ABSTRACT
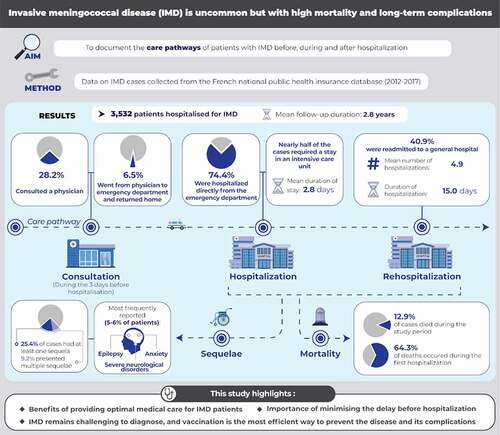
Invasive meningococcal disease (IMD) carries a high burden in terms of mortality, long-term complications, and cost, which can be significantly reduced by vaccination. The objectives of this case–control study were to document the care pathways of patients with IMD before, during, and after hospitalization and to assess in-hospital complications and long-term sequelae. Cases consisted of all people hospitalized for IMD in France between 2012 and 2017. Controls were matched by age, gender, and district of residence. Data were extracted from the French national public health insurance database on demographics, hospitalizations, mortality and potential sequelae of IMD. Overall, 3,532 cases and 10,590 controls were assessed and followed up for 2.8 years (median). During hospitalization, 1,577 cases (44.6%) stayed in an intensive care unit, 1,238 (35.1%) required mechanical ventilation, and 43 (1.2%) underwent amputation; 293 cases (8.3%) died in hospital and a further 163 (4.6%) died following discharge; 823 cases (25.4% of survivors) presented ≥1 sequela and 298 (9.2%) presented multiple sequelae. The most frequently documented sequelae were epilepsy (N = 205; 5.8%), anxiety (N = 196; 5.5%), and severe neurological disorders (N = 193; 5.5%). All individual sequelae were significantly more frequent (p < .0001) in cases than controls. Hearing/visual impairment and communication problems were conditions that presented the highest risk for cases compared to controls (risk ratios >20 in all cases). In conclusion, this study highlights the importance of providing optimal medical care for patients with IMD, of minimizing the delay before hospitalization, and of effective prevention through comprehensive vaccination programs.
This study highlights
Benefits of providing optimal medical care for IMD patients.
Importance of minimising the delay before hospitalization.
IMD remains challenging to diagnose, and vaccination is the most efficient way to prevent the disease and its complications.
Introduction
Colonization of the nasopharynx by Neisseria meningitidis affects around 10% of the general population in Europe. In rare cases, the bacterium can cause invasive meningococcal disease (IMD), particularly in infants and young children ≤2 years old and in adolescents/young adults.Citation1–3 In Europe, the incidence of IMD has fallen over the last two decades following the introduction of comprehensive vaccination programs in many countriesCitation4 and is currently around 1/100,000 per annum.Citation5 In France, around 600 new cases of all ages are reported each year.Citation6,Citation7 The principal age groups affected are young children ≤5 years of age (around 30% of all cases, of which two-thirds were due to serogroup B), adolescents and young adults aged 16–24 years (around 18% of cases; 40% due to serogroup B and 20% to serogroup C) and older adults aged ≥65 years (around 16% of cases, with serogroup Y accounting for a third of these cases).Citation8
Although IMD is uncommon, it carries a substantial burden, which could be reduced by more widespread vaccination, particularly in individuals who are at particular risk. In Europe, the case fatality rate during hospitalization is estimated to be around 6–8%,Citation9 with additional deaths occurring in the years following the initial hospitalization due to long-term complications.Citation10 The most common clinical manifestations of IMD are meningitis and sepsis, the latter frequently presenting as purpura fulminans, sometimes requiring limb amputation.Citation2,Citation10 Following IMD episodes, serious sequelae may remain or develop that require lifetime management,Citation10,Citation11 of which the most frequent are hearing impairment, cognitive impairment, and psychological problems.Citation10 In addition, irreversible skin scarrng may occur following purpura fulminans.Citation2,Citation12
Given the potentially high long-term burden of IMD, timely hospitalization and appropriate care are essential. Although practice guidelines on the management of IMD are available in many countries, including France,Citation13 data on how patients with IMD are actually managed in everyday practice are limited.Citation14 In particular, it would be useful to document how many patients are hospitalized in a timely manner, how many need to be managed in an intensive care facility, and how many need long-term care for sequelae. A better understanding of patient management may help identify aspects of care, which could be optimized with the aim of limiting the burden of disease. In particular, this information could be of use for public health institutions to justify the interest of routine meningococcal vaccination programs.
Large health insurance databases represent a useful source for collecting data on the clinical management of rare diseases such as IMD. A number of such studies have been performed previously in a number of countries, including Belgium, Germany, France, Spain, and the United States.Citation15–23 The national public health insurance database in France (Système National des données de santé; SNDS) is of particular interest in this respect as it covers >99% of the country’s population (>66 million individuals) and thus allows exhaustive inclusion of all IMD cases in the country. We have previously extracted data on all patients hospitalized for IMD in France over a six-year period (2012–2017), and reported on their characteristics and risk profiles, and the associated economic burden.Citation7,Citation23 In the present study, we have documented the care pathways of these patients before, during, and after hospitalization for the initial episode, including an assessment of the burden of in-hospital stays and complications requiring acute management during the index stay, as well as long-term sequelae of IMD.
Materials and methods
This observational case–control study was conducted using the SNDS national health insurance database in France. The design and methodology of the study have been described in detail previously.Citation7 All individuals hospitalized between January 1, 2012 and December 31, 2017 with a diagnosis of acute IMD were defined as cases. The case definition was based solely on the presence of an ICD-10 code for IMD (A39.0 to A39.9; meningococcal infection) in the hospital discharge summary. This code explicitly requires the pathogen to have been formally isolated and identified. However, the results of the laboratory tests themselves (such as the serogroup) are not documented in the SNDS database. The index date for a case was defined as the date of first hospital admission. Three control subjects without IMD, randomly selected in the SNDS database, were matched to each case with respect to age, gender, and administrative district of residence. Controls were not required to have been hospitalized, although this may have been the case by chance. The index date for a case was attributed to their matched controls. Cases and controls were followed until December 31, 2017 (or death). Different steps of the care pathway were documented, including physician consultation prior to hospitalization, the hospital stay, complications requiring acute management during the index stay, mortality, hospital readmissions, long-term sequelae, and recurrence.
Data collection
Data were extracted from the SNDS database on demographics, hospitalizations, physician consultations, mortality, and potential sequelae of IMD (as well as the same medical conditions in controls). Demographic information was limited to age, gender, and municipality of residence.
Hospitalizations
Information in the SNDS database was available for stays in general hospitals, rehabilitation centers, long-term facilities and psychiatric units. In this context, general hospitals correspond to hospitals dedicated to short-term stays in medical, surgical, or obstetric wards. In addition, home care coordinated from the hospital was also documented.
The route of admission to a general hospital (referral by a general practitioner, visit to an emergency department, or transfer from another care facility) was documented. However, the specific reason for any consultation to a general practitioner or to an emergency department prior to hospitalization was not documented in the SNDS database. Any physician consultations in the three days prior to admission were identified. The period of three days was chosen, since this is the period over which symptoms of IMD can be expected to develop in the majority of patients.Citation24
For the index hospitalization for IMD, the total length of stay was determined. If the patient had been hospitalized consecutively in different structures (e.g. an intensive care unit, a medical ward and a rehabilitation center), these stays were aggregated to determine the total stay duration. Certain procedures were considered as markers of the severity of the IMD episode (intensive care/reanimation, mechanical ventilation, catecholamine administration, dialysis, or amputation) and these were analyzed individually.
If the patient was discharged to another care structure (e.g. a rehabilitation center), this was also documented, together with the duration of stay in the relay care structure.
All hospitalizations occurring during the follow-up period were identified. The reason for hospitalization was identified from the hospital discharge summary. For cases, subsequent hospitalizations with an ICD-10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) diagnostic code for a meningococcal infection (A39.0 to A39.9) mentioned as the primary diagnosis on the hospital discharge summary, and occurring at least 30 days after the index hospitalization were considered to represent recurrence. A threshold of 30 days was applied in order to ensure that the hospitalization was probably related to a recurrent infection, rather than rehospitalization from the original episode, as previously described.Citation25 In addition, hospitalizations for non-meningococcal bacterial meningitis were identified.
Mortality
Any deaths occurring during the follow-up period were documented for all cases and controls. The date, but not the cause of death, was available.
Sequelae
Certain medical conditions considered as potential sequelae of IMD were identified in the SNDS database for both cases and controls. Sequelae considered included immediate and irreversible events, such as amputation, which were identified from hospital discharge records and sequelae appearing and identified after discharge. The type of sequelae considered here followed the findings of a previous case–control study performed in the United Kingdom (UK) National Meningococcal RegistryCitation26 and those of a Canadian meningococcal surveillance program,Citation27 completed by other sequelae reported in a recent systematic review of the subject.Citation11 Sequelae were identified in the SNDS database from hospital discharge summaries by the corresponding ICD-10-CM diagnostic code or CCAM (Classification Commune des Actes Médicaux) – a procedure code from the common classification for medical procedures used in France since 2005 – or from records of medication delivery in a community pharmacy. The disease classification system developed in 2015 by the French national general health insurance fund was used to assign these medical conditions.Citation28 From the items extracted, sequelae potentially related to IMD were identified specifically using a previously described algorithm.Citation29 A list of codes used to identify individual sequelae is provided in Supplementary Table S1.
The conventions used in order to distinguish sequelae from comorbidities rely on the timing of the first occurrence with respect to the index hospitalization.Citation23 To qualify as sequelae, post-discharge medical conditions had to be documented in the SNDS database for the first time at a date after the index date. The SNDS database was searched during the year preceding the index IMD event to ensure that they were not already present before the event. This first date at which they were documented was in general required to fall within the three months following the index hospitalization, except for motor deficits (6 months), epilepsy and mental retardation (18 months), or bilateral hearing loss, severe hearing loss requiring a cochlear implant, and attention deficit hyperactivity disorder (36 months), for which a broader time window was permitted. These conditions were also identified in the control group if they were documented for the first time following the matched index date.
Statistical analysis
Data are presented by three age classes (<25 years, 25–59 years and ≥60 years). The <25 year age class was selected as it corresponds to the age group for whom MenC vaccination is available in France and the ≥60 year age class as this is a population at risk for IMD, with a high case fatality rate, and in whom the incidence of IMD is rising in France.Citation7 In addition, for certain variables, the <25 age group was broken down into five narrower age groups (<1 year, 1–4 years, 5–14 years, 15–19 years and 20–24 years).
Continuous variables are presented as mean values ± standard deviations (SD) or median values with their interquartile range (IQR), and compared between cases and controls using the Wilcoxon test. Categorical variables are presented as frequency counts and percentages, and compared between cases and controls using the chi-squared (χ2) test or Fisher’s exact test as appropriate. The relative risks of rehospitalization, recurrence, or developing sequelae in cases and controls were expressed as risk ratios (RRs) with their 95% confidence intervals (CIs). Mortality rates in cases and controls were compared using Kaplan–Meier actuarial survival analysis and compared using the logrank test. The findings are presented graphically as Forest plots. All statistical analyses were performed using SAS software, Version 9.5 (SAS Institute, Inc., Cary, NC, USA).
Ethics
The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments, as well as with relevant international and French regulatory requirements. Patient data on the SNDS database was anonymized using irreversible double encryption. Access to the SNDS is regulated by a Committee of Expertise for Research, Studies and Evaluations in the field of Health (CESREES), to which the present study protocol was submitted for approval. Since this study was retrospective on an anonymized database and had no influence on patient care, ethics committee approval was not required. The use of the SNDS database for this type of study is regulated by the French national data protection agency (Commission Nationale de l’Informatique et des Libertés), which approved the protocol.
Results
Study population
Overall, 3,532 individuals were hospitalized for IMD between 2012 and 2017 and constituted the cases. The mean age of the cases was 29.7 ± 27.6 years (median: 21 years [IQR: 4–52]). The breakdown of the study population is presented by age class in . Around half of the cases were male (N = 1,849; 52.3%). Overall, 3,530 cases were matched in a 1 to 3 ratio to 10,590 controls. The remaining two cases could not be matched but were retained in the analysis. Cases were followed up for a mean duration of 2.8 ± 1.9 years (median: 2.8 years [IQR: 0–6.0 years]) and controls for 3.0 ± 1.9 years (median: 3.0 years [IQR: 0–6.0 years]).
Table 1. Age distribution of the cases
Index hospitalization
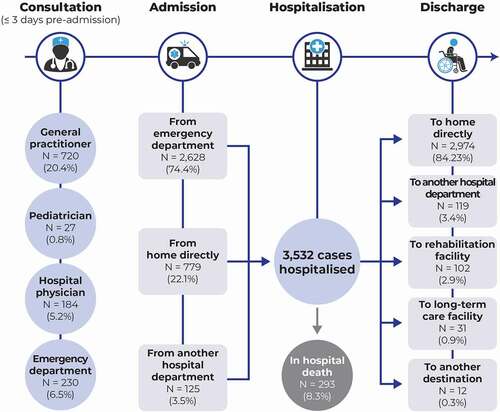
Over the three days prior to hospital admission, 995 cases (28.2%) had consulted at least one community- or hospital- based physician, principally a general practitioner (). Of these, 230 cases (6.5%) made an emergency department visit in the three days prior to hospitalization and then returned home before being hospitalized definitively. Overall, 2,628 cases (74.4%) were hospitalized directly from the emergency department. Of the remaining 904 patients who did not transit by the emergency department, 779 (21.1%) were hospitalized directly from home.
Figure 1. Patient trajectory for management of invasive meningococcal disease. For one patient, discharge destination was unknown.
The mean duration of the index hospitalization for the cases was 14.8 days (median: 8 days; IQR: 6–14 days). However, the stay duration was highly dependent on age, with cases aged ≥60 years being hospitalized for a mean duration of 25 days (). On the other hand, the stay duration was similar for infants, older children and young adults (). During the index hospitalization, nearly half the cases, regardless of age, required a stay in an intensive care unit or a reanimation unit. The median length of stay in a reanimation unit was 2.8 ± 7.6 days. Mechanical ventilation was required in 1,238 patients (35.1%), principally in adults, and around one-fifth required catecholamine administration. In addition, 43 cases (1.2%) had limbs amputated during the index hospitalization.
Table 2. Characteristics of index hospitalization stay, by age group
Table 3. Hospital stay duration and in-hospital mortality in children, adolescents, and young adults during the six-year study period
When discharged, the majority of cases (84.2%) went home directly (). In addition, 102 (2.9%) were discharged to a rehabilitation facility, where they stayed for a mean duration of 15.6 days.
Mortality
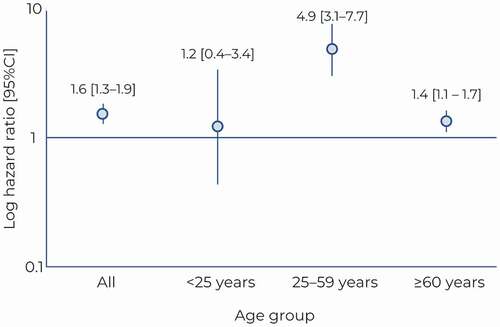
Over the follow-up period, 456 (12.9%) cases died. Almost two-thirds of these deaths (293 deaths; 8.3% of cases and 64.3% of all deaths) occurred during the index hospitalization. In-hospital mortality was no higher in infants than in older children and young adults (). A further 163 (4.6%) died following discharge from the index hospitalization. For the 10,590 controls, 344 patients (3.2%) died over the follow-up period. The mortality rate was compared between cases who survived the index hospitalization and their controls, and found to be significantly higher in cases compared to controls, with a hazard ratio (HR) of 1.6 [95% CI: 1.3–1.9; p < .0001). The mortality risk was highest for the 25–59-year age group, with a nearly five-fold elevation risk compared to controls (hazard ratio: 4.9 [95% CI: 3.1–7.7]). In contrast, no significant elevation in mortality risk was observed in the age group <25 years. The variation of this hazard ratio with age is presented in .
Hospitalizations during the follow-up period
During the follow-up period, 1,448 cases (41%) were readmitted to a general hospital at least once, whatever the reason (). Although the proportion of patients hospitalized during the follow-up period did not differ significantly between cases and controls, cases were hospitalized more frequently (mean number of hospitalizations: 4.9 ± 14.1 in cases and 2.8 ± 6.4 in controls; p < .0001) and for longer durations (total duration of hospitalizations: 15.0 ± 52.2 days in cases and 7.7 ± 19.8 days in controls; p < .0001). In addition, cases were more frequently readmitted to rehabilitation facilities and more frequently required home care ().
Table 4. Hospitalizations in cases and controls during the follow-up period
Sequelae
For the 3,239 patients who survived the index hospitalization, sequelae were documented in 823 cases (25.4%), of whom 298 cases (9.2%) presented multiple sequelae (). In comparison, these same medical conditions were documented in 6.5% of controls (N = 687), with 0.9% presenting more than one condition (N = 96). In cases, the most frequently documented sequelae, reported in between 5% and 6% of cases, were epilepsy, anxiety, and severe neurological disorders ().
Table 5. Sequelae of interest
Figure 3. Sequelae of interest. Data are presented as a Forest plot illustrating the risk ratios for the occurrence of each type of sequela between cases and controls, with their 95% confidence intervals (95% CI).
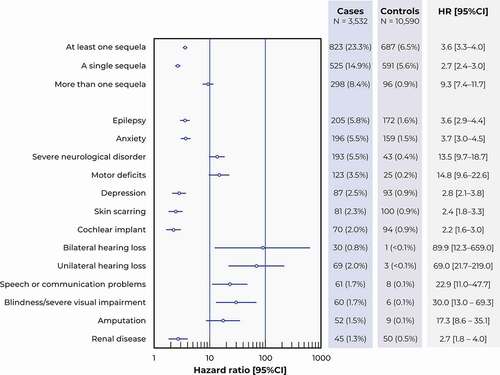
All types of sequelae were significantly more frequent (p < 0.0001) in cases than controls, with RRs ranging from 2.2 to 69.0 (). Those conditions for which cases presented the highest risk compared to controls were unilateral and bilateral hearing loss, severe visual impairment, and speech or communication problems (RRs >20 in all cases, with a lower limit to the 95% CI >10).
In cases, the frequency of these sequelae varied with age. In general, frequency increased with age, and this was also the case for each individual sequela, with the exception of skin scarring and amputation ().
Table 6. Frequency of sequelae as a function of age class
Cases developing sequelae were more frequently rehospitalized than cases of IMD without sequelae (). In contrast, overall mortality rates did not differ according to the presence or absence of sequelae (). After adjusting for age, no increased mortality risk was observed in cases with sequelae compared to controls (hazard ratio: 1.02 [95% CI: 0.75–1.40]) ().
Table 7. Association between number of sequelae and proportion of patients hospitalized or death
Readmissions to hospital for disease recurrence
A total of 35 cases (1.0%) were hospitalized for a recurrence of IMD at some stage during the follow-up period. The median age of these recurrent meningococcal cases was 8 years [IQR; 0–22], with the majority of cases (77.1%, N = 27) being under 25 years of age. The mean duration between discharge from the index hospitalization and rehospitalization for a recurrence was 143.1 ± 135.4 days (median: 91 days [IQR: 49–211]). Three of these recurrent cases, one with a human immunodeficiency virus infection, one with asplenia, and one with otherwise unspecified damage to the immune system, were documented with some form of immunodeficiency. When rehospitalizations for all forms of bacterial meningitis (not restricted to IMD) were considered, 57 cases (1.6%) were rehospitalized.
Vaccination
During the follow-up period (after the index hospitalization), 598 cases (16.9%) received at least one anti-meningococcal vaccine. The majority of these 520 cases (26.4% of the age group) were under 25 years of age. Seventy-nine cases received a MenB vaccine, 488 cases a MenC vaccine and 86 a polyvalent ACWY vaccine. The mean age at vaccination was 18.4 ± 19.1 years for MenB vaccine, 7.4 ± 13.9 years for MenC vaccine and 24.2 ± 24.2 years for polyvalent ACWY vaccine.
Discussion
This study documented the immediate and long-term outcome of IMD in hospitalized patients in France between 2012 and 2017. During the index hospitalization, we observed an in-hospital mortality rate of 8.3%, an in-hospital amputation rate of 1.2%, and an occurrence rate for long-term sequelae of 23.3%. Although the incidence of IMD was highest in the age group <25 years, mortality and long-term sequelae rates in our study were higher in the two older age groups (25–59 years and ≥60 years) than in the age group <25 years. This pattern of lower incidence but greater severity in older adults is seen in several other infectious diseases such as pneumococcal disease or influenza.Citation30,Citation31
The majority of patients were hospitalized directly following a visit to an emergency department. However, prior to hospital admission, 28.2% of cases had consulted a physician previously and had been sent home. Given its rarity and the non-specificity of the initial symptoms, IMD is easily misdiagnosed.Citation24,Citation32 Failure to diagnose IMD in a timely manner may have serious consequences due to rapid progression of the infection, which may lead to death in as early as 24 hours.Citation24 General practitioners are the physician category most frequently consulted in the period leading up to hospitalization, and it is important that they advise patients or their parents to be vigilant for symptoms which are “red flags” and to go to an emergency department as soon as possible if these symptoms appear or are aggravated.
Moreover, 44.6% of cases required admission to an intensive care unit. This proportion was higher than reported in the UK IMD Patient Registry (31%),Citation33 with an intermediate proportion reported in the Netherlands (40%);Citation34 these differences may be explained by differences in management strategies or service provision between countries. Severe complications arising during hospitalization included amputation and dialysis. Both these were rare events (~1% of cases each), consistent with previous studies.Citation26,Citation27 However, around one-third of cases admitted to an intensive care unit required mechanical ventilation.
The proportion of patients readmitted for disease recurrence was low (1.0%). This proportion is lower than that reported in many, but not all, earlier studies, which range from 1.3% to 9.0%.Citation35–38 However, the latter studies included patients from the pre-vaccination era and some included patients with pneumococcal meningitis as well, and, for these reasons, are difficult to compare with the present study. More recently, a study from the German national reference laboratory reported a lower recurrence rate of 0.2%.Citation25 Given the low absolute number of recurrent cases (35 over the six-year follow-up period in the present study, and 15 over a median observation period of 9.4 years in the German study), it is not clear whether this difference is a real one. However, many factors, including the N. meningitidis strain distribution in a particular country, the prevalence of immune deficiency states, the extent of vaccination of survivors of the initial IMD episode, and the number of strains against which the cohort had been vaccinated may be expected to influence the recurrence rate. In our study, the post-episode vaccination rate was rather low (16.9%), and this may contribute to the number of recurrent cases that we observed. Systematic vaccination following an initial IMD episode, as recommended in certain countries (such as Spain), could help reduce the recurrence rate.
Recurrent episodes of IMD should always trigger exploration for immune deficiencies and, in particular, in the complement pathway both in the patient and in any siblings, since patients with hereditary terminal complement pathway deficiencies have been reported to be at risk for recurrent IMD.Citation39
Following the index hospitalization, cases with IMD remained at significantly greater mortality risk than controls (HR: 1.6, [95% CI: 1.3–1.9]). This excess mortality is somewhat higher than that reported in a study of >5,000 cases from the Danish National Hospital Register followed for up to 25 years, which reported a crude mortality RR of 1.27 [95% CI: 1.12–1.45].Citation40 However, the one-year mortality rate (8.3%) was comparable to the mortality rate during the index hospitalization in the present study. The mortality rates can also be compared with those reported in a similar recent study of a German medical insurance database.Citation15 The latter reported a mortality rate of 4.3% at 30 days and of 5.5% at 1 year. These rates are around twofold lower compared to the present findings from France. However, such a comparison should be interpreted with caution, since the German sample was considerably smaller (164 cases), with a different age distribution (and, potentially, a different serogroup distribution). Indeed, a report using exhaustive national surveillance data from Germany covering the period 2012 to 2015 estimated the case fatality rate at one year at 9.6%.Citation41 The most recent English data (2019–2020) report a case fatality rate of 7% at the national level.Citation42 Finally, a meta-analysis of 48 national estimates published in 2019 found that case fatality rates ranged from 4.1% to 20.0% and provided a pooled estimate of 8.3% [95% CI: 7.5–9.1].Citation43
Around one-quarter of all cases hospitalized for IMD developed long-term sequelae, which required specific management. Multiple sequelae were also frequent, documented in 298 cases (9.2%). All of the medical conditions evaluated were documented significantly more often in cases than in matched controls, suggesting that they were indeed consequences of IMD, rather than spurious associations. The proportion of patients with sequelae (23.3%) was very comparable to that observed in the German medical insurance database (23.5%).Citation15 A number of systematic reviews of the long-term sequelae of IMD have been published in recent years.Citation10,Citation11,Citation44 Although the individual studies that were evaluated reported very different complication rates (depending on how the data were collected, the observation period considered, and the sequelae studied), there was general agreement across studies that hearing loss, neurological disorders such as seizures, behavioral or psychological disorders, and skin scarring were common. The findings of the present study are consistent with this, with epilepsy, anxiety, severe neurological disorders all being documented in around 5% of cases.
The study highlights the high burden of IMD in terms of morbidity and mortality, and we have described elsewhere the high cost to the health system of management of long-term sequelae of IMD.Citation7 Although this burden could be reduced by effective vaccination programs, meningococcal vaccination coverage in France has been low compared to other European countries.Citation45 Furthermore, unlike for example in Spain,Citation46 ScotlandCitation47 or Germany,Citation48 the introduction of a vaccination program in France was not followed by a reduction in the incidence of IMD.Citation45 Since the end of the recruitment period for the present study (December 31, 2017), MenC vaccination has been rendered obligatory in France for all infants born on or after January 1, 2018 and Men B vaccination of all infants from the age of two months is now (since 2021) recommended by the French health authorities. It is to be hoped that these measures will lead to a reduction in the burden of IMD over the coming decade, and the findings of the present study will be of use for benchmarking future trends.
The strengths of the study include the large sample size, which is at the upper end of the range of cohort studies published to date. The SNDS database covers >99% of French residents, which is an important advantage compared to health insurance databases which are, for example, employment-based. This is of relevance for IMD, as this is a disease in which socioeconomic status affects both the incidence and outcome of the disease.Citation7,Citation49–51 Countrywide databases such as the SNDS and the Danish National Hospital Register thus avoid recruitment biases due to socioeconomic factors. For this reason, the approach permits exhaustive capture of all sequelae presented by patients hospitalized for IMD using a standard methodology. The data collected could be of use to inform future epidemiological and economic modeling studies in France, which have, until now, had to rely on fragmentary data from different sources in other countries to document sequelae of IMD.Citation52
Several limitations of the study are inherent to use of the SNDS database. These include underreporting or potentially inaccurate reporting of diseases. This is unlikely to be a major issue for case identification, since the incidence of IMD matches that documented through mandatory surveillance monitoring in France,Citation6 although it may well be relevant for certain complications or long-term sequelae of IMD. In this respect, certain sequelae previously identified in the UK IMD Patient RegistryCitation26 could not be identified with confidence from the ICD-10-CM or CCAM codes due to a high risk of underreporting, namely separation anxiety and attention deficit hyperactivity disorder. Patients are rarely hospitalized for these disorders and, for this reason, they cannot be identified from the primary diagnosis listed on the hospital discharge summary. According to registry and cohort studies,Citation26,Citation53–55 mental health disorders are common in patients who have experienced an episode of IMD, and the fact that they are unlikely to have all been identified will probably lead to underestimation of the total number of cases with sequelae. In addition, the SNDS database does not contain information on the meningococcal serogroup, which may influence the severity, age distribution, and presentation of IMD.Citation34,Citation44 Severity, determined, for example, with a rating scale, is not documented, except through proxy variables such as admission to an intensive care unit. Furthermore, no information is available on when or how antibiotic treatment was initiated, which is a critical aspect of care in patients with IMD.Citation13,Citation56 Finally, other important and frequent consequences of IMD, such as cognitive impairment and lower educational achievementCitation26,Citation57 cannot be assessed in medical insurance databases. Again, absence of information on these events will potentially lead to an underestimate of the total proportion of cases with long-term sequelae.
In conclusion, this study confirms the high short- and long-term burden of IMD, with potentially disabling and costly sequelae persisting in around a quarter of affected individuals. This highlights the importance of providing optimal medical care for patients with IMD. Optimal care should include timely and accurate diagnosis in order to minimize the delay before hospitalization. The data collected may also help policymakers plan the long-term health resource requirements and costs associated with IMD infections. elaborates on the findings in a form that healthcare professionals could share with patients and their families.
Authors’ contribution
All authors participated in the design or implementation or analysis, and interpretation and development of this manuscript. All authors had full access to the data and gave final approval before submission.
Supplemental Material
Download MS Word (18.9 KB)Acknowledgments
The authors would like to thank Kinga Meszaros (GSK, Belgium) for her participation in the scientific committee and involvement in the discussions and Valérie Grange (GSK, France) for her input during the development of the present manuscript. Joffrey Baneton (Intys Consulting c/o GSK) assisted as operational and project manager during the conduct of the study. Medical writing support was provided by Adam Doble (Foxymed, on behalf of GSK). The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK. Lyes Derouiche (Business & Decision Life Sciences platform) coordinated manuscript development and editorial support.
Disclosure statement
CP, EB, GN, and VL-P are employed by the GSK group of companies. EB and GN hold shares in the GSK group of companies. CW-O received honoraria for lectures from the GSK group of companies and from AstraZeneca, MedImmune, Pfizer, Sanofi-Pasteur, Seqirus outside of the presented work. M-KT reported his institution received fees from the GSK group of companies for the work presented here and from the GSK group of companies, Pfizer and Sanofi-Pasteur for activities outside the presented work. M-KT reported a patent (630133) issued. SB and CE reported that their institution (CEMKA) received grants from the GSK group of companies to perform the study related to the present publication. All authors declare no other financial and non-financial relationships and activities.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2021764.
Additional information
Funding
References
- Feldman C, Anderson R. Meningococcal pneumonia: a review. Pneumonia (Nathan). 2019;11:3. doi:10.1186/s41479-019-0062-0.
- Nadel S, Ninis N. Invasive meningococcal disease in the vaccine era. Front Pediatr. 2018;6:321. doi:10.3389/fped.2018.00321.
- Stephens DS. Neisseria meningitidis. Infect Control. 1985;6:37–11. doi:10.1017/S0195941700062482.
- Whittaker R, Dias JG, Ramliden M, Kodmon C, Economopoulou A, Beer N, Pastore Celentano L, Kanitz E, Richter L, Mattheus W, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine. 2017;35:2034–41. doi:10.1016/j.vaccine.2017.03.007.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11:17. doi:10.1186/1478-7954-11-17.
- Parent du Chatelet I, Deghmane AE, Antona D, Hong E, Fonteneau L, Taha MK, Lévy-Bruhl, D . Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006-2015. J Infect. 2017;74:564–74. doi:10.1016/j.jinf.2017.02.011.
- Taha MK, Weil-Olivier C, Bouée S, Emery C, Nachbaur G, Pribil C, Loncle-Provot, V. Risk factors for invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2021;17:1858–66. doi:10.1080/21645515.2020.1849518.
- Taha MK, Gaudelus J, Deghmane AE, Caron F. Recent changes of invasive meningococcal disease in France: arguments to revise the vaccination strategy in view of those of other countries. Hum Vaccin Immunother. 2020;1–6. doi:10.1080/21645515.2020.1704580.
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi:10.1016/j.vaccine.2009.04.063.
- Strifler L, Morris SK, Dang V, Tu HA, Minhas RS, Jamieson FB, Deeks SL, Crowcroft NS, Sander B. The health burden of invasive meningococcal disease: a systematic review. J Pediatric Infect Dis Soc. 2016;5:417–30. doi:10.1093/jpids/piv065.
- Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7:421–38. doi:10.1007/s40121-018-0213-2.
- Buysse CM, Oranje AP, Zuidema E, Hazelzet JA, Hop WC, Diepstraten AF, Joosten KFM. Long-term skin scarring and orthopaedic sequelae in survivors of meningococcal septic shock. Arch Dis Child. 2009;94:381–86. doi:10.1136/adc.2007.131862.
- Société de Pathologie Infectieuse de Langue Française. 17e Conférence de Consensus en Thérapeutique Anti-Infectieuse. Prise en charge des méningites bactériennes aiguës communautaires (à l’exclusion du nouveau-né). 2008.
- Pearce J, Peters M, May N, Marshall H, Hein C, Grantham H. Care of the patient with invasive meningococcal disease by prehospital emergency medical service clinicians: a scoping review. BMJ Open. 2020;10:e033447. doi:10.1136/bmjopen-2019-033447.
- Huang L, Heuer OD, Janssen S, Hackl D, Schmedt N. Clinical and economic burden of invasive meningococcal disease: evidence from a large German claims database. PLoS One. 2020;15:e0228020. doi:10.1371/journal.pone.0228020.
- O’Brien JA, Caro JJ, Getsios D. Managing meningococcal disease in the United States: hospital case characteristics and costs by age. Value Health. 2006;9:236–43. doi:10.1111/j.1524-4733.2006.00113.x.
- Davis KL, Bell TJ, Miller JM, Misurski DA, Bapat B. Hospital costs, length of stay and mortality associated with childhood, adolescent and young adult meningococcal disease in the US. Appl Health Econ Health Policy. 2011;9:197–207. doi:10.2165/11587330-000000000-00000.
- Davis KL, Misurski D, Miller JM, Bell TJ, Bapat B. Cost of acute hospitalization and post-discharge follow-up care for meningococcal disease in the US. Hum Vaccin. 2011;7:96–101. doi:10.4161/hv.7.1.13692.
- Hanquet G, Christensen H, Agnew E, Trotter C, Robays J, Dubois C, Van de Sande, S, Thiry, N . A quadrivalent vaccine against serogroup B meningococcal disease: a cost-effectiveness study. Health Technology Assessment KCE Report 231. Belguim (Brussels): KCE Belgian Health Care Knowledge Centre; . 14 2014 October Accessed 03 November 2021 https://kce.fgov.be/sites/default/files/atoms/files/KCE_231_Meningococcal_disease_Report.pdf
- Karve S, Misurski D, Miller J, Davis KL. Costs of sequelae associated with invasive meningococcal disease: findings from a US managed care population. Health Outcomes Res Med. 2011;2:e215–26. doi:10.1016/j.ehrm.2011.08.001.
- Gil-Prieto R, García-García L, Alvaro-Meca A, González-Escalada A, Viguera Ester P, Gil de Miguel A. The burden of hospitalizations for meningococcal infection in Spain (1997-2008). Vaccine. 2011;29:5765–70. doi:10.1016/j.vaccine.2011.05.089.
- Montero JM, Prieto RG, Alejandre CG, Meca LA, Portugal P, de Miguel AG. Hospital admissions for meningococcal infection in Spain (1997-2005). J Infect. 2009;58:15–20. doi:10.1016/j.jinf.2008.10.009.
- Weil-Olivier C, Taha MK, Emery C, Bouée S, Beck E, Aris E, Loncle-Provot V, Nachbaur G, Pribil C. Healthcare resource consumption and cost of invasive meningococcal disease in France: a study of the national health insurance database. Infect Dis Ther 2021;10:1607–23. doi:10.1007/s40121-021-00468-w.
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367:397–403. doi:10.1016/S0140-6736(06)67932-4.
- Krone M, Lâm TT, Claus H, Vogel U. Recurrent invasive meningococcal infections - quantifying the risk, Germany, 2002 to 2018. Euro Surveill. 2020;25 1900565 . doi:10.2807/1560-7917.ES.2020.25.25.1900565.
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11(9):774–83. doi:10.1016/S1474-4422(12)70180-1.
- Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R . The disease burden of invasive meningococcal serogroup B disease in Canada. Pediatr Infect Dis J. 2013;32:e20–5. doi:10.1097/INF.0b013e3182706b89.
- Quantin C. Etude des algorithmes de définition de pathologies dans le système national d’information inter-régimes de l’assurance maladie (SNIIRAM). France,Paris: Caisse nationale d’Assurance maladie des travailleurs salariés; 2015.
- Beck E, Klint J, Neine M, Garcia S, Meszaros K. Cost-effectiveness of 4CMenB infant vaccination in England: a comprehensive valuation considering the broad impact of serogroup B invasive meningococcal disease. Value Health. 2021;24:91–104. doi:10.1016/j.jval.2020.09.004.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi:10.1111/1469-0691.12461.
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, et al. Influenza. Nat Rev Dis Primers. 2018;4:3. doi:10.1038/s41572-018-0002-y.
- Haj-Hassan TA, Thompson MJ, Mayon-White RT, Ninis N, Harnden A, Smith LF, Perera R, Mant DC. Which early ‘red flag’ symptoms identify children with meningococcal disease in primary care? Br J Gen Pract. 2011;61(584):e97–104. doi:10.3399/bjgp11X561131.
- Parikh SR, Campbell H, Gray SJ, Beebeejaun K, Ribeiro S, Borrow R, Ramsay ME, Ladhani SN. Epidemiology, clinical presentation, risk factors, intensive care admission and outcomes of invasive meningococcal disease in England, 2010-2015. Vaccine. 2018;36:3876–81. doi:10.1016/j.vaccine.2018.02.038.
- Loenenbach AD, van der Ende A, de Melker HE, Sanders EAM, Knol MJ. The clinical picture and severity of invasive meningococcal disease serogroup W compared with other serogroups in the Netherlands, 2015-2018. Clin Infect Dis. 2020;70:2036–44. doi:10.1093/cid/ciz578.
- Adriani KS, van de Beek D, Brouwer MC, Spanjaard L, de Gans J. Community-acquired recurrent bacterial meningitis in adults. Clin Infect Dis. 2007;45:e46–51. doi:10.1086/520682.
- Drummond DS, de Jong AL, Giannoni C, Sulek M, Friedman EM. Recurrent meningitis in the pediatric patient–the otolaryngologist’s role. Int J Pediatr Otorhinolaryngol. 1999;48:199–208. doi:10.1016/S0165-5876(99)00022-1.
- Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr., Swartz MN. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. doi:10.1056/NEJM199301073280104.
- igurdardóttir B, Björnsson OM, Jónsdóttir KE, Erlendsdóttir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med. 1997;157:425–30. doi:10.1001/archinte.1997.00440250077009.
- Rosain J, Hong E, Fieschi C, Martins PV, El Sissy C, Deghmane AE, Ouachée M, Thomas C, Launay D, de Pontual L. Strains responsible for invasive meningococcal disease in patients with terminal complement pathway deficiencies. J Infect Dis. 2017;215:1331–38. doi:10.1093/infdis/jix143.
- Roed C, Omland LH, Engsig FN, Skinhoj P, Obel N. Long-term mortality in patients diagnosed with meningococcal disease: a Danish nationwide cohort study. PLoS One. 2010;5:e9662. doi:10.1371/journal.pone.0009662.
- Hellenbrand W. (Invasive Meningococcal Disease 2012 - 2015). Invasive Meningokokken-Erkrankungen 2012 – 2015. Epidemiologisches Bulletin. 2016;471–84.
- Public Health England. Meningococcal disease: laboratory confirmed cases in England in 2019 to 2020. United Kingdom (London): Public Health England.12 January2021. Accessed 03 November 2021. https://www.gov.uk/government/publications/meningococcal-disease-laboratory-confirmed-cases-in-england-in-2019-to-2020
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–82. doi:10.1016/j.vaccine.2019.04.020.
- Vyse A, Anonychuk A, Jakel A, Wieffer H, Nadel S. The burden and impact of severe and long-term sequelae of meningococcal disease. Expert Rev Anti Infect Ther. 2013;11:597–604. doi:10.1586/eri.13.42.
- Gaudelus J, Cohen R, Leboucher B, Stahl JP, Denis F, Pujol P, Longfier L, Martinot A. Meningococcal C vaccine coverage in France in infants, children, and adolescents. Med Mal Infect. 2019;49:180–86. doi:10.1016/j.medmal.2019.01.014.
- Garrido-Estepa M, León-Gómez I, Herruzo R, Cano R. Changes in meningococcal C epidemiology and vaccine effectiveness after vaccine introduction and schedule modification. Vaccine. 2014;32:2604–09. doi:10.1016/j.vaccine.2014.03.010.
- Mooney JD, Christie P, Robertson C, Clarke SC. The impact of meningococcal serogroup C conjugate vaccine in Scotland. Clin Infect Dis. 2004;39:349–56. doi:10.1086/421947.
- Hellenbrand W, Elias J, Wichmann O, Dehnert M, Frosch M, Vogel U. Epidemiology of invasive meningococcal disease in Germany, 2002-2010, and impact of vaccination with meningococcal C conjugate vaccine. J Infect. 2013;66:48–56. doi:10.1016/j.jinf.2012.09.008.
- Burgess S. Social and environmental influences affecting the risk of development of meningococcal disease: considerations for prehospital care. J Emerg Prim Health Care. 2006;4:1–10.
- Heyderman RS, Ben-Shlomo Y, Brennan CA, Somerset M. The incidence and mortality for meningococcal disease associated with area deprivation: an ecological study of hospital episode statistics. Arch Dis Child. 2004;89:1064–68. doi:10.1136/adc.2003.036004.
- Stuart JM, Middleton N, Gunnell DJ. Socioeconomic inequality and meningococcal disease. Commun Dis Public Health. 2002;5:327–28.
- Lecocq H, Parent Du Châtelet I, Taha MK, Lévy-Bruhl D, Dervaux B. Epidemiological impact and cost-effectiveness of introducing vaccination against serogroup B meningococcal disease in France. Vaccine. 2016;34:2240–50. doi:10.1016/j.vaccine.2016.03.020.
- Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics. 2009;123:e502–9. doi:10.1542/peds.2008-0581.
- Garralda ME, Gledhill J, Nadel S, Neasham D, O’Connor M, Shears D. Longer-term psychiatric adjustment of children and parents after meningococcal disease. Pediatr Crit Care Med. 2009;10:675–80. doi:10.1097/PCC.0b013e3181ae785a.
- Gottfredsson M, Reynisson IK, Ingvarsson RF, Kristjansdottir H, Nardini MV, Sigurdsson JF, Schneerson, R, Robbins, JB, Miller, MA,. Comparative long-term adverse effects elicited by invasive group B and C meningococcal infections. Clin Infect Dis. 2011;53:e117–24. doi:10.1093/cid/cir500.
- van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib, SL, Mourvillier, B, Ostergaard, C, Pagliano, P, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37–62. doi:10.1016/j.cmi.2016.01.007.
- Roed C, Omland LH, Skinhoj P, Rothman KJ, Sorensen HT, Obel N. Educational achievement and economic self-sufficiency in adults after childhood bacterial meningitis. JAMA. 2013;309:1714–21. doi:10.1001/jama.2013.3792.