 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Japanese encephalitis is the main cause of viral encephalitis in Asia. In a previous single-arm vaccine trial, an inactivated chromatographically purified Japanese encephalitis Vero cell vaccine (CVI-JE; JEVACTM) was safe and immunogenic in 152 Thai children aged 1–3 years receiving a 2-dose primary immunization and booster dose 1 year later. We conducted a 5-year follow-up assessment of the persistence of the immune response the 144 children remaining in this cohort after first booster dose. Immunity was assessed by 50% plaque reduction neutralization test annually for up to 5 years post-booster. Seroprotection rates (95%CI) decreased from 100% (97.1–100) at 1 year post-booster to 93% (85.0–98.3) at 5 years post-booster. No serious vaccine-related adverse events or Japanese encephalitis infections were reported. A 2-dose primary immunization and booster 1 year later with CVI-JE provided long-lasting immunity in the majority of children.
Introduction
Japanese encephalitis (JE) is a mosquito-borne viral encephalitis caused by the Japanese encephalitis virus (JEV) (Flavivirus: Flaviviridae). Transmission is maintained in a natural enzootic cycle between predominately Culex mosquitoes and water birds with pigs as an amplifying host while humans are considered to be an incidental, dead-end host.Citation1,Citation2 In Asia and the Western Pacific, JEV transmission is endemic in tropical and subtropical areas and is seasonally epidemic in temperate areas of most countries. Most infections occur in rural areas, but urban transmissions were reported.Citation3,Citation4 Although vaccination programs have greatly reduced the disease burden, limited laboratory diagnostic resources and incomplete case reporting make estimated incidence uncertain, and JEV remains the leading cause of viral childhood encephalitis in Asia.Citation5–7
The main control strategy is human vaccination. In Thailand, mouse-brain-derived inactivated (MBDI) vaccine was phased out by 2013 partly due to severe adverse event following immunization, including hypersensivity reactions (18–64 per 10,000 doses), and rare neurologic reactions, such as acute disseminated encephalomyelitis (1 per 50,000 to 1,000,000 vaccines).Citation8,Citation9 Currently, two live attenuated vaccines based on the JE SA14-14-2 strain (CD JEVAXTM and the chimeric IMOJEVTM) and one chromatographically purified Vero cell inactivated Japanese encephalitis vaccine (CVI-JE) based on Beijing-3 strain (JEVACTM) are the other licensed vaccines in Thailand. These have better safety profiles than MBDI vaccine. However, live attenuated vaccines are contraindicated in pregnant and breastfeeding mothers, individuals with HIV infection, and individuals with congenital or acquired immune deficiency impairing cellular immunity.
Studies in China and Thailand proved CVI-JE is safe and immunogenic. A study of 375 Chinese children aged 8 months to 10 years given a 2-dose primary immunization (day 0 and day 7) reported a seroconversion rate of 90.4%.Citation10 A study of 152 Thai children aged 1–3 years given a 2-dose primary immunization (day 0 and day 7–28) had seroprotection rates (SPRs) of 100% 28 days after completing the primary series, 89.7% at one year later pre-booster, and 100% SPR 28 days post-booster at 1 year.Citation11 Both studies reported self-limiting mild localized to systemic reaction adverse events following immunization (AEFI).Citation10,Citation11
CVI-JE was licensed in China in 2008 and Thailand in 2014. The current recommendation in Thailand is a 2-dose primary immunization 7–28 days apart from 6 months of age and a booster 1 year later. However, data on long-term seroprotection and antibody persistence of CVI-JE are lacking. Thus, we herein report the antibody response of CVI-JE up to 5 years after primary immunization and booster 1 year later.
Materials and methods
Study design and subjects
The details of our previous non-randomized, open-label, single-arm trial of the safety, reactogenicity, and immunogenicity of a 2-dose primary immunization and booster 1 year later up to the 28-day post-booster response using freeze-dried CVI-JE (Liaoning Cheng Da Biotechnology Co., Ltd., Shenyang, China) have previously been reported.Citation11 Each dose was 0.5 mL. The study was registered with Clinical Trial registration number of NCT01408537. The combined schedule of visits for both the previous and the present study is shown in .
Figure 1. Disposition of participants.
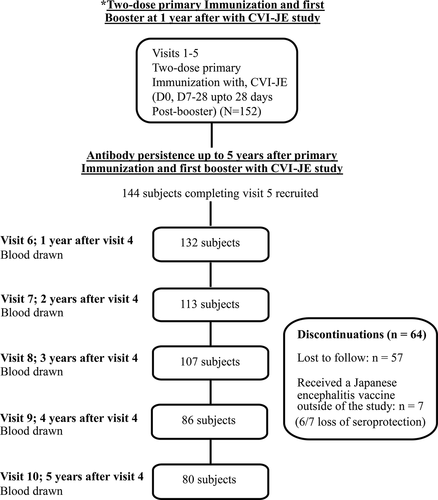
In the present study, we assessed the JE antibody persistence for up to 5 years post-booster dose by annual follow-up visits for 60 months between 2011 and 2016 in all the 144 participants completing the protocol of our previous study up to the 28-day post-booster visit.Citation11 We recruited from two hospital sites in Bangkok, Thailand: the Well Baby Clinic, Outpatient Department, Nopparat Rajathanee Hospital and the Department of Tropical Pediatrics, Faculty of Tropical Medicine, Mahidol University. We carried out the study in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. The institutional and ethical review boards of the participating sites approved the study protocol. Signed informed consent was obtained from the parents or legal guardians of the participating children.
Procedures and assessments
At each annual visit, blood was drawn for immunogenicity assessment and physical examination was performed. Participants and parent/legal guardians were asked about any illnesses, including JE, since the previous visit to evaluate any vaccine-related serious adverse events (SAEs).Citation12 The study investigators evaluated whether these were related to the study vaccine.
JE neutralizing antibody titers were assayed with the 50% plaque reduction neutralization test (PRNT50; Center for Vaccine Development, Mahidol University, Thailand), for which the method has previously been described.Citation11 This method used the homologous strain to the CVI-JE vaccine (Beijing-3) as the challenge virus. The lower limit of quantitation of the test was 10 [1/dil]. This is also the accepted threshold for seropositivity (≥10 [1/dil]) and the correlate of protection against JE (≥10 [1/dil]).Citation13 A titer <10 [1/dil] was defined as seronegative. Any participants who were found to have a titer measurement below the protective threshold during the 5-year follow-up were offered revaccination with a JE vaccine licensed in Thailand and were discontinued from the study.
Statistical analysis
No sample size calculation was performed as this was a 5-year follow-up of the remaining participants who completed the protocol of the previous study.Citation11 All analyses were descriptive without hypothesis testing. The primary analysis included all available long-term follow-up data except for participants seropositive at the baseline of the previous study.Citation11 Proportions and their 95%CIs were calculated by Clopper-Pearson exact binomial method.Citation14 GMTs and 95%CI were calculated from log10 titers with an assumption of normal distribution, followed by antilog transformations to report results on the original scale. PRNT50 values below the lower limit of quantification of the test of 10 [1/dil] were replaced by 5 [1/dil] (half the limit of seropositivity).
A sensitivity analysis was performed to avoid bias in estimating the persistence of seroprotection in participants with low neutralizing titers who withdrew from the study or participants who received another JE vaccination and then were discontinued from the study. If a participant was seronegative at the last recorded visit and withdrew from the study for any reason or missed a following visit or blood sample, the participant was assumed to be seronegative at all proceeding follow-up visits. If a participant was seronegative at a visit and then was seropositive later for any reason, the participant was still considered to be seronegative at all proceeding follow-up visits. A further sensitivity analysis was performed by Kaplan–Meier analysis to estimate the proportion of participants maintaining seroprotection over the 5-year follow-up.
Results
Study population
A total of 144 children aged 1–3 years previously receiving two primary doses and one booster dose 1 year later completed the protocol of the previous study up to visit 5 (28-day post-booster)Citation11 and were recruited into the present study. Of these, 5 were seropositive at the baseline of the previous study and were excluded from the primary analysis and sensitivity analysis of immunogenicity. Thus, 139 children were included in this analysis. All 144 children recruited into the present study were included safety analysis. Seventy-six participants discontinued during the 5-year follow-up, and no withdrawals occurred due to safety-related events. Their dispositions and characteristics of the study participants are summarized in and
Table 1. Baseline characteristics of participants at 28 days post-booster (N = 144)
Immunogenicity assessments
Seroprotection rates
All 139 participants were seropositive at 28 days post-booster as reported in our previous study.Citation11 In the present study, SPRs (95%CI) in the primary analysis remained high for throughout the 5-year follow-up period (). These SPRs decreased from 100% (97.1–100) at year 1 to 93.8% (85.0–98.3) at year 5 (). Sensitivity analysis SPRs were similar to primary analysis results from years 1 to 3 of follow-up (). However, sensitivity analysis SPRs (95%CIs) at years 3 to 5 of follow-up post-booster were lower than those of primary analysis SPRs at 83.5% (74.9–90.1), 72.4% (62.5–81.0), and 66.3% (55.7–75.8), respectively ().
Table 2. Primary analysis of seroprotection rates of CVI-JE primed and boosted children aged 1–3 years up to 5 years post-booster
Kaplan–Meier SPRs by years 1–5 of 100%, 93.7% (95%CI 89.4–98.0), 85.4% (95%CI 78.9–91.9), 75.4% (95%CI 67.2–83.6), and 70.8% (95%CI 62.0–79.6), respectively.
Persistence of neutralizing antibodies
The immune response for the primary immunization and booster 1 year later up to 28 days post-booster have been reported previously.Citation11 At 28 days post-booster, geometric mean titers (GMTs) (95%CI) were 622 (510–757).Citation11 In the present study, GMTs in both the primary analysis and sensitivity analysis remained above the seroprotection threshold for JE of ≥ 10 [1/dil] throughout the 5-year follow-up period although sensitivity analysis GMTs were lower in years 3–5 ().
Table 3. Geometric mean titers of CVI-JE primed and boosted children aged 1–3 years up to 5 years post-booster
The GMTs of the 5 participants seropositive at the baseline of the previous study are shown in .
Table 4. Neutralizing antibody titers of 5 subjects seropositive at baseline of the previous study.Citation11
Serious adverse events
Neither cases of JE nor vaccine-related serious adverse events were reported during the 5-year follow-up period.
Discussion
We report the first study of antibody persistence for up to 5 years after a 2-dose primary immunization and booster 1 year later with CVI-JE in children aged 1–3 years. A protective immune response and GMTs above the threshold considered protective were observed in the majority of the participants. No vaccine-related serious adverse events occurred, and no cases of JE were observed during the 5-year follow-up post-booster.
In the present study, primary analysis SPRs gave less conservative estimates than sensitivity analysis SPRs that more accurately estimate of the performance of the vaccine due to the including any seronegative participants in the denominator for calculating the SPR in subsequent visits, and all post-booster comparison are after the first booster dose. We compare sensitivity analysis by the same method from reports in the literature where possible. Primary analysis SPRs at 28 days post-booster reported in our previous study were 100% and remained higher than 85% throughout the 5-year follow-up period in the present study. However, estimated SPR by the sensitivity analysis in years 4 and 5 were much lower at around 72% and 66%, respectively, and the Kaplan–Meier SPRs by years 4 and 5 were around 75% and 71%, respectively. Our results are similar to comparable studies of 5-year follow-up after 1-dose primary immunization with a live, attenuated JE chimeric vaccine (JE-CV) in children aged 12–18-month in Thailand and the Philippines, which reported sensitivity analyses SPRs of 59–67% at 5 years post-booster.Citation15,Citation16 The 5-year post-booster primary analysis SPRs of the present study were also comparable to studies in which JE-CV booster was given after primary immunization with either MBDI vaccine or JE-CV in children in the Philippines and Thailand, all of which had apparent SPRs of > 90%.Citation15,Citation17 CVI-JE had a similar primary analysis SPR (95%CI) compared with the inactivated Vero cell vaccine IC51 (IXIAROTM) in children aged 1–3 years at 2 years post-booster at 92.7% (86.1–96.8) vs 100% (94.6–100.0), respectively.Citation18 The primary analysis SPR (95%CI) of CVI-JE post-booster was slightly lower compared to a study in Korean children vaccinated with Beijing-1 strain inactivated Vero cell vaccine (ENCEVACTM) at 87.7% (78.5–93.9) vs 100% (91.6–100), respectively, and similar to a Nakayama strain MBDI vaccine at 4 years post-booster.Citation19
GMTs of CVI-JE in the present study declined rapidly over the first 2 years of follow-up, before appearing to generally plateau. GMTs were still above the threshold considered protective at 5 years post-booster although they were considerably lower from day 28 post-booster to 2- or 5-year post-booster than any studies in which either JE-CV or IC51 was the booster.Citation15,Citation17,Citation18 A slight rise in apparent GMT in the present study was seen between years 3 and 4 of follow-up, which is consistent with previous long-term JE vaccine immunogenicity reports in endemic settings.Citation15,Citation16 This may have been caused by natural boosting from wild-type JEV or other flaviviruses, which may cross-react with the JE PRNT50 test.Citation20 Natural boosting from wild-type JEV or other flaviviruses may contribute to CVI-JE antibody persistence in flavivirus-endemic settings.
There were limitations to the present study. The single-arm trial design did not allow for head-to-head comparison of the long-term immunogenicity and safety of CVI-JE with other contemporary JE vaccines. Results between testing laboratories may vary because no standardized protocol for the PRNT exists. Also, different endemic settings and study timings may be a source of heterogeneity between studies due to natural boosting from JEV or other flaviviruses. Therefore, interpretations of non-head-to-head comparisons should be treated with caution.Citation21,Citation22
Conclusion
A 2-dose primary immunization and booster 1 year later in children aged 1–3 years with CVI-JE provided long-lasting immunity for the majority of participants up to 5 years post-booster. No JE cases and no related SAEs were observed.
Acknowledgments
The authors would like to thank the children and parents who participated in this project. The authors would like to thank Dr. Saranath Lawpoolsri from Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS), Faculty of Tropical Medicine, Mahidol University, for performing the statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pearce JC, Learoyd TP, Langendorf BJ, Logan JG. Japanese encephalitis: the vectors, ecology and potential for expansion. J Travel Med. 2018;25(suppl_1):S16–6. doi:10.1093/jtm/tay009.
- Oliveira ARS, Cohnstaedt LW, Strathe E, Etcheverry L, McVey DS, Piaggio J, Cernicchiaro N. Meta-analyses of Japanese encephalitis virus infection, dissemination, and transmission rates in vectors. Am J Trop Med Hyg. 2018;98(3):883–90. doi:10.4269/ajtmh.17-0622.
- Kumari R, Kumar K, Rawat A, Singh G, Yadav NK, Chauhan LS. First indigenous transmission of Japanese encephalitis in urban areas of National Capital Territory of Delhi, India. Trop Med Int Health. 2013;18(6):743–49. doi:10.1111/tmi.12104.
- Xu Y, Zhaori G, Vene S, Shen K, Zhou Y, Magnius LO, Wahren B, Linde A. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J. 1996;15(11):1018–24. doi:10.1097/00006454-199611000-00017.
- Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89(10):766–74, 774A–774E. doi:10.2471/BLT.10.085233.
- Heffelfinger JD, Li X, Batmunkh N, Grabovac V, Diorditsa S, Liyanage JB, Pattamadilok S, Bahl S, Vannice KS, Hyde TB, et al. Japanese encephalitis surveillance and immunization - Asia and Western Pacific regions, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(22):579–83. doi:10.15585/mmwr.mm6622a3.
- Caldwell JP, Chen LH, Hamer DH. Evolving epidemiology of Japanese encephalitis: implications for vaccination. Curr Infect Dis Rep. 2018;20(9):30. doi:10.1007/s11908-018-0635-8.
- Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag. 2015;11:435–48. Published 2015 Mar 19. doi:10.2147/TCRM.S51168.
- Global Vaccine Safety. Information sheet observed rate of vaccine reactions Japanese encephalitis. Geneva (Switzerland): World Health Organization. 2019 Oct 5 [accessed 2019 Nov 22] https://www.who.int/vaccine_safety/initiative/tools/vaccinfosheets/en/ .
- Libao Z, Xin Z, Xutao W, Ligang W, Hui L, Miaomiao L. Adverse reaction and immunogenicity of inactivated Japanese encephalitis vaccine prepared on Vero cells. Chin J Biological. 2009;8:809–11.
- Chanthavanich P, Limkittikul K, Sirivichayakul C, Chokejindachai W, Hattasingh W, Pengsaa K, Surangsrirat S, Srisuwannaporn T, Kaewma B, Yoksan S, et al. Immunogenicity and safety of inactivated chromatographically purified Vero cell-derived Japanese encephalitis vaccine in Thai children. Hum Vaccin Immunother. 2018;14(4):900–05. doi:10.1080/21645515.2017.1414763.
- Food and Drug Administration. Code of Federal Regulations Title 21, Sec.600.80. Postmarketing reporting of adverse experiences, 2017. [ accessed 19 November 2019] http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=600.80 .
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23(45):5205–11. doi:10.1016/j.vaccine.2005.07.002.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Pancharoen C, Boaz M, Bouckenooghe A, Feroldi E. Long-term follow-up of Japanese encephalitis chimeric virus vaccine: immune responses in children. Vaccine. 2016;34(46):5664–69. doi:10.1016/j.vaccine.2016.09.018.
- Kosalaraksa P, Watanaveeradej V, Pancharoen C, Capeding MR, Feroldi E, Bouckenooghe A. Long-term immunogenicity of a single dose of Japanese encephalitis chimeric virus vaccine in toddlers and booster response 5 years after primary immunization. Pediatr Infect Dis J. 2017;36(4):e108–e113. doi:10.1097/INF.0000000000001494.
- Capeding MR, Alberto ER, Bouckenooghe A, Laot TM, Chansinghakul D, Monfredo C, Machabert T, Feroldi E. Five-year antibody persistence following a Japanese encephalitis chimeric virus vaccine (JE-CV) booster in JE-CV–primed children in the Philippines. J Infect Dis. 2018;217(4):567–71. doi:10.1093/infdis/jix601.
- Kadlecek V, Borja-Tabora CF, Eder-Lingelbach S, Gatchalian S, Kiermayr S, Sablan B Jr, Kundi M, Taucher C, Dubischar KL. Antibody persistence up to 3 years after primary immunization with inactivated Japanese encephalitis vaccine IXIARO in Philippine children and effect of a booster dose. Pediatr Infect Dis J. 2018;37(9):e233–e240. doi:10.1097/INF.0000000000002124.
- Yun KW, Lee HJ, Park JY, Cho HK, Kim YJ, Kim KH, Kim NH, Hong YJ, Kim DH, Kim HM, et al. Long-term immunogenicity of an initial booster dose of an inactivated, Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) and the safety and immunogenicity of a second JE-VC booster dose in children previously vaccinated with an inactivated, mouse brain-derived Japanese encephalitis vaccine. Vaccine. 2018;36(11):1398–404. doi:10.1016/j.vaccine.2018.01.075.
- Nealon J, Taurel AF, Yoksan S, Moureau A, Bonaparte M, Quang LC, Capeding MR, Prayitno A, Hadinegoro SR, Chansinghakul D, et al. Serological evidence of Japanese encephalitis virus circulation in Asian children from dengue-endemic countries. J Infect Dis. 2019;219(3):375–81. doi:10.1093/infdis/jiy513.
- Ferguson M, Johnes S, Li L, Heath A, Barrett A. Effect of genomic variation in the challenge virus on the neutralization titres of recipients of inactivated JE vaccines - report of a collaborative study on PRNT50 assays for Japanese encephalitis virus (JE) antibodies. Biologicals. 2008;36:111–16. doi:10.1016/j.biologicals.2007.07.002.
- WHO Expert Committee on Biological Standardization. Fifty-eighth report. Geneva (Switzerland): WHO Technical Report Series; 2011. Report No: 963.
