ABSTRACT
Due to COVID-19, vaccinations dropped in 2020 and 2021. We estimated the impact of reduced recombinant zoster vaccine (RZV) use on herpes zoster (HZ) cases, complications, and quality-adjusted life-year (QALY) losses among older adults. Various scenarios were compared with Markov models using data from national sources, clinical trials, and literature. Missed series initiations were calculated based on RZV distributed doses. In 2020, 3.9 million RZV series initiations were missed, resulting in 31,945 HZ cases, 2,714 postherpetic neuralgia cases, and 610 lost QALYs. Scenarios further projected disease burden increases if individuals remain unvaccinated in 2021 or the same number of initiations are missed in 2021. Health professionals should emphasize the importance of vaccination against all preventable diseases during the COVID-19 era.
Introduction
Herpes zoster (HZ [i.e., shingles]), caused by reactivation of the varicella-zoster virus (VZV, chickenpox), is a common ailment in adult populations. Nearly one in three individuals in the United States (US) develop HZ at some point in their lifetime, with an estimated 1 million individuals diagnosed with HZ annually.Citation1
HZ is characterized by a painful rash that typically appears on one side of the back, chest, or abdomen, associated with one or more affected dermatomes. Some patients describe the pain as an intense burning sensation, which can last for months or even years after the rash goes away. This long-lasting pain is called postherpetic neuralgia (PHN), and it is the most common complication of HZ.Citation2 Other complications include ocular, neurological, cutaneous, and non-pain complications.Citation2 HZ impacts sleep, work, and an individual’s ability to perform other daily activities.Citation3,Citation4
Recombinant zoster vaccine (RZV, Shingrix, GSK) is licensed in the US and recommended by the Advisory Committee on Immunization Practices to prevent HZ in adults ≥ 50 years, as well as adults ≥ 19 years who are or will be immunodeficient or immunosuppressed due to disease or therapy.Citation5,Citation6 In adults aged ≥ 50 years, vaccine efficacy in preventing HZ has been shown to be greater than 90%.Citation7–9 Vaccine efficacy in reducing HZ burden of illness due to pain and HZ burden of interference on activities of daily living was also greater than 90% in individuals aged ≥ 50 years.Citation10
With the COVID-19 pandemic, non-COVID-19 vaccination rates have declined for various reasons, including lockdown restrictions. However, given that HZ is caused by the reactivation of latent VZV [i.e., chickenpox], even individuals who are isolated are still at risk of HZ. To address the issue of decreased vaccination coverage, the Centers for Disease Control and Prevention’s (CDC) guidance on HZ vaccination specifies that HZ vaccination should not be delayed or discontinued because of the pandemic unless a patient is suspected or confirmed to have COVID-19 because this preventive care is essential for older adults.Citation11 The guidance also notes that health care providers and eligible patients should strive to administer the two RZV doses within the recommended interval of 2 to 6 months.Citation11
In this study, we explored the impact of decreased HZ vaccination by estimating the number of HZ cases, complications, and quality-adjusted life-year (QALY) losses that would have been avoided if vaccination coverage had continued at the same rate as observed prior to the pandemic period.
Methods
Model overview
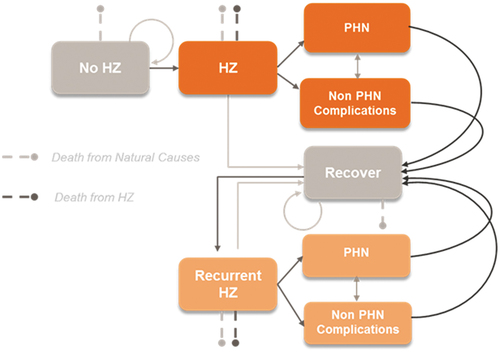
A multi-cohort Markov model estimated the impact of missed RZV vaccinations on avoidable HZ cases, PHN cases, and complication cases among US adults aged ≥ 50 years (). The model includes a 1-year cycle length, with five age-specific cohorts (50–59, 60–64, 65–69, 70–79, and ≥ 80 years) that are followed for a period of 1–2 years after their missed vaccinations, depending on the scenario. The age distribution of the population in the model is reflective of the population of adults ≥ 50 years of age in the US. A multicohort model, instead of a single cohort model, was employed to better account for the increasing risk of HZ as individuals age as well as varying risk of HZ-related complications and QALY losses by age. Specific details on the model’s underlying structure, inputs, data sources, and assumptions have been previously described.Citation12 The base-case analysis compares scenarios with and without missed RZV vaccinations between April through December 2020, with a 1-year follow-up period.
Model inputs
Model inputs related to the age distribution of the US population, mortality rates, HZ epidemiology, baseline utilities, and QALY losses per case of HZ and per dose of RZV (for adverse event-related QALY losses) were obtained from standard US sources and published literature.Citation12 RZV year 1 efficacy values against HZ and PHN were obtained from clinical trial data,Citation7,Citation8 consistent with the previously published model.Citation12 Two-dose RZV waning estimates were updated with more recent data on long-term persistence, which resulted in annual waning rates of 1.5% and 2.3% among individuals < 70 years and ≥ 70 years, respectively.Citation13,Citation14 In the scenario without missed vaccinations, 1-dose RZV coverage was assumed to be 100%, and 2nd dose compliance was set at 80% based on recent data on RZV series completion.Citation15 An assumption of 100% first dose coverage was used, as the analyses focused on the incremental outcomes resulting from vaccination of the population estimated to have missed series initiations in 2020 (or 2021 in scenario analyses), rather than outcomes for the entire US population. The model assumed that the RZV doses were administered two months apart as per labelCitation16 and that the series completion rate did not change during the pandemic.
Missed doses were estimated using data on RZV distributed doses and an assumed 43% reduction in RZV vaccinations during the pandemic, based on data reported by the CDC on reductions in RZV use among Medicare beneficiaries during specific weeks in 2020 as compared with the corresponding weeks in 2019.Citation17 Hong et al.Citation17 reported that RZV use decreased by 62% during the week following the national emergency declaration (week of March 13, 2020) and continued decreasing to a low of an 89% decrease (week of April 12, 2020) before rebounding to a decrease of 43% by the end of the study period (week of July 12, 2020). Missed RZV series initiations were then calculated from the missed doses assuming an 80% two-dose completion rate.Citation15
Analyses
The base-case analysis assumed a 43% reduction in RZV use between April through December of 2020 and followed individuals with a missed series initiation for a period of 1 year. Because data on reduced RZV use were not available through the end of 2020, a one-way sensitivity analysis was also conducted, assuming a more conservative 30% reduction in RZV use between April and December 2020. Two scenario analyses were also conducted:
Scenario analysis 1: assuming that individuals with missed RZV series initiations in 2020 remain unvaccinated in 2021
Scenario analysis 2: assuming that individuals with missed RZV series initiations in 2020 remain unvaccinated in 2021 (as above) and that the same number of individuals missed RZV series initiations in 2021
For both scenario analyses, the follow-up period was 2 years, with QALYs discounted at 3% per year.
Results
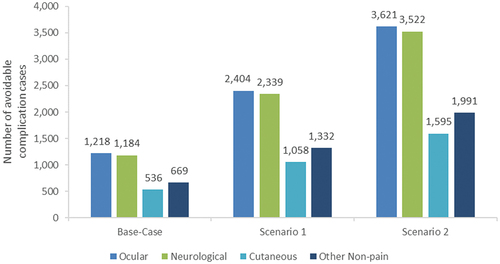
Assuming a 43% reduction in RZV use due to the COVID-19 pandemic, an estimated 21 million RZV doses were expected to be distributed in 2020, including approximately 16.5 million doses between April through December 2020. Of the 9.2 million RZV series initiations expected between April and December 2020, an estimated 5.4 million series were initiated, and 3.9 million series initiations were missed (). In the base-case analysis, these missed RZV series initiations resulted in projections of 31,945 avoidable HZ cases, 2,714 avoidable PHN cases, 3,607 avoidable complication cases, and 610 avoidable QALY losses over the 1-year follow-up period (). Among the avoidable complication cases, ocular complications were most frequently projected (33.8%), followed by neurological complications (32.8%), other non-pain complications (18.5%), and cutaneous complications (14.9%) ().
Table 1. Base-case and scenario analysis results: avoidable cases and QALY losses due to missed RZV vaccinations
Figure 2. Estimated missed RZV series initiations in base-case, sensitivity, and scenario analyses. RZV = recombinant zoster vaccine.
Figure 3. Base-case and scenario analysis results: avoidable complication cases due to missed RZV vaccinations. RZV = recombinant zoster vaccine Note: Scenario analysis 1 assumes individuals with missed RZV series initiations in 2020 remain unvaccinated in 2021. Scenario analysis 2 assumes that individuals with missed RZV series initiations in 2020 remain unvaccinated in 2021 (as above) and that the same number of individuals missed RZV series initiations in 2021.
In a one-way sensitivity analysis assuming 30% RZV reduction during the pandemic, an estimated 13.5 million RZV doses were expected to be distributed between April and December 2020. During this period, 7.5 million RZV series initiations were expected, with approximately 2.2 million initiations missed (). As a result of these missed RZV series initiations, the model projected 18,020 avoidable HZ cases, 1,531 avoidable PHN cases, 2,035 avoidable complication cases, and 344 avoidable QALY losses over the 1-year follow-up period.
Results from Scenario Analysis 1 indicate that if the estimated 3.9 million individuals who missed RZV series initiations in 2020 remain unvaccinated in 2021, avoidable HZ cases, PHN cases, complication cases, and QALY losses will all increase over a follow-up period of 2 years ( and ). Additionally, if these 3.9 million individuals remain unvaccinated in 2021 and the same number of 3.9 million additional RZV series initiations are missed in 2021 (Scenario Analysis 2), projected outcomes over this 2-year follow-up period increase further to a high of 95,062 avoidable HZ cases, 8,070 avoidable PHN cases, 10,730 avoidable complication cases, and 1,795 avoidable QALY losses ( and ).
Discussion
The current study estimated that 31,945 avoidable HZ cases were expected as a result of the decrease in HZ vaccination rates due to the pandemic in the US. These HZ cases included over 2,700 avoidable PHN cases, over 3,600 avoidable complication cases, and over 600 avoidable QALY losses that were projected by the model, highlighting the added HZ-related burden attributed to the COVID-19 pandemic beyond the substantial morbidity and mortality posed by primary COVID-19 cases. Over the short term, increases in HZ-related burden were consistently observed across the various sensitivity and scenario analyses conducted (e.g., with avoidable HZ cases over a 1- to 2-year follow-up period ranging from 18,020 to 95,062 across analyses).
Previous analyses have reported reductions in child, adolescent, and adult vaccination coverage in the US due to the COVID-19 pandemic.Citation17–19 However, to our knowledge, this is the first analysis that has modeled the impact of missed vaccinations for other vaccine-preventable diseases on disease-related health outcomes. This type of analysis is particularly relevant for HZ, where individuals remain at risk of developing HZ regardless of COVID-related mitigation efforts (e.g., lockdown restrictions, social distancing, use of face masks).
The current analysis focused on short term HZ-related health outcomes resulting from missed RZV vaccinations during the pandemic and did not model the impact of these avoidable HZ cases on associated health care resource use (HCRU) and costs. Additional data on healthcare seeking behaviors during the pandemic would be needed to estimate the impact of reduced RZV use on HZ-related HCRU and costs. In addition to the burden experienced by unvaccinated individuals who experienced HZ, these avoidable cases may have also been competing for scarce health care resources during the pandemic, which is not captured in the current analysis. Additional data are also needed to improve understanding of the impact of the pandemic on HZ-related outcomes over the longer term, depending on whether HZ vaccination coverage continues to lag into 2021 and beyond.
In this study, no increased risk of HZ during the COVID-19 pandemic has been assumed. However, previous studies have proposed a potential association between HZ and the COVID-19 pandemic.Citation20–22 Several hypotheses could be postulated on why there may be an increased risk of HZ during lockdowns and restrictions. Increased levels of stress and depression have been shown to be associated with an increased risk of HZ,Citation23,Citation24 while mental health domains such as anxiety and depression are risk factors for developing PHN.Citation25,Citation26 In addition, COVID-19 induced T-cell dysfunction, and aberrant IL-17 signaling have been suggested to also increase the risk of developing HZ.Citation27,Citation28
Findings from the current study indicate that, in the US, reduced RZV use during the pandemic likely resulted in avoidable cases of HZ. Since HZ is a global disease and the COVID-19 pandemic has spread worldwide, the global impact of reduced RZV use during the pandemic is likely significant. In addition to vaccination against COVID-19, the results underscore the need for older adults to continue to be vaccinated against other preventable diseases, including HZ. It is important that vaccine recipients and health care providers are informed of the disease risk and impact of avoiding vaccinations to ensure that vaccine coverage levels are restored to pre-pandemic levels and maintained or improved over the long term.
Abbreviations
Contributorship
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and publication of scholarly work in medical journal.
Trademark
Shingrix is a trademark owned by or licensed to the GSK group of companies.
Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Diego Collin coordinated manuscript development and editorial support. Esther van de Vosse provided medical writing support.
Disclosure statement
All authors are employed by the GSK group of companies. DC, EML, NL, and SP hold shares in the GSK group of companies. The authors declare no other financial and non-financial relationships and activities.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC). Epidemiology and prevention of vaccine-preventable diseases: herpes zoster; 2020a [accessed 2021 Aug 17]. https://www.cdc.gov/vaccines/pubs/pinkbook/herpes-zoster.html .
- Centers for Disease Control and Prevention (CDC). Shingles (herpes zoster); 2019 [accessed 2021 Aug 17]. https://www.cdc.gov/shingles/about/index.html .
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ. 2011;14 (5):639–5. doi:10.3111/13696998.2011.607482.
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, Chan ISF, Choo P, Levin MJ, Johnson G, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23(6):490–96. doi:10.1097/AJP.0b013e318065b6c9.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. Morb Mortal Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- Centers for Disease Control and Prevention (CDC). Advisory committee on immunization practices (ACIP); 2021 [accessed 2021 Dec 10]. https://www.cdc.gov/vaccines/acip/recommendations.html .
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–32. doi:10.1056/NEJMoa1603800.
- Curran D, Kim JH, Matthews S, Dessart C, Levin MJ, Oostvogels L, Riley ME, Schmader KE, Cunningham AL, McNeil SA, et al. Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc. 2021;69(3):744–52. doi:10.1111/jgs.16917.
- Curran D, Oostvogels L, Heineman T, Matthews S, McElhaney J, McNeil S, Diez-Domingo J, Lal H, Andrews C, Athan E, et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74(8):1231–38. doi:10.1093/gerona/gly150.
- Centers for Disease Control and Prevention (CDC). Vaccines and preventable diseases: frequently asked questions about Shingrix; 2020b [accessed 2021 Aug 17]. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/faqs.html .
- Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. doi:10.1016/j.vaccine.2018.07.005.
- Boutry C, Hastie A, Shi M, Diez-Domingo J, Tinoco JC, Yu C-J, Pirrotta, P, Kalema, G, Schuind, A . 8. The adjuvanted recombinant zoster vaccine (RZV) confers long-term protection against herpes zoster: interim results of an extension study (ZOSTER-049) of Two Clinical Trials (ZOE-50 and ZOE-70). Open Forum Infect Dis. 2020;7:S4–S5 doi:10.1093/ofid/ofaa417.007.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu C-J, Andrews C, Beytout J, Caso C, Cheng H-S, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase III clinical trials (ZOE-50 and ZOE-70). Clin Infect Dis. 2021. doi:10.1093/cid/ciab629.
- Patterson BJ, Chen C-C, McGuiness CB, Glasser LI, Sun K, Buck PO. Early examination of real-world uptake and second-dose completion of recombinant zoster vaccine in the United States from October 2017 to September 2019. Hum Vaccines Immunother. 2021;17(8):2482–87 doi:10.1080/21645515.2021.1879579.
- GlaxoSmithKline Biologicals: SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted), suspension for intramuscular injection; 2017 [accessed 2021 Aug 26]. https://www.fda.gov/media/108597/download.
- Hong K, Zhou F, Tsai Y, Jatlaoui TC, Acosta AM, Dooling KL, Kobayashi M, Lindley MC. Decline in receipt of vaccines by medicare beneficiaries during the COVID-19 pandemic—United States, 2020. Morb Mortal Wkly Rep. 2021;70(7):245–49. doi:10.15585/mmwr.mm7007a4.
- Brooks HE, McLendon LA, Daniel CL. The impact of COVID-19 on pediatric vaccination rates in Alabama. Prev Med Rep. 2021;22:101320. doi:10.1016/j.pmedr.2021.101320.
- Murthy BP, Zell E, Kirtland K, Jones-Jack N, Harris L, Sprague C, Schultz J, Le Q, Bramer CA, Kuramoto S, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations—10 US Jurisdictions, March–September 2020. Morb Mortal Wkly Rep. 2021;70(23):840–45. doi:10.15585/mmwr.mm7023a2.
- Wang B, Guo S, Yao Y, Li Y, Zhang G. Dermatologists may need to pay more attention to herpes zoster during the pandemic of COVID-19. Infect Dis. 2020;52(12):917–18. doi:10.1080/23744235.2020.1797158.
- Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir J Med Sci. 2021;1–5.
- Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli DRB, Martelli-Júnior H. Increased number of herpes zoster cases in Brazil related to the COVID-19 pandemic. Int J Infect Dis. 2021;104:732–33. doi:10.1016/j.ijid.2021.02.033.
- Choi HG, Kim E-J, Lee YK, Kim M. The risk of herpes zoster virus infection in patients with depression: a longitudinal follow-up study using a national sample cohort. Medicine. 2019;98(40): e17430.
- Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 7(1): 2020, ofaa005 doi:10.1093/ofid/ofaa005.
- Dworkin RH, Hartstein G, Rosner HL, Walther RR, Sweeney EW, Brand L. A high-risk method for studying psychosocial antecedents of chronic pain: the prospective investigation of herpes zoster. J Abnorm Psychol. 1992;101(1):200–05. doi:10.1037/0021-843X.101.1.200.
- Curran D, Schmidt-Ott R, Schutter U, Simon J, Anastassopoulou A, Matthews S. Impact of herpes zoster and postherpetic neuralgia on the quality of life of Germans aged 50 or above. BMC Infect Dis. 2018;18(1):496. doi:10.1186/s12879-018-3395-z.
- Diez-Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID-19 increase the risk of herpes zoster? A narrative review. Dermatol Ther (Heidelb.) 2021;11(4):1119–26.
- Yu X, Li L, Chan MTV, Wu WKK. Bioinformatic analyses suggest augmented interleukin-17 signaling as the mechanism of COVID-19-associated herpes zoster. Environ Sci Pollut Res. 2021; doi:10.1007/s11356-021-15567-x.