ABSTRACT
This study aimed to evaluate the immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine (PCV13). In total, 1200 infants were randomized into two groups with a 1:1 allocation and received a three-dose series of tested PCV13 or control PCV13 at ages 2, 4 and 6 months, respectively, and a booster dose at 12–15 months. Blood samples were collected before and 30 days after primary and booster vaccination. Serotype-specific antibodies were measured using ELISA for immunoglobulin G (IgG) and OPA for functional antibodies. Safety data were collected for 30 days after each inoculation. Results showed that post primary vaccination seropositive rates of all 13 serotypes except type 3 were not significantly different between two groups. The seropositive rate for type 3 in Group T was significantly higher than Group C (P < .0001). For all 13 serotypes except type 7 F, the GMCs in Group T were significantly higher than Group C. The GMC for type 7 F in Group T (P < .0009) was significantly lower than Group C. The frequencies of overall adverse events (P = .0064) and solicited adverse reactions (P = .0019) in Group T were significantly lower than Group C. Post booster vaccination, seropositive rates for all serotypes in Group T were 100.00%. For all serotypes except type 23 F, IgG GMCs in Group T were significantly higher than Group C. Totally, 21 subjects reported SAEs and all but one were considered irrelevant or probably irrelevant to vaccination. In conclusion, the tested PCV13 showed non-inferior immunogenicity and had a good safety profile compared with control vaccine.
Introduction
Streptococcus pneumococcal is the major pathogenic bacteria of human pneumoniae, meningitis, bacteremia and otitis media. Invasive pneumococcal disease (IPD) is related with significant morbidity and mortality.Citation1–3 Streptococcus pneumoniae is genetically diverse, with more than 90 distinct capsular polysaccharides which have already been identified.Citation4,Citation5 Before pneumococcal conjugate vaccines (PCVs) were prevalently introduced in 2000, 700,000 deaths and 14 million cases of diseases in children under 5 years old were caused by Streptococcus pneumoniae every year.Citation6 A variety of related pneumococcal vaccines have been developed to prevent pneumococcal infection and proved to be probably cost effective in some regions.Citation7 It is of great importance to improve vaccine coverage to eliminate the burden of pneumococcal infection. Currently, several pneumococcal conjugate vaccines (such as PCV7,10,13,15) are used to prevent infants against pneumococcal infection. After introduction of PCV-13, there was significant decrease of IPD due to vaccine-related serotype.Citation8,Citation9
In China, Beijing Minhai Biotechnology Co., LTD. had developed a new PCV13 and obtained official approval for clinical trials in June, 2014. An open-label phase 1 trial had been performed among 80 adults and children aged from 2 months to 55 years, in which the safety and tolerability of the new PCV13 had been preliminarily proved. In this study, we evaluated its immunogenicity and safety administered among healthy infants aged 2 months compared with a control PCV13. The results were described hereinafter.
Materials and methods
Ethics statement
Ethic approval (Approval No.: JSJK2015-A002-02) had been granted by the Institutional Review Board of Jiangsu Provincial Center for Disease Control and Prevention. Written informed consent had been obtained before each participant’s entry into the study. The research was performed in compliance with the principles of the Declaration of Helsinki, and followed the standards of Good Clinical Practice and Chinese regulatory requirements stipulated by the National Medical Products Administration (NMPA).
Study design and participants
This was a randomized, double blind, positive-controlled phase III clinical trial of a new PCV13 administered in a prime-boost regimen (Clinical Trials.gov identifier: NCT02494999). It was conducted in three study sites-Lianshui County, Huaiyin District and Hongze District, Jiangsu Province, China, between June 2016 and December 2018. Eligible participants were healthy infants, aged 42–77 days. Those who (1) were preterm infants or low birth weight infants, (2) had any administration history of pneumococcal polysaccharide or conjugate vaccine, (3) had a medical history of culture-confirmed invasive disease caused by Streptococcus pneumonia, (4) had allergic history or serious adverse reaction history after vaccination, (5) with congenital malformation, developmental disorder, genetic defects or severe malnutrition, (6) with epilepsy, seizure or mental disease, (7) were known or suspected immune deficiency or immune suppression, (8) were diagnosed coagulation abnormalities or significant bruising or blood clotting disorder, (9) had immunosuppressive therapy, cytotoxic therapy, inhaled corticosteroids within 6 months prior to the study entry were excluded from the study.
A total of 1200 infants were randomly assigned at 1:1 ratio into two study groups- Group T received tested PCV13 and Group C received positive control PCV13 separately. Eligible participants were vaccinated three doses intramuscularly at Months 0, 2, and 4 and a booster dose at Month 10. Baseline demographics was collected during enrollment interview.
Vaccines
The tested PCV13 (batch number 20170301) was produced by Beijing Minhai Biotechnology Co., LTD and the positive control PCV13 (Prevnar 13, batch number TS15224N73146) was manufactured by Pfizer Pharmaceuticals. Both of them contained 0.125 mg of aluminum as aluminum phosphate adjuvant and were supplied in prefilled 0.5 mL syringes. In tested PCV13, each 0.5 mL dose contained 1.8 μg pneumococcal polysaccharide serotype 1, 2.1 μg pneumococcal polysaccharide serotypes 3 and 4, 1.75 μg pneumococcal polysaccharide serotypes 5 and 7 F, 1.85 μg pneumococcal polysaccharide serotype 6A, 4.4 μg pneumococcal polysaccharide serotype 6B, 2.3 μg pneumococcal polysaccharide serotype 9 V, 1.35 μg pneumococcal polysaccharide serotype 14, 3.65 μg pneumococcal polysaccharide serotype 18 C, 1.6 μg pneumococcal polysaccharide serotype 19A, 1.25 μg pneumococcal polysaccharide serotype 19 F and 2.35 μg pneumococcal polysaccharide serotype 23 F; and serotypes 1, 5, 6A, 9 V, 19A, 19 F, and 23 F capsular polysaccharide were conjugated to a tetanus toxoid carrier protein while serotypes 3, 4, 6B, 7 F, 14 and 18 C were conjugated to a diphtheria toxoid carrier protein. Unlike tested PCV13, control PCV13 contained 2.2 μg pneumococcal polysaccharide serotypes 1, 3, 4, 5, 6A,7 F, 9 V, 14, 18 C, 19A, 19 F, and 23 F and 4.4 μg pneumococcal polysaccharide serotype 6B per 0.5 mL dose; and each of the polysaccharides was covalently conjugated to CRM 197, a nontoxic variant of diphtheria toxin.
Immunogenicity assessment
Serum samples were collected at four different time points: immediately before the first dose, 30 days after the third dose, immediately before the booster dose, and 30 days after the booster vaccination. For all participants serum concentrations of anticapsular polysaccharide immunoglobulin G (IgG) for each of the 13 pneumococcal serotypes were measured at the previously mentioned time points, using the standardized enzyme-linked immunosorbent assay (ELISA) method.Citation10 In addition, the opsonophagocytic assay (OPA)Citation11 for each serotype were performed to test functional antibodies within a subset comprising of approximate 100 participants in each group (around 200 infants in total).
Safety assessment
Each participant had been observed for 30 minutes after each dose of inoculation. Any immediate and noted adverse events (AEs) were recorded by investigators on safety diary cards at site. Parents or legal guardians of each participant were trained how to observe and document local reactions (redness, pain, induration, rash, swelling, and pruritus), systemic reactions (decreased appetite, fever, diarrhea, cough, crying, vomiting and fatigue) and axillary temperatures for 7 days after leaving the site. Additionally, other AEs within 30 days after each dose together with concomitant medications to treat or to prevent symptoms were also collected. All AEs were assessed by trained investigators and classified according to the guidelines predefined in the study protocol. Serious adverse events (SAEs) had been collected throughout the research period until one month after booster immunization.
Statistical analysis
Full analysis set (FAS) complied with the principles of intent-to-treat (ITT) and comprised all participants who received at least one dose of vaccine and one follow-up visit. Per-protocol analysis set (PPS) comprised ITT participants who completed all study visits and had available serological data. Immunogenicity analysis consisted of all participants who belonged to PPS. Safety analysis set (SS) included participants who received at least one dose of study vaccines and whose safety data were recorded.
The primary immunogenicity endpoints were the proportion of participants reaching the serotype-specific IgG concentration threshold of 0.35 μg/mL (seropositive rate) and the geometric mean IgG concentration (GMC) measured 30 days after the primary series. To evaluate differences between two groups, the sequential testing of the non-inferiority of tested PCV13 for each pneumococcal serotype had been performed. If the lower limit of the two-sided 97.5% confidence interval (CI) calculated using the exact binomial method for the difference in proportion (Group T-Group C), was >-10%, or the lower bound of the two-sided 97.5% CI of the IgG GMC ratio (Group T/Group C), was >0.5, the non-inferiority would be declared. Only after all of 13 serotypes had been confirmed to be non-inferior would the tested PCV13 be assumed noninferior to the control PCV13. On the premise of noninferiority, if the lower limit of the two-sided 97.5% CI was >0%, or the lower bound of the two-sided 97.5% CI of the IgG GMC ratio >1, the superiority for each serotype would be declared.
The secondary immunogenicity endpoints were the proportion of participants reaching the serotype-specific IgG concentration threshold of 1.0 μg/mL 30 days after the primary and booster immunization, the IgG GMC measured 30 days after the booster dose and the geometric mean IgG antibody increase (GMI) 30 days after the primary and booster immunization. In addition, the proportions of participants reaching the OPA titer threshold of 1:8 and the geometric mean OPA titer (GMT) after primary and booster immunization in predefined subgroups were also served as secondary immunogenicity end points.
All statistical analyses were performed by an independent statistician using SAS 9.3 software. Chi-square test and two-sided Fisher’s exact test were used to compare the seropositive rates, the incidences of local and systemic ARs for each dose, respectively, and for all in total as well. The GMC and GMT of each serotype between two groups were compared using paired Student’s t-test. A p value equal to or < 0.05 indicated a statistically significant difference.
Results
Participant characteristics
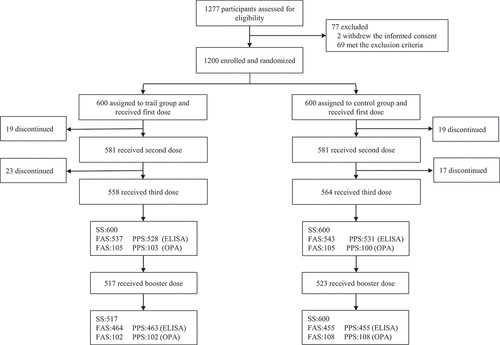
Of 1277 infants assessed for eligibility in the trial, 1200 healthy infants were enrolled and randomly assigned into one of two study groups- Group T and Group C. In each 600 participants received the first dose of tested PCV13 or control PCV13 respectively. In sum, 1122 (93.5%) children completed primary vaccination: 558 (93.0%) children in Group T and 564 (94.0%) children in Group C; 1040 (86.7%) children completed booster vaccination: 517 (86.2%) children in Group T and 523 (87.2%) children in Group C (). Rates of drop out in the following visits were similar between the two groups. The baseline demographics including age, sex, body length, weight, and axillary temperature were equally comparable ().
Table 1. Baseline demographics of study population
At baseline, seropositive rates of Group T and Group C, ranging from 6.59% to 96.42%, were similar for twelve serotypes (all except serotype 19 F-Group T was significantly lower than Group C). IgG GMCs between two groups had no significant difference for eleven serotypes (all except serotype 1 and serotype 5-Group T was significantly lower than Group C, 0.14 VS. 0.17 and 0.09 VS. 0.12) (). The percentages of participants with IgG GMC≥1.0 μg/mL were similar for all 13 serotypes (Supplementary Table 1). The percentages of participants with OPA≥1:8 (range 3.88%~69.90%) between two groups were similar for twelve serotypes (all except serotype 19A-Group T was significantly higher than Group C, 40.78% VS. 26.00%) (). OPA GMTs between two groups had no significant difference for eleven serotypes (all except serotype 14 and serotype 19A-Group T was significantly higher than Group C, 27.71 VS. 15.23, 6.01 VS. 4.06) (Supplementary Table 2). In general levels of serotype-specific antibodies were comparable between two study groups.
Table 2. Type-specific seropositive rates, GMC and GMI pre- and postprimary vaccination
Table 3. Type-specific seropositive rates, GMC and GMI pre- and postbooster vaccination
Anti-pneumococcal IgG response
One month post primary vaccination (Month 5), seropositive rates of Group T and Group C were at least 93.41% for all serotypes in two groups, which had no significant difference for twelve serotypes (all except serotype 3-Group T was higher than Group C, 100.00% vs. 93.41%). For twelve serotypes IgG GMCs of Group T were higher than those of Group C and the differences were statistically significant (all P values <.05); while for serotype 7 F IgG GMC of Group T was significantly lower than Group C (5.60 vs. 6.54). For eleven serotypes IgG GMIs of Group T were higher than those of Group C (all P values <.05) while there were no significant difference for serotype 7 F and serotype 14 (). Percentages of participants in Group T with IgG GMC ≥1.0 μg/mL for five serotypes-serotype 3, 4, 5, 9 V, and 23 F were significantly higher than those in Group C (all P values <.05) (Supplementary Table 1).
Prebooster vaccination (Month 10), for two serotypes-3 and 4 both seropositive rates in Group T were significantly higher than Group C, for serotype 7 F seropositive rate in Group T was significantly lower than Group C (98.06% vs. 100.00%). For eight serotypes-3, 4, 5, 6B, 9 V,19A, 19 F, and 23 F, IgG GMCs of Group T were higher than Group C and for two serotypes-7 F and 14 IgG GMCs in Group T were significantly lower than Group C (). For eight serotypes-4, 5, 6A, 6B, 9 V, 19A, 19 F, and 23 F percentages of participants with IgG GMC ≥1.0 μg/mL in Group T were significantly higher than Group C, for two serotypes-7 F and 18 C percentages of participants with IgG GMC ≥1.0 μg/mL in Group T were significantly lower than Group C (all P values <.05) (Supplementary Table 1).
Table 4. Percentage of participants with OPA≥1:8 pre- and postprimary and booster vaccination
Post booster vaccination (Month 11), seropositive rates for all serotypes of Group T were 100.00% while seropositive rates for three serotypes-3, 6A, 18 C of Group C were 99.56%, 99.78%, and 99.78% separately. All differences of seropositive rates between two groups were not statistically significant. For 12 serotypes IgG GMCs of Group T were significantly higher than those of Group C (all P values <.05); only for serotype 23 F there was no significant difference when IgG GMCs of two groups were compared. For twelve serotypes IgG GMIs of Group T were higher than those of Group C (all P values <.05); only for serotype 23 F IgG GMI of Group T was lower than that of Group C (). Percentages of participants in Group T with IgG GMC ≥1.0 μg/mL for four serotypes- serotype 3, 4, 5, and 18 C were significantly higher than those in Group C (all P values <.05) (Supplementary Table 1).
OPA responses
Post primary vaccination (Month 5), there were no significant differences when comparing percentages of participants with OPA≥1:8 (range 96.00%~100.00%) between two groups (). For four serotypes-1, 3, 4, and 5, OPA GMTs of Group T were higher than those of Group C and for five serotypes-6A, 6B, 7 F, 19A, and 23 F OPA GMTs of Group T were lower than those of Group C (all P values <.05). For four serotypes-1, 3, 4, and 5, OPA GMIs of Group T were higher than those of Group C and for five serotypes-6A, 7 F, 14, 18 C, and 19A OPA GMIs of Group T were lower than those of Group C (all P values <.05) (Supplementary Table 2).
There was no significant difference between two groups when percentages of participants with OPA≥1:8 before (range 90.20%~100.00%) and 30 days after (range 96.30%~100.00%) booster vaccination for all serotypes were compared (). Before booster vaccination (Month 10), for four serotypes-3, 4, 5, and 19 F OPA GMTs of Group T were higher than Group C and three serotypes-6A, 7 F and 23 F OPA GMTs of Group T were lower than Group C (all P values equal to or <0.0193). Post booster vaccination (Month 11), for four serotypes-3, 5, 9 V, and 14 OPA GMTs of Group T were higher than Group C and for another four serotypes-6A, 6B, 18 C, 23 F OPA GMTs of Group T were lower than those of Group C(all P values equal to or <0.0181). For two serotypes-7 and 14 OPA GMIs of Group T were higher than Group C and for three serotypes-5, 6A, and 19 F OPA GMIs of Group T were lower than those of Group C (all P values <.05) (Supplementary Table 3).
Safety analysis
Overall, after primary immunization solicited adverse reactions (ARs) were reported in 72.00% of participants in Group T and 79.83% in Group C- the difference between two groups was statistically significant (P = .0019). In Group T 17 subjects had Grade 3 injection-site ARs (redness, induration and swelling) and 4 subjects had Grade 3 systemic ARs (fever and diarrhea) while in Group C 16 subjects had Grade 3 injection-site ARs (redness, induration and swelling) and 6 subjects had Grade 3 systemic ARs (fever, diarrhea and crying). Fewer subjects in Group T reported common ARs (redness, swelling and fever) than those in Group C (all P values <.05). All adverse events (AEs) were mild and mainly classified in Grade 1 and Grade 2. No Grade 4 AE was reported. Five subjects in Group T reported SAEs and thirteen in Group C. None of the SAEs were considered related to study vaccines ().
Table 5. Overall profiles of adverse events after primary and booster vaccination
As for safety after each inoculation, the frequencies of overall AEs after the first dose were 52.50% in Group T and 58.50% in Group C respectively-the difference was statistically significant (P = .0420). After the second dose solicited ARs (43.55% vs. 49.91%, P = .0342) reported in Group T were significantly fewer than Group C while unsolicited ARs (6.88% vs. 3.79%, P = .0258) and pain (2.58% vs. 0.86%, P = .0397) occurred more frequently in Group T than in Group C. Participants who reported swelling after each dose of primary immunization in Group T were fewer than Group C (4.83% vs. 11.00% P = .0001, 5.85% vs. 9.12% 0.0443, 3.58% vs. 9.40% 0.0001, respectively) (Supplementary Table 4).
After booster immunization there was a significantly difference of the frequency of solicited ARs between Group T and Group C (38.49% vs. 47.61%, P = .0032). In Group T 11 subjects had Grade 3 solicited ARs while in Group C 21 subjects had Grade 3 solicited ARs – the difference was not statistically significant. All AEs were mainly Grade 1 and Grade 2 and no Grade 4 AE occurred. One subject in Group T reported SAE and two in Group C. The one case of SAE in Group T, diagnosed as febrile convulsion and acute upper respiratory tract infection, was considered relevant to study vaccines ().
Discussion
The results reported establish the immunologic noninferiority and comparable safety of tested PCV13 relative to positive control PCV13 given as a 3-dose series at ages 2, 4, and 6 months respectively, with an additional dose administered at 12–15 months. One month post primary vaccination (Month 5), for serotype 3 seropositive rate in Group T was significantly higher than Group C (100.00% vs. 93.41, P < .0001) and for the other 12 serotypes seropositive rates in two groups were similar. For 12 serotypes IgG GMCs of Group T were significantly higher than Group C(all P values equal to or <0.0038); for serotype 7 F IgG GMC of Group T was lower than Group C (P = .0009). Hence, for all serotypes seropositive rates of Group T were noninferior to those of Group C. Meanwhile, for all serotypes IgG GMC of Group T were noninferior to those of Group C. Based on the noninferiority, for serotype 3 seropositive rate of Group T was declared superior to Group C. For all serotypes except 7 F IgG GMCs of Group T were also tested to be superior to Group C. These probably had relation with different carrier proteins contained in the two study vaccines but the exact mechanism underlying for the difference of the immunogenicity is still unclear to our knowledge.
After combining the immunogenicity data at four different time points – immediately before the first dose, 30 days after the third dose, immediately before the booster dose, and 30 days after the booster vaccination, we found an overall increase-decrease-reincrease trend of the anti-pneumococcal IgG response in both groups. This indicated that levels of type-specific serological antibodies waned after primary immunization as time went by and booster immunization elicited prospective immunity enhancement. As for control PCV13, among 13 serotypes seropositive rates for serotype 3 were lowest and IgG GMCs for serotype 14 were highest both after primary series and after booster vaccination. There were higher immunogenic levels after booster vaccination than after primary series. These data were in line with findings of previous studies among healthy infants in prime-boost regimens.Citation12
On the other hand, the frequencies of overall AEs and solicited ARs post primary immunization in Group T were significantly lower than Group C. The most common injection-site ARs were redness, induration and swelling; the frequencies of redness and swelling reported in Group T were both significantly lower than those in Group C. The most common systemic AR was fever and significantly fewer participants reported fever in Group T than Group C. None unsolicited adverse events were related with vaccination assessed by clinical investigator. Post booster immunization the frequencies of overall AEs, solicited ARs and systemic ARs in Group T were significantly lower than Group C. As for AE symptoms, the frequencies of fever, crying and fatigue in Group T were also significantly lower than Group C. Consequently the tested PCV showed better safety profile post booster immunization, which might be attributed to its less total content of pneumococcal polysaccharide in each 0.5 mL dose (28.25 μg vs. 30.8 μg). Similarly with results of other trials among Chinese infants, AEs were mainly mild to moderate and were reported more frequently after the first dose than any other dose.Citation13,Citation14 Totally 21 subjects reported SAEs during the whole research period. All SAE cases except one were assessed irrelevant or probably irrelevant to study vaccines.
This study had two notable advantages. Firstly, it was a rigorous comparative study of PCV13 because it had chosen Prevnar 13 as positive control. The 2, 4, 6 + 12-month dosing schedule was the same as the administration schedule approved in China which allowed for direct comparison between two study vaccines. Secondly, for each serotype not only IgG binding but also functional antibody responses were examined in this study, which provided additional data of immunologic support among Chinese infants.
To put our study results in perspective, some limitations have to be addressed. First, the persistent immunogenicity of tested PCV 13 remains unknown. A 5-year follow-up study is in process among a subset of our study population. Second, in order to investigate the long-term safety surveillance systems are also needed to monitor rare adverse events or diseases after immunization. Additionally, all 1200 subjects in our study were healthy infants from Jiangsu Province, China. In the future clinical trials may be performed among larger populations with different demographic characteristics such as races or nutrient status of children.
In conclusion, in this study the tested PCV13 showed noninferior immunogenicity and had a good safety profile in prime-booster regimen compared with control vaccine. It could be a promising vaccine to prevent infants against pneumococcal disease attributable to pneumococcal serotypes contained in the vaccine.
Abbreviations
Author contributions
Y.H. and G.L. designed the trial and the study protocol, Q.L. and G.L. contributed to the critical review and revision of the report. W.W., Q.L., J.Z., J.Z., J.C., S.X., H.M. led and participated in the site work, including the recruitment, follow-up. W.W. and Q.L. contributed to the data collection, data management, and statistical analysis and wrote the paper.
Supplemental Material
Download MS Word (42.3 KB)Disclosure statement
Guifan Li is an employee of Beijing Minhai Biotechnology Co., LTD. All other authors have no conflicts.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2019498.
Additional information
Funding
References
- GBD 2015 Mortality and Causes Of Death CollaboratorsWang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM et al . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;385(9963):117–9. doi:10.1016/S0140-6736(14)61682-2.
- Askim S, Mehl A, Paulsen J, DeWan AT, Vestrheim DF, Åsvold BO, Damås JK, Solligård E. Epidemiology and outcome of sepsis in adult patients with Streptococcus pneumoniae infection in a Norwegian county 1993–2011: an observational study. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-1553-8.
- Stockmann C, Ampofo K, Byington CL, Filloux F, Hersh AL, Blaschke AJ, Cowan P, Korgenski K, Mason EO, Pavia AT, et al. Pneumococcal meningitis in children: epidemiology, serotypes, and outcomes from 1997–2010 in Utah. Pediatrics. 2013;132(3):421–28. doi:10.1542/peds.2013-0621.
- Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28(3):871–99. doi:10.1128/CMR.00024-15.
- Eletu SD, Sheppard CL, Thomas E, Smith K, Daniel P, Litt DJ, Lim WS, Fry NK. Development of an extended-specificity multiplex immunoassay for detection of streptococcus pneumoniae serotype-specific antigen in urine by use of human monoclonal antibodies. Clin Vaccine Immunol. 2017;24:e00262–17. doi:10.1128/CVI.00262-17.
- World Health Organization (WHO). Estimated Hib and pneumococcal deaths for children under 5 years of age, 2000, Mar 2014.
- Chen C, Cervero Liceras F, Flasche S, Sidharta S, Yoong J, Sundaram N, Jit M. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. 2019;7(1):e58–67. doi:10.1016/S2214-109X(18)30422-4.
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;3099:1–9. doi:10.1016/S1473-3099(15)70044-7.
- Fu J, Yi R, Jiang Y, Xu S, Qin P, Liang Z, Chen J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae causing invasive diseases in China: a meta-analysis. BMC Pediatr. 2019;19(1):424. doi:10.1186/s12887-019-1722-1.
- Wernette CM, Frasch CE, Madore D, Carlone G, Nahm MH. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–19. doi:10.1128/CDLI.10.4.514-519.2003.
- Hu BT, Yu X, Jones TR, Kirch C, Harris S, Hildreth SW, Madore DV, Quataert SA. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2005;12(2):287–95. doi:10.1128/CDLI.12.2.287-295.2005.
- Ruiz-Aragón J, Márquez Peláez S, Molina-Linde JM, Grande-Tejada AM. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in infants: a meta-analysis. Vaccine. 2013;31(46):5349–58. doi:10.1016/j.vaccine.2013.09.008.
- Zhu F, Hu Y, Li J, Ye Q, Young MM, Liang JZ, Gruber WC, Giardina PC, Scott DA. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine administered in a 3 + 1 versus 2 + 1 dose schedule among infants in China. Pediatr Infect Dis J. 2019;38(11):1150–58. doi:10.1097/INF.0000000000002458.
- Zhu F, Hu Y, Liang Q, Young M, Zhou X, Chen Z, Liang JZ, Gruber WC, Scott DA. Safety and tolerability of 13-valent pneumococcal conjugate vaccine in healthy Chinese adults, children and infants. Ther Adv Drug Saf. 2015;6(6):206–11. doi:10.1177/2042098615613985.