD-L1 was significantly related to the efficacy. As mentioned earlier, although patients with high PD-L1 expression were more likely to benefit, they were also more likely to develop irAEs. Goodman et al.Citation78 reported for the first time that higher TMB was associated with a good prognosis in immunotherapy of a variety of tumors. Patients with MMR-deficient/ MSI-high also had better immunotherapy efficacy.Citation79 TMB is the total number of non-synonymous somatic mutations in the coding region of the genome, this mutation changes the amino acid sequence of the protein encoded by the affected gene, resulting in the formation of mutation associated neogenes (Manas).Citation80 In addition, MMR-deficient cancers produce a large amount of Manas, which are recognized by the immune system.Citation81 We speculate that these Manas may be similar or the same as the antigen structure on the surface of some normal tissues and organs of human body. The immune system attacks normal tissues and organs after using ICIs, resulting in autoimmune diseases. Whether these Manas can cross-react with the host and cause irAEs deserves further study.
Imaging parameters
Imaging is often used in the evaluation of the anti-tumor efficacy of cancer patients. At present, there are few researches related to the prediction of irAEs by imaging. Positron emission tomography (PET) is often used for staging. A study has shown that it can predict the occurrence of thyroiditis irAEs in lung cancer. Increased fluro deoxyglucose (FDG) uptake in the thyroid of patients with thyroiditis irAEs can be observed on PET images.Citation82 The study also confirmed the ability of 18F-FDG PET to detect thyroiditis irAEs before serum TSH increases.Citation82 Colen et al.Citation83 used radiomics to predict the occurrence of pneumonia irAEs. They retrospectively analyzed the radiographic pretreatment baseline computed tomography (CT) scans of 290 patients treated with ICIs. Two of the 290 cases developed pneumonia irAEs. The control group included 30 randomized patients without pneumonia irAEs. The results showed that higher skewness and angular variance of sum of squares were related to pneumonia irAEs, and the accuracy was 100%. There are only two patients with pneumonia irAEs in this trial, and we look forward to the results of the large sample study.
Conclusion and future prospects
Clarifying the underlying mechanism of irAEs is the key to finding early biomarkers of irAEs. The specific pathophysiological mechanism of irAEs in patients treated with ICIs is not yet clear. Some studies have shown that there may be the following mechanisms: 1. the blocking of CTLA-4 on regulatory T cells (Treg) may lead to Treg depletion, change Treg function and regulate T cell bank, resulting in the emergence of autoreactive T cells, thus affecting B cell function and increasing the production of autoantibodies;Citation84 2. PD-1 blockade may reactivate exhausted/anergic T cells, resulting in pathogenic/self-reactive T cells;Citation84 3. Blockade of PD-1 and CTLA-4 enhances the effector function of T cells, and may produce pathogenic T cells, overrelease cytokines, alter B cell number/function, and increase autoantibodies, leading to inflammation and autoimmunity;Citation84 4. After tumor cell death, self and tumor antigens can be produced, which are ingested by antigen-presenting cell and migrated to lymph nodes and original T cells. In normal tissues, self-antigens are recognized by autoreactive T cells, cytokines and autoantibodies increase, resulting in tolerance and tissue destruction;Citation85 5. During the treatment of ICIs, CTLA-4 expressed in normal tissues is also combined, leading to the destruction of organ tolerance.Citation85 Various mechanisms may eventually point to the activation of autoimmunity, which can occur in different organ systems of the human body. When it occurs in the skin, it is skin toxicity, when it occurs in the heart, it is cardiotoxicity, and it can also occur in multiple organ systems at the same time, varying according to the organ in which it occurs. In clinical work, we should comprehensively judge patients based on their individual characteristics, symptoms, biological indicators, imaging parameters, etc., and implement close follow-up and full management of patients receiving ICIs.
Due to a variety of real-world clinical problems, it is difficult to find clinically valuable biomarkers for the early prediction of irAEs. Firstly, the study of current biomarkers for the early prediction of irAEs is still in its infancy. Most biomarkers are not detected in routine clinical tests, and the high cost of testing for these markers may limit their application. Second, many studies are also focused on a single type of cancer or specific irAEs, which also limits the application of biomarkers in other cancer types or irAEs. In addition, many biomarkers predict irAEs of any grade, while there is little clinical significance in predicting grade 1 and 2 irAEs. Finally, some organs have a low incidence of toxicity, and because of the small sample size, it is difficult to find biomarkers for early prediction. The more practical clinical test item is blood cell count, in which NRL and PRL are mostly used to judge the prognosis.Citation86–88 Recently, some researchers have studied their relationship with irAEs. Pavan et al.Citation51 proposed that NLR and PLR could predict the occurrence of irAEs, but Khoja et al.Citation52 considered this to be irrelevant. This difference in opinions may be caused by the different types of cancers, drugs, and detection times of NLR and PLR. It has also been proposed that certain indicators in the blood cell count can predict irAEs in specific organs. Kurimoto et al.Citation29 pointed out that an early reduction in platelet count may predict the occurrence of thyroid irAEs. Fujisawa et al.Citation26 found that increased white blood cell count and decreased relative lymphocyte count were associated with grade 3 or 4 irAEs and lung/GI irAEs. Blood count is a relatively routine and low-cost test. Although controversial, blood count can also be used as a reference index for clinicians.
Population characteristic risk factors provide important clues for clinicians and indicate which patients need to be monitored. Some researchers have found that female sex, age, smoking, and higher BMI were risk factors for irAEs,Citation28,Citation63,Citation64,Citation68,Citation72 but Kartolo et al.Citation65 believed that these four factors were not related to irAEs. The tumor type in this study was mainly melanoma, while other studies mainly focused on NSCLC, and there were fewer subjects than in other studies. The statistical analysis of the data mainly adopted the mean value, and the analysis of single factors lacked stratified analysis, all of which may affect the results. Two other studies on age also came to inconsistent conclusions. The four clinical trials included by Marur et al.Citation67 were not randomized, and less than a quarter of patients were ≥75 years old but stratified by age. In the three KEYNOTE trials analyzed by Nosaki et al.Citation66 there was no stratification by age, and the total number of older and younger patients differed significantly, but the incidence of irAEs in older patients was similar to the results observed in the overall pooled population.Citation89–91 Cortellini et al.Citation72 believe that individuals with a BMI ≥25 are prone to irAEs. In this study, there were significantly fewer patients with BMI <18.5 than the other groups. Obesity and emaciation are both malnutrition, and nutritional status plays an important role in the treatment of tumors.Citation92,Citation93 It is worth exploring whether patients with a lower BMI are also prone to irAEs. At present, there are few studies exploring the relationship between nutrition and irAEs, and we suspect that this may be a potential future direction. Whether sex, age, smoking, and BMI are related to irAEs is still controversial.
Many biomarkers and risk factors have been used to predict the occurrence of irAEs. By searching the literature, we found that increased white blood cell count, decreased relative lymphocytes, early B cell changes and ECOG PS ≥2 could predict the occurrence of grade 3 or higher irAEs, and the detection of IFN-γ by QFT-Plus could detect severe irAEs on day 22 ± 7 at the earliest. Myocarditis and pneumonia are common severe irAEs, cTnT can predict myocarditis, white blood cells and lymphocytes can predict pneumonia. Most of the current studies are retrospective studies and lack a validation cohort. However, the validation cohort studies are prone to bias, including immediate time bias, and other information bias including notation bias, which are all challenges we will face. Some studies even lack a control group without irAEs, which reduces the reliability of these conclusions. Therefore, a large number of prospective studies are needed to verify and investigate biomarkers, especially the early predictors of grade 3–5 irAEs, which may be the direction of future research and will have important clinical value.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–12. doi:10.1056/NEJMra1703481.
- Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels GA, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J clin oncol. 2017;35(34):3815–22. doi:10.1200/JCO.2016.72.1167.
- Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, Kähler KC, Loquai C, Reinmuth N, Steins M, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi:10.1016/j.ctrv.2016.02.003.
- Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. doi:10.1056/NEJMoa1609214.
- Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5(7):1043–47. doi:10.1001/jamaoncol.2019.0402.
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi:10.1126/science.aan4236.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–08. doi:10.1126/science.aao3290.
- Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79. doi:10.1093/annonc/mdx108.
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi:10.1186/s40425-019-0805-8.
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. doi:10.1126/scitranslmed.3008002.
- Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ, Yao Y, Jiang -L-L, Sun H, Qin T-J, Guo H, et al. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res. 2020;39(1):284. doi:10.1186/s13046-020-01749-x.
- El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1–12. doi:10.1016/j.critrevonc.2017.09.002.
- Kottschade LA. Incidence and management of immune-related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep. 2018;20(3):24. doi:10.1007/s11912-018-0671-4.
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–28. doi:10.1001/jamaoncol.2018.3923.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030.
- Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 2017;69:687–99. doi:10.1002/art.40043.
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82. doi:10.1001/jamaoncol.2017.3064.
- Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, Pennell NA, Velcheti V. Safety and efficacy of PD-1/PD-L1 inhibitors in treatment-naive and chemotherapy-refractory patients with non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer. 2018;19(3):e335–e48. doi:10.1016/j.cllc.2018.01.002.
- Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72(1):315–29. doi:10.1002/hep.31227.
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264–iv6. doi:10.1093/annonc/mdy162.
- Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34(1):108–17. doi:10.1093/ndt/gfy105.
- Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. doi:10.1016/j.jacc.2018.02.037.
- Hsu C, Marshall JL, He AR. Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. The Oncologist. 2020;25(2):105–11. doi:10.1634/theoncologist.2018-0162.
- Hasan Ali O, Berner F, Bomze D, Fässler M, Diem S, Cozzio A, Jörger M, Früh M, Driessen C, Lenz TL, et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer. 2019;107:8–14. doi:10.1016/j.ejca.2018.11.009.
- Hasan Ali O, Bomze D, Ring SS, Berner F, Fässler M, Diem S, Abdou M-T, Hammers C, Emtenani S, Braun A, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol. 2020;82(4):854–61. doi:10.1016/j.jaad.2019.08.045.
- Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, Hata H, Gosho M, Tanaka R, Yamaguchi K, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88(2):225–31. doi:10.1016/j.jdermsci.2017.07.007.
- Shahabi V, Berman D, Chasalow SD, Wang L, Tsuchihashi Z, Hu B, Panting L, Jure-Kunkel M, Ji -R-R. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med. 2013;11(1):75. doi:10.1186/1479-5876-11-75.
- Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, Kobayashi Y, Ishiguro T, Takaku Y, Kurashima K, et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2021;12(2):153–64. doi:10.1111/1759-7714.13736.
- Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, Takeshima K, Furukawa Y, Morita S, Yamamoto Y, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020;111(5):1468–77. doi:10.1111/cas.14363.
- Yano S, Ashida K, Sakamoto R, Sakaguchi C, Ogata M, Maruyama K, Sakamoto S, Ikeda M, Ohe K, Akasu S, et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur J Cancer. 2020;130:198–203. doi:10.1016/j.ejca.2020.02.049.
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471–80. doi:10.2337/dbi18-0002.
- Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology. 2019;58(3):476–80. doi:10.1093/rheumatology/key358.
- Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J clin oncol. 2018;36(17):1714–68. doi:10.1200/JCO.2017.77.6385.
- Matsui Y, Makino T, Ishii N, Hashimoto T, Shimizu T. Detection of IgG antibodies to BP180 NC16a and C-terminal domains and LAD-1 in nivolumab-associated bullous pemphigoid. Eur J Dermatol. 2019;29(5):554–55. doi:10.1684/ejd.2019.3618.
- Master SR, Robinson A, Mills GM, Mansour RP. Cardiovascular complications of immune checkpoint inhibitor therapy. J Clin Oncol. 2019;37(15_suppl):2568. doi:10.1200/JCO.2019.37.15_suppl.2568.
- Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano J-P, Balko JM, Bonaca MP, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89. doi:10.1016/S1470-2045(18)30608-9.
- Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi:10.1016/S0140-6736(18)30533-6.
- Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83. doi:10.1038/nm955.
- Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J clin oncol. 2017;35(7):709–17. doi:10.1200/JCO.2016.68.2005.
- Gauci ML, Boudou P, Baroudjian B, Vidal-Trecan T, Da Meda L, Madelaine-Chambrin I, Basset-Seguin N, Bagot M, Pages C, Mourah S, et al. Occurrence of type 1 and type 2 diabetes in patients treated with immunotherapy (anti-PD-1 and/or anti-CTLA-4) for metastatic melanoma: a retrospective study. Cancer Immunol Immunother. 2018;67(8):1197–208. doi:10.1007/s00262-018-2178-0.
- Iwama S, Kobayashi T, Arima H. Clinical characteristics, management, and potential biomarkers of endocrine dysfunction induced by immune checkpoint inhibitors. Endocrinol Metab. 2021;36(2):312–21. doi:10.3803/EnM.2021.1007.
- Cunningham-Bussel A, Wang J, Prisco LC, Martin LW, Vanni KMM, Zaccardelli A, Nasrallah M, Gedmintas L, MacFarlane LA, Shadick NA, et al. Predictors of rheumatic immune-related adverse events and de novo inflammatory arthritis after immune checkpoint inhibitor treatment for cancer. Arthritis Rheumatol. 2021. doi:10.1002/art.41949.
- Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, Enk A, Lorenz H-M, Hassel JC. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother. 2018;67(2):175–82. doi:10.1007/s00262-017-2069-9.
- Liew DFL, Leung JLY, Liu B, Cebon J, Frauman AG, Buchanan RRC. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheum Dis. 2019;22(2):297–302. doi:10.1111/1756-185X.13444.
- Liu Y, Jaquith JM, McCarthy-Fruin K, Zhu X, Zhou X, Li Y, Crowson C, Davis JM, Thanarajasingam U, Zeng H, et al. Immune checkpoint inhibitor-induced inflammatory arthritis: a novel clinical entity with striking similarities to seronegative rheumatoid arthritis. Clin Rheumatol. 2020;39(12):3631–37. doi:10.1007/s10067-020-05162-9.
- Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14(10):569–79. doi:10.1038/s41584-018-0074-9.
- Huang YT, Chen YP, Lin WC, Su WC, Sun YT. Immune checkpoint inhibitor-induced myasthenia gravis. Front Neurol. 2020;11:634. doi:10.3389/fneur.2020.00634.
- Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020;19(4):479–88. doi:10.1080/14740338.2020.1738382.
- Yshii LM, Gebauer CM, Pignolet B, Mauré E, Quériault C, Pierau M, Saito H, Suzuki N, Brunner-Weinzierl M, Bauer J, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain. 2016;139(11):2923–34. doi:10.1093/brain/aww225.
- Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, Guarneri V, Aprile G, Conte P, Bonanno L, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. The Oncologist. 2019;24(8):1128–36. doi:10.1634/theoncologist.2018-0563.
- Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, Hogg D, Butler MO, Joshua AM. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med. 2016;5(10):2792–99. doi:10.1002/cam4.878.
- Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145(10):2625–31. doi:10.1007/s00432-019-03002-1.
- Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, Yoshino K, Matsushita S, Funakoshi T, Hata H, et al. Serum levels of soluble CD163 and CXCL5 may be predictive markers for immune-related adverse events in patients with advanced melanoma treated with nivolumab: a pilot study. Oncotarget. 2018;9(21):15542–51. doi:10.18632/oncotarget.24509.
- Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, Bacchiocchi A, Kluger H, Wei W, Halaban R, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest. 2018;128(2):715–20. doi:10.1172/JCI96798.
- Hirashima T, Kanai T, Suzuki H, Yoshida H, Matsusita A, Kawasumi H, Nasu S, Tanaka A, Morishita N, Kawahara K, et al. Significance of pre-treatment interferon-gamma release in patients with non-small-cell lung cancer receiving immune checkpoint inhibitors. Anticancer Res. 2020;40(12):6971–78. doi:10.21873/anticanres.14721.
- Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-mccutchen Y, Lu R, Xie Y, Li Q, Wakeland E, et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br J Cancer. 2019;120(1):63–68. doi:10.1038/s41416-018-0155-1.
- Jing Y, Liu J, Ye Y, Pan L, Deng H, Wang Y, Yang Y, Diao L, Lin SH, Mills GB, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun. 2020;11(1):4946. doi:10.1038/s41467-020-18742-9.
- Sugisaka J, Toi Y, Taguri M, Kawashima Y, Aiba T, Kawana S, Saito R, Aso M, Tsurumi K, Suzuki K, et al. Relationship between programmed cell death protein ligand 1 expression and immune-related adverse events in non-small-cell lung cancer patients treated with pembrolizumab. Jma J. 2020;3(1):58–66. doi:10.31662/jmaj.2019-0005.
- Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47(7–8):1650–60. doi:10.1016/j.molimm.2010.02.008.
- Fujimura T, Kakizaki A, Furudate S, Aiba S. A possible interaction between periostin and CD163+ skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid. Exp Dermatol. 2017;26(12):1193–98. doi:10.1111/exd.13157.
- Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, Barber DL. CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 2016;12(5):e1005667. doi:10.1371/journal.ppat.1005667.
- Takada S, Hirokazu H, Yamagishi K, Hideki S, Masayuki E. Predictors of the onset of type 1 diabetes obtained from real-world data analysis in cancer patients treated with immune checkpoint inhibitors. Asian Pac J Cancer Prev. 2020;21:1697–99. doi:10.31557/APJCP.2020.21.6.1697.
- Duma N, Abdel-Ghani A, Yadav S, Hoversten KP, Reed CT, Sitek AN, Enninga EAL, Paludo J, Aguilera JV, Leventakos K, et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: are we all equal? Oncologist. 2019;24(11):e1148–e55. doi:10.1634/theoncologist.2019-0094.
- Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol. 2018;25(5):e403–e10. doi:10.3747/co.25.4047.
- Nosaki K, Saka H, Hosomi Y, Baas P, de Castro G Jr., Reck M, Wu Y-L, Brahmer JR, Felip E, Sawada T, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–95. doi:10.1016/j.lungcan.2019.07.004.
- Marur S, Singh H, Mishra-Kalyani P, Larkins E, Keegan P, Sridhara R, Blumenthal GM, Pazdur R. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol. 2018;45:220–25. doi:10.1053/j.seminoncol.2018.08.007.
- Okada N, Matsuoka R, Sakurada T, Goda M, Chuma M, Yagi K, Zamami Y, Nishioka Y, Ishizawa K. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: a single-institution retrospective study. Sci Rep. 2020;10(1):13773. doi:10.1038/s41598-020-70743-2.
- Nakanishi Y, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, Miyamoto S, Tsutani Y, Iwamoto H, Fujitaka K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig. 2019;57(5):451–59. doi:10.1016/j.resinv.2019.05.002.
- Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K, Yoshida H, Akai M, Mio T, Hirai T. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847–55 doi:10.1111/1759-7714.12759.
- Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, Han C, Long Y, Li Y, Zheng X, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018;7(8):4115–20. doi:10.1002/cam4.1579.
- Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M, Zoratto F, et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/ Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer. 2020;128:17–26. doi:10.1016/j.ejca.2019.12.031.
- Wang S, Cowley LA, Liu XS. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules. 2019;24(18):3214. doi:10.3390/molecules24183214.
- Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J clin oncol. 2017;35(7):785–92. doi:10.1200/JCO.2015.66.1389.
- Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi:10.1016/j.ejca.2016.02.025.
- Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, Hisakane K, Takahashi S, Tanaka T, Takeuchi S, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer. 2020;11(4):1052–60. doi:10.1111/1759-7714.13364.
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J clin oncol. 2016;34(34):4102–09. doi:10.1200/JCO.2016.67.2477.
- Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–608 doi:10.1158/1535-7163.MCT-17-0386.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi:10.1056/NEJMoa1500596.
- Adam T, Becker TM, Chua W, Bray V, Roberts TL. The multiple potential biomarkers for predicting immunotherapy response-finding the needle in the haystack. Cancers (Basel). 2021;13(2):277. doi:10.3390/cancers13020277.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi:10.1126/science.aan6733.
- Eshghi N, Garland LL, Nia E, Betancourt R, Krupinski E, Kuo PH. (18)F-FDG PET/CT can predict development of thyroiditis due to immunotherapy for lung cancer. J Nucl Med Technol. 2018;46:260–64. doi:10.2967/jnmt.117.204933.
- Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, Hajjar J, Suarez-Almazor ME, Alshawa A, Hong DS, et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Invest New Drugs. 2018;36(4):601–07. doi:10.1007/s10637-017-0524-2.
- June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles‘ heel of cancer immunotherapy? Nat Med. 2017;23(5):540–47. doi:10.1038/nm.4321.
- Von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66(6):779–93. doi:10.1093/clinchem/hvaa081.
- Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the Neutrophil to Lymphocyte Ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18(5):1417–21. doi:10.22034/APJCP.2017.18.5.1417.
- Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T, Nomura S, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–40. doi:10.1007/s10147-018-1250-2.
- Gu X, Sun S, Gao XS, Xiong W, Qin S, Qi X, Ma M, Li X, Zhou D, Wang W, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep. 2016;6(1):23893. doi:10.1038/srep23893.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. doi:10.1016/S0140-6736(15)01281-7.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi:10.1056/NEJMoa1606774.
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. doi:10.1016/S0140-6736(18)32409-7.
- Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579(7800):507–17. doi:10.1038/s41586-020-2124-0.
- Fillon M. Exercise and nutrition may prolong the lives of patients with colon cancer. CA Cancer J Clin. 2018;68(5):319–21. doi:10.3322/caac.21430.