ABSTRACT
Human papillomavirus (HPV) is one of the most common causes of sexually transmitted diseases, and the main etiology of cervical cancer. This study was aimed to assess type-specific cervical HPV prevalence and their association with HPV-specific antibodies in a cohort of female university students. HPV genotyping was performed by amplifying and sequencing a fragment of the L1 protein. A BLAST search was performed to identify HPV types. HPV-specific IgG antibodies were measured by ELISA in serum samples. A total of 129 women participated, with an average age of 21.75 years. The prevalence of vaginal HPV infection was 74.42%. The most predominant high-risk HPV types were 18 (13.95%), 31 (10.85%), and 16 (9.3%). We found that early age at coitarche and a higher number of sexual partners were significantly associated with a high prevalence of HPV infection. In addition to sexual behavior, we observed that the presence of serum-specific IgG antibodies against HPV can impact the prevalence of the virus. Seropositivity to HPV-16 and HPV-18 was associated with a lower prevalence of HPV-16, but not for other HPV types. Of note, there was a lower proportion of HPV-specific seropositivity in women who had the presence of the same HPV type in a cervical specimen, suggesting an immunoregulatory mechanism associated with the viral infection. In conclusion, the prevalence of HPV in university women was higher than expected and it was associated with early age of sexual debut, an increasing number of sexual partners, and a low proportion of HPV seropositivity.
Introduction
Human Papillomavirus (HPV) infection is among the most common sexually transmitted infections. Papillomaviruses are small non-enveloped viruses with around 8 kb of circular double-stranded DNA genomes including a non-coding regulatory long control region (LCR), and eight open reading frames (ORF): L1 and L2 that encodes capsid proteins; E1, E2, E4, E5, E6, and E7 that encodes proteins involved in replication, transcription, and transformation.Citation1 HPV types are established when the DNA sequence of the L1 differs by at least 10%. More than 170 types of HPV have been identified; approximately 40 of them infect the epithelium in the genital tract, the mucosa of the upper respiratory tract, and the skin developing epidermodysplasia verruciformis.Citation2,Citation3 HPV infection has a central role in common dermatologic and sexually transmitted diseases as well as in one of the most frequent cancers worldwide.Citation4,Citation5 The risk of being infected by HPV at least once in a lifetime among both men and women is calculated as 50%.Citation6 However, in the majority of cases, HPV infection is transient or asymptomatic and is resolved spontaneously.Citation7 According to their oncogenic potential, HPV are classified as low-risk (wart-causing) and high-risk (cancer-causing) viruses.Citation7 Persistent infection with high-risk types is associated with precancerous and cancerous lesions.Citation8 High-risk HPV types 16, 18, 31, 33, and 35 have been associated with cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancers and with pre-cancer lesions.Citation9,Citation10 HPV types 16 and 18 are the most common high-risk types worldwide and are considered to be responsible for more than 70% of all cervical cancer cases.Citation11,Citation12 In Mexico, previous studies have identified HPV types 16, 18, 31, 45, 51, 58, and 59 as the most prevalent in cervical samples.Citation13–16
Prophylactic HPV vaccination was introduced in 2006 with the development of two vaccines: the bivalent vaccine (Cervarix) containing HPV 16 and 18 antigens, and the quadrivalent vaccine (Gardasil) containing HPV 6, 11, 16, and 18 antigens. Recently, a nonavalent vaccine (Gardasil 9) was approved, protecting against infection from HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. These vaccines are made with L1 viral proteins that assemble into morphologically similar particles to the virus but do not contain its genome, the so-called virus-like particles (VLP). Different studies have demonstrated the efficiency of HPV vaccination in preventing infection with the types of HPV they target when given before initial exposure to the virus. High-risk HPV-16 and 18 are virtually preventable by vaccination.Citation17,Citation18 In Mexico, vaccination against HPV was first introduced in 2008 with low coverage to girls aged 12–16 years using a 0–6 month schedule; 1 year later, it changed to an extended dosing schedule that targets girls aged 9–12 years for the first 2 doses, applied 6 months apart, followed by a third dose 60 months later.Citation19 The vaccine was included in the national vaccine program until 2012.Citation9 The coverage has been increased over time; according to the last reported data in 2018, about 1 million doses were applied in Mexico.Citation20 However, this number is still very low considering the whole population of 125 million persons, from who 5.7% are females between 9 and 14 years old.Citation21
Despite the demonstrated benefits of HPV immunization, there are still many obstacles to implement the vaccination schedules in high- and low-income countries. Those barriers are multifactorial and include limitations in costs, infrastructure, and even social stigma.Citation22 Due to these difficulties in less developed countries the prevalence of HPV infections remains high. Mexico is within the regions with a high rate of HPV infection.Citation9,Citation12 Several studies in different regions have shown the highest prevalence of HPV in women younger than 25 years of age and college women because of their risky sexual behavior, lack of knowledge of HPV infection, low rate of vaccination, and other cultural factors.Citation23–25 Because of the need for data that support the actual status of the prevalence of HPV infection in our country as well as the levels of specific antibodies in individuals at high risk of HPV infection the current study was designed to identify HPV prevalence, genotypes, and seropositivity to help establish appropriate preventive strategies for a high-risk population.
Material and methods
Study population
One hundred twenty-nine female students who attended gynecological consultation at the Clinica Universitaria de Salud Integral Iztacala (CUSI) from March 2017 to April 2018 were included. All the subjects that provide informed consent were eligible for participation. This study was conducted with the approval of the Institutional Review Board of Facultad de Estudios Superiores Iztacala (FESI). The clinical data for the study was extracted from the clinical history of each individual.
Preparation of DNA from cervical cell swabs
Porous foam-tipped swabs (Epicenter Biotechnologies, Madison, WI, USA) were used for the collection of cervical exfoliated epithelial cells from patients. Cervical DNA samples were extracted with DNA Extraction Kits (Epicenter Biotechnologies). Samples were processed according to the manufacturer’s instructions.
DNA genotyping
Viral DNA was amplified with polymerase-chain reaction in the L1 region of the HPV genome, with human beta-actin as the reference gene. The sequences of primers used in this were three consensus primer pairs, L1C1 ⁄ L1C2 (Fw: 5ʹCGTAAACGTTTTCCCTATTTTTTT 3’, Rev 5ʹTACCCTAAATACTCTGTATTG 3’), GP5+ ⁄ 6+ (5’ TTTGTTACTGTGGTAGATACTAC3’, Rev 5’ GAAAAATAAACTGTAAATCATATTC 3’, and actin (5’ GCACAGGGACATAATAATGG 3’, Rev 5’ CGTCCAAAAGGAAACTGATC 3’). Polymerase chain reaction amplification was done in a 50 μL reaction mixture. Thermal cycling conditions were as follows: denaturation at 95°C for 10 minutes followed by 40 cycles of denaturation at 95°C for 45 seconds, annealing at 50°C for 55 seconds, and extension at 72°C for 1 minute. The amplification program was followed by a final extension step at 72°C for ten minutes. The PCR products were electrophoretically migrated on 1.5% agarose gels and the bands corresponding to the specific amplicons were cut off from gels. Then DNA was extracted using the WizardRSV Gel and PCR Clean-Up System. Every product was used as a template to conduct a sequencing reaction with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Austin, TX, USA), according to the manufacturer’s procedures. The sequences were analyzed using the ABI PRISM 310 system (Applied Biosystems). The sequence data obtained by automated DNA sequencing were analyzed using the BLAST search (http://www.ncbi.nlm.nih.gov/ BLAST/, accessed in 2017–2018) for HPV genotypes. DNA sequencing identified the most dominant genotype in a given specimen, and it served as a confirmatory assay.
HPV-negative detected samples were confirmed by qPCR using the consensus primer set GP5+/GP6+. All qPCR was carried out using an StepOne Real-Time PCR System (Applied Biosystems) with Maxima SYBR Green/ROX qPCR Kit (Thermo Scientific, USA). Reactions comprised 1× SYBR Green mix, 50 nM primer pairs, and 5 μl of template. The following cycling conditions were employed: initial denaturation 95°C 15 minutes, followed by 40 cycles of 95°C 15 seconds, 46°C 1 minute, 72°C 1 minute. A final extension of 72°C 10 minutes, and melting curve of 95°C 15 seconds, 60°C 1 minute, 95°C 15 seconds transition were incorporated.
HPV ELISA
Antibodies against HPV were measured by a VLP-based direct enzyme-linked immunoabsorbent assay (ELISA) base on the recommendations of the World Health Organization.Citation26 Maxisorp Nunc-Immuno plates (Thermo Fisher Scientific, MA, USA) were coated with 1 µg/ml of either purified recombinant HPV-16 (Abcam, Cat. ab119880) or HPV-18 (Abcam, Cat. ab119881) L1 proteins in phosphate-buffered saline (PBS), pH 7.2 at 4°C overnight. The plates were blocked with ELISA Assay Diluent (Biolegend, Cat. 421203) at 4°C overnight. Plates were incubated with serum samples diluted 1:1000 in ELISA Assay Diluent for 2 hrs at 4°C. After washing the wells 5 times with PBS containing 0.1% Tween 20 (Sigma-Aldrich), HRP-conjugated goat anti-human IgG (Abcam, Cat. ab6858) diluted 1:7500 was added and incubated at room temperature for 1 hr. Following an additional washing cycle, TMB ELISA Substrate (Abcam, Cat. ab171527) was added and incubated at room temperature for 15 minutes, followed by the addition of 1 M H2SO4. The absorbance was measured at 490 nm. To control the inter, and intra-assay variation it was included a positive control serum, identified during the optimization process of the immunoassay, diluted in the same way as the specimens in every ELISA plate. For data analysis, it was first calculated the net mean OD value for each sample by subtracting the mean blank OD. Then a normalized absorbance ratio (NAR) was obtained by dividing the net mean OD value of each sample by the net mean OD from the positive control serum included in the same plate, similar to the approach reported by Ramanakumar et al.Citation27 aimed to reduce the effect of inter-, and intra-assay variations. The seropositivity cut points were determined by three standard deviations above the mean values obtained from a pool of HPV-negative samples.
Heatmap analysis
The heatmap analysis of seropositivity for HPV16 and HPV18 versus vaccination and infections status was made with the free online software Heatmapper (http://www.heatmapper.ca).Citation28
Statistical analysis
The statistical analysis was performed with the GraphPad Prism 7.0 statistics package software. The study data were expressed in terms of arithmetic mean, standard deviation, number, and percentage. Differences between prevalence were analyzed by Fisher’s exact test. P-values less than 0.05 were considered statistically significant (*P < .05; **P < .01; ***P < .001).
Results
HPV prevalence
A total of 129 female university students were recruited voluntarily. The analyzed group consisted of students from the following careers: Biology (25%), Optometry (12%), Medicine (18.4%), Psychology (30.2%), and Odontology (14.4%). The mean age of the study population was 21.75 ± 2.86 with a range of 17 to 35 years. The clinical characteristics of the patients are grouped in (supplementary material). The overall incidence of HPV infection in cervical samples was 74.4% (96 students) (). Among the HPV-positive women, 70 were positive for a high-risk HPV type, accounting for 72.9% (70/96) of the HPV infection, and 26 students were positive for low-risk HPV types. The different HPV genotypes assessed had different prevalence. The frequencies of infection with low-risk HPV types 6 and 11 were 16.3% (21 students) and 3.9% (5 students), respectively (). Concerning genotype distribution of high-risk types viruses, the higher prevalence was for HPV-18 (14%). HPV-31 was the second most frequent genotype (11%), followed by HPV-16 (9%), HPV-51 (9%), and HPV-59 (5%). Moreover, we also detect the presence of the HPV genotypes 39, 45, 53, 66, 68, and 90 in very low frequencies (data not shown). Twenty-three HPV negative samples were further evaluated by qPCR. As expected, even the higher sensitivity of the fluorescent qPCR method, the 23 samples analyzed were confirmed as HPV-negative (data not shown). We did not find multiple HPV genotypes in any sample.
Table 1. Clinical characteristics of the participants. IUD = Intrauterine device
Figure 1. The overall prevalence of HPV genotypes in female university students. a) The infection prevalence is expressed as the number of individual positives for each genotype. From 129 samples, 74.41% (96 students) were positive for HPV (gray bar). b) Comparison of the most common HPV genotypes detected in all samples. The prevalence of most common high-risk HPV was HPV-18 13.95%; HPV-31 10.85%; HPV-16 9.3%, HPV-51 9.3%; and HPV-59 5.4%. c) Age distribution of sexual debut among students. The median age of coitarche for HPV- was 17.5 and for HPV+ girls was 17 years, which were used as a cutoff value for stratification of the girls by age. Fisher’s exact test showed that girls over 17 years old have a higher risk of HPV infection (P = .016). d) Comparison of the distribution of sexual partners among HPV- and HPV+ girls showed a statistically significant association between HPV infection and an increased number of lifetime sexual partners (Fisher’s exact test, P = .038). The cutoff value was the median number of lifetime sexual partners that was 3 for HPV- girls and 3.5 for HPV+ girls.
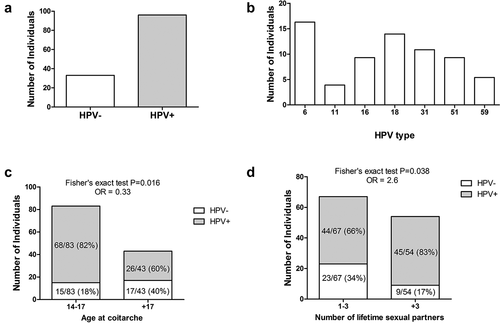
The average age of sexual debut among students was 17.1 ± 1.9 years old. Among the HPV negative and positive cases, the average age at coitarche was 17.8 ± 2, and 16.9 ± 1.9, respectively. To analyze whether there was an association between the age of sexual debut and genital HPV infection, we stratified the population of girls into two age groups, the cutoff value for age distribution was the median age that was 17.5 for the HPV- group and 17 for HPV+ girls. We found a statistically significant association between early age at coitarche and the presence of HPV infection, the frequency of infection at early age (14–17 years) of sexual debut was 82% in comparison with 60% when girls were above 17 years old (, Fisher’s exact test P = .016). Regarding the number of lifetime sexual partners, the mean was 4.16 ± 3.85 for the whole group. For HPV-negative girls, the mean number of sexual partners was 3.6 ± 3.6, and for the HPV+ group was 4.4 ± 3.9. There was a significant association between HPV infection and the number of lifetime sexual partners (, Fisher’s exact test P = .038). The cutoff value for the distribution was the median number of sexual partners that was 3 for HPV- girls and 3.5 for HPV+ girls.
Seroprevalence and HPV infection
Of the 129 individuals enrolled, 38 reported having received at least one vaccination against papillomavirus, while 86 were not vaccinated, and 5 have no record. The girls without vaccination records were excluded from the analysis. The percentage of vaccination was 30.6. Among vaccinated women 17 had received one dose; the same number had received two doses, and 4 had three doses. Sexual behavior analysis indicates that the median age of sexual debut and the median number of lifetime sexual partners were not significantly different between vaccinated and unvaccinated girls (T-test P = .245 and P = .822, respectively). In addition, vaccine effectiveness (VE) was analyzed against incidence of infections as follows: VE = (Risk among unvaccinated group – Risk among vaccinated group)/Risk among unvaccinated group. In our study, the vaccine effectiveness against incident HPV 16/18 infection was 58.1%; nevertheless, a cross-protective HPV infection was not observed since the vaccine effectiveness was 4.9% for all HPV genotypes infection.
Next, we compare the prevalence of the main HPV types found in our cohort in unvaccinated girls, and those with one, two or three doses of HPV vaccine. We found a downward trend in the prevalence of infection as the number of doses of the vaccine increased for HPV6, HPV16 and HPV31 (). In contrast, for HPV18 there were not differences observed for one and two doses, but there was an increase in the prevalence in girls with three doses of the vaccine. For HPV51 there was a decrease of the prevalence in girls with one dose of the vaccine, but not differences were observed in the group with two doses, and similarly to HPV18 in girls with three doses there was an increase in the prevalence.
Figure 2. HPV-vaccination decrease the prevalence of some HPV types. a) The effect of HPV vaccination was evaluated in girls who received one, two or three doses of the vaccine in comparison with non-vaccinated girls. The percentages of infections were for HPV6 16% (12/75 girls), 12% (2/17), 12% (2/17) and 0% for unvaccinated, one dose, two doses and three doses of the vaccine, respectively. For HPV16 were 9% (7/75),18% (3/17), 6% (1/17), and 0%; for HPV18 were 12% (9/75), 18% (3/17), 12% (2/17), and 50% (2/4); for HPV31 were 15% (11/75), 12% (2/17), 6% (1/17), and 0%; and for HPV51 were 8% (6/75), 0%, 12% (2/17), and 25% (1/4). Data were analyzed by Fisher’s exact test. P-values less than 0.05 were considered statistically significant (*P < .05; **P < .01; ***P < .001). b) Percentage of seropositive individuals for HPV-16-specific IgG antibodies (left graph), and IgG anti-HPV-18 antibodies (right graph) in girls based on their vaccination status. Forty-three percent (32 of 75) of unvaccinated girls were seropositive for HPV16 and 41% (31/75) for HPV18. Forty-four percent (7/16) of vaccinated girls were seropositive for HPV16 after one dose of the HPV vaccine, 56% (9/16) for two doses, and 75% (3/4) for three doses. For HPV18 38% (6/16) of vaccinated girls were seropositive for one dose of the vaccine, while 56% (9/16) and 75% (3/4) were seropositive after two and three doses, respectively. c) Prevalence (percentage) of specific HPV infection in girls that were seropositive for HPV16 (left graph) or HPV18 antibodies (right graph). White bars represent unvaccinated girls and gray bar vaccinated girls. Data were analyzed by Fisher’s exact test. P-values less than 0.05 were considered statistically significant (*P < .05; **P < .01; ***P < .001).
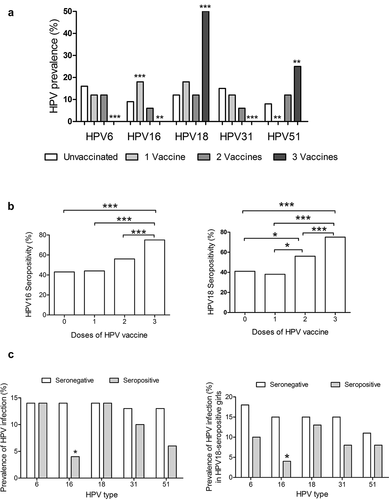
The increased rate of infection for HPV18 after vaccination was unexpected, but it could be related to a decay in the levels of specific antibodies along the time according to some reports. For that reason, we decided to evaluate the presence of specific serum IgG antibodies against HPV16 and HPV18. The seropositivity observed between unvaccinated and vaccinated girls showed an evident impact of HPV vaccination from two doses of the vaccine, and it increased with the third dose (). The prevalence of HPV infection was affected by the presence of specific antibodies against the virus; the analysis with specific HPV types showed similar frequencies of infection in seronegative girls: HPV6, HPV16, and HPV18 have a prevalence of 14% (9/63 girls), and for HPV 31 and HPV51 the prevalence was of 13% (8/63). In contrast, for seropositive girls there was a significant reduction for HPV16 with only 2 of 51 girls infected (4%), for HPV 31 and HPV51 there was a slight decrease in the prevalence, but it was not statistically significant (); no effect was observed for other HPV types. For HPV-18 seropositivity, even though there were no significant changes in the prevalence of HPV-18 infection, it was observed a slight decrease, similarly for HPV6, HPV31, and HPV51 in seropositive girls (right graph in ).
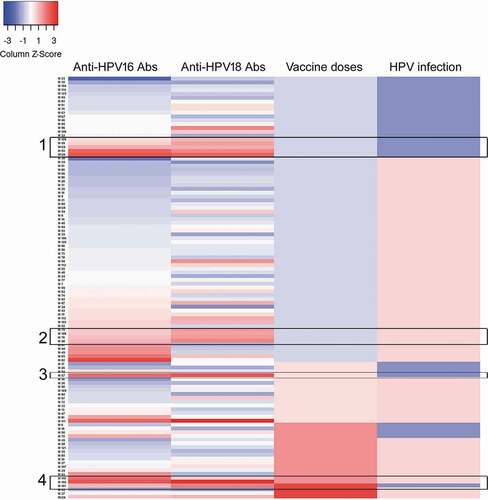
Of note, we observed in HPV-18 seropositive girls a significant decrease in the prevalence of HPV16 infection (), which could probably be due to the presence of specific antibodies for HPV-16 in the same individuals. For that reason, we perform a heatmap that shows the seroprevalence for both types of antibodies in all the individuals analyzed together with the infection and vaccination status (). There were 34 girls that showed seropositivity for both HPV16 and HPV18, while 17 girls were seropositive only for HPV16, and 15 girls seropositive only for HPV18. Interestingly, the double seropositivity can be acquired by both natural infection according to data displayed in the rectangles 1 and 2, or by vaccination as observed in the rectangles 3 and 4 ().
Figure 3. Heatmap analysis of seroprevalence, infection, and vaccination. Analysis of the levels of specific antibodies against HPV16 and 18, and their association with the presence of HPV infection and vaccination status were analyzed by heatmap. For the antibody levels the scoring star with blue color for negative or low levels and change to red color for higher amounts of antibodies detected by ELISA. For the vaccination status, the blue color represents unvaccinated girls and the increasing intensity in red represents 1, 2, or 3 doses of the HPV vaccine. Finally, for HPV infection blue color represent HPV- girls and light red color represent girls with HPV infection (any HPV). Each horizontal line represents one girl, and the sample number is indicated in the left side of the graph. Rectangles 1 and 2 show unvaccinated double seropositive girls, while rectangles 3 and 4 show vaccinated double seropositive girls.
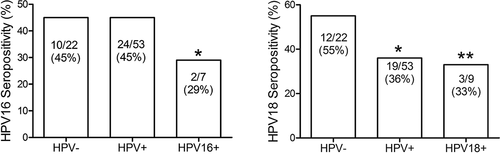
Finally, to assess whether the presence of HPV infection could affect the immune response against the virus we compare in the group of unvaccinated girls the seroconversion rate between HPV-, HPV+ or specific HPV for the antibodies measured (). We found that seroconversion was decreased for anti-HPV16 antibodies when the girls were positive for HPV16, but not for other genotypes (Fisher exact test, P = .027). Similarly, this effect was also observed for HPV18 seroconversion in the presence of HPV18 infection (P = .002), however for HPV18 the inhibitory effect in the seroconversion was also observed with the group HPV+ that include all genotypes of HPV detected.
Figure 4. HPV seropositivity according to cervical HPV infection status. Percentages of HPV16 or HPV18 seropositive girls, which were grouped in those negative for HPV (HPV-), positive for any HPV (HPV+), and positive only for HPV-16 (HPV-16+) or HPV-18 (HPV-18+). Data were analyzed by Fisher’s exact test. P-values less than 0.05 were considered statistically significant (*P < .05; **P < .01).
Discussion
Human papillomavirus is an infectious agent of epithelial tissue with high clinical relevance for its association with the generation of cervical carcinoma. HPV is considered to be among the most common sexually transmitted infections. The HPV infection is detected frequently in young women; the epidemiological data indicated that at least 70% of sexually active women are infected at least once during their lifetimes.Citation8 In this study, a group of women attending an outpatient clinic for gynecological exams were assessed for molecular detection of HPV in cervical samples, showing that the overall HPV infection rate in the students that were analyzed was 74.4%. In comparison to other studies, the frequency reported in our research was higher than the world prevalence of 10.4%, and some regional rates such as Asia (8%),Citation12,Citation29 Africa (22.1%), Central America, Mexico (20.4%), and North America (11.3%).Citation30,Citation31 However, the population in our study is considered at high risk for HPV infection according to several studies in which college young women had a high prevalence. This population has been reported to have a prevalence of 30–50%.Citation23–25 Moreover, it is important to consider the fact that young women attending clinics often do so because of signs or symptoms of a genital tract infection, which increases the possibility of having a sexually transmitted infectious disease. The high incidence of HPV infection reported in this study is closely related to the different risk factors associated with the participants such as the coitarche age between 14 and 17 years of age (65%), multiple sexual partners (4 on average). Domínguez et al. mention that the main risk factors for HPV infection in women under 25 years of age are both the early sexual intercourse debut and having multiple sexual partners.Citation32 Another reason to which we attribute the high incidence of HPV infection was due to the fact that a significant percentage of the patients attended a gynecological evaluation for both presence of condylomatosis or another infection as observed in supplementary Table 1.
Understanding the HPV prevalence and genotype distribution is important for the planning of diagnostic and preventive strategies toward HPV-related diseases. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 are the most common in the general female population worldwide, accounting for 70% of HPV infections in the presence of normal cytological findings.Citation10 However, some reports have shown that the prevalence and genotype distribution of HPV varies greatly all over the world.Citation12 In our cohort, the HPV infection was associated mainly with high-risk viruses (72.9%), which increase the possibility of cervical cancer development. Some studies have reported that HPV-58 is the most frequent genotype in the southern regions of Mexico, with a prevalence of 55.9%.Citation14 Further, Gallegos-Bolaños et al. in 2017 reported that the HPV-16, 33, 51, and 52 were the most prevalent types in Mexico CityCitation33. In our study, we found that HPV-18 showed the highest prevalence (14%) followed by genotypes 31, 51, and 16. The discrepancy in prevalence with the Gallegos-Bolaños study might be due to population differences because our study included women from Mexico City and the State of Mexico. In line with this hypothesis, it has been observed that depending on the geographical region, some viral types are more frequently found than others. For example, HPV types 33 and 31 are more prevalent in Europe and the USA, types 35 and 45 in Africa, and types 52 and 58 are more frequently observed in Asia.Citation12,Citation34 Moreover, the age range was also different between both studies, which also can account for the different prevalence observed. Regarding sexual behavior, we found an association of HPV prevalence with the early age of sexual debut and with a higher number of lifetime sexual partners, these sexual behaviors have been previously reported as a risk factor for HPV infection in different populations.Citation35,Citation36 Taking into account the proportion of girls that had an early sexual debut (65.9%), and the proportion of those who have had more than three sexual partners (46.6%), the population analyzed here can be considered as a high-risk population for HPV infection, which is confirmed by the high prevalence of the virus observed.
In addition to sexual behavior, immunization against HPV is critical to prevent dissemination of the viral infection, especially in young populations. The low vaccination rate (30%) reported in this study could be in part responsible for the high prevalence observed for HPV infection. The low HPV vaccination coverage in our cohort reflects the fact that the HPV vaccine was introduced in Mexico in 2008 with very low coverage, and it was included in the national vaccine program until 2012 with only 52 thousand doses applied.Citation9,Citation20 Between 2013 and 2018 an average of 1.4 million doses were administered every year,Citation20 this amount covered less than one-third of the female population between 9 and 14 years of age, which was assessed at 6.5 million in the year 2020.Citation21
Based on the effectiveness of HPV vaccine in our study, we found that the efficacy of vaccination is lower than other reports. Basu et al. showed that a single dose of HPV vaccine provides a similar protection against persistent infection from HPV 16 and 18 to that provided by two or three doses. The authors mention that adjusted vaccine efficacy against incident HPV 16/18 infection was 63.5% with a single dose with very low variation with two or three doses.Citation37 In our study, the effectiveness was 58.96% against incident HPV 16/18. We could attribute these differences to some sociodemographic characteristics of the participants, for example, in our study, the coitarche age was before 20 years of age and most patients have had multiple sexual partners, which increases the risk to HPV exposure compared to the low-risk exposure of women studied by Basu et al. The sociodemographic differences between the participants could explain the differences in vaccine effectiveness in both populations. It would be interesting to increase the number of participants to assess the vaccine effectiveness, in both, incident and persistent HPV 16/18 infection.
The impact of HPV vaccination in our cohort was evident from the second dose of the vaccine, in contrast with other studies that reported that one dose can induce seroconversion in most individuals, especially with HPV16.Citation38,Citation39 A possible explanation is a decay in the levels of the specific antibodies to HPV16 and HPV18 along the time as it has been described in other reports, where the seropositivity decay after 18 and 36 months.Citation40,Citation41 This possibility may be the main reason in our cohort because the average age of the girls was 22 years, meaning that they have around 10 years post vaccination. According to the study of Sankaranarayanan et al. in 2016, the levels of antibodies against HPV in girls with one dose of the HPV-vaccine can decrease below the limit of seropositivity after 36 months of vaccination.Citation41
Another factor that affects the seropositivity is the fact that in previous studies they have excluded girls with positive infection at the time of recruitment, this condition in our cohort is unknown and it could affect the development of the immune response according to some reports that showed that HPV infection delays the seroconversion and even prevent it.Citation42,Citation43 Indeed, in one report the efficacy of the vaccine among individuals with cervical HPV16 or HPV18 DNA detected at enrollment was only 25% compared with 85% for women who were negative for HPV.Citation44 To assess this possibility, we compare the seroconversion rate between HPV-, HPV+, HPV16+, or HPV18+ girls (). We only performed this analysis with unvaccinated girls to avoid the seroconversion due to vaccination. In agreement with the previous reports mentioned early, we found a decreased rate in the seroconversion when the girls were infected by the same HPV genotype of the antibodies analyzed. This delay or decrease in seroconversion could be due to some immune-regulatory mechanisms associated with the virus such as the induction of anti-inflammatory cytokines like IL-10 in keratinocytes, macrophages, and Langerhans cells.Citation45 Moreover, it has also been reported an increased frequency of regulatory T cells in cervical lesions that had persistent HPV16 infection.Citation46
Of note, the prevalence of HPV-16 infection was significantly decreased in girls that were HPV-16 seropositive in comparison with those that were negative for HPV-16-specific antibodies, but the prevalence of other HPV types was unaffected. This clearly shows that the induction of specific antibodies to the L1 protein of HPV-16 is protective against the infection with HPV16, but they are not protective against other types of HPV. This is in line with other clinical studies.Citation47–50 In contrast, the prevalence of HPV-18 infection in seronegative and seropositive girls for HPV-18 specific antibodies was similar between both groups. This might reflect a less effective immunization for HPV18 antigens or shorter protective levels of neutralizing antibodies, in support of this presumption in other reports the levels of antibodies against HPV18 are lower than those observed for HPV16 after vaccination, and they have a faster decay effect along the time.Citation39–41 Interestingly, we observed that HPV18 seropositive girls had a reduction in the number of HPV16 infections, suggesting the presence of seropositivity for this virus that could be associated with vaccination, which includes both viruses. In a heatmap analysis, we corroborate that 34 of 49 girls that were positive for HPV18-antibodies were also positive for antibodies to HPV16, nevertheless, this double seroconversion is not only associated with vaccination, but also with natural infection. This suggests that unvaccinated girls, that were seropositive for both HPV16 and HPV18 viruses were infected by both viruses or there is a crossed immune response, this last possibility could also explain why HPV18 seropositive girls did not have a significant reduction in the rate of infection because crossed immune response is less effective than direct immune response against the specific HPV.Citation51–53
This study has some limitations that should be considered. Though there was statistically significant analysis between variables, the number of patients included in the study was low. Only some risk factors related to sexual behavior were recorded during enrollment like the number of lifetime sexual partners and age of sexual debut, but others like the type of sexual activity, use of condoms, number of current sexual partners are missing.
In conclusion, this study shows that the incidence of high-risk HPV infection was higher than that reported previously in some regions of Mexico, being the most common genotypes HPV-18, HPV-31, and HPV-51 for female college students. The prevalence and genotype distribution of HPV provides the basis for designing HPV prevention programs including vaccination. The observation that HPV-specific antibodies are mainly protective for the virus used in immunization is very important for the application of HPV vaccines in each geographic region where the viral types can change, and it suggests that the new nonavalent vaccine would be more beneficial in our population, that have a prevalence of diverse HPV types. Furthermore, the coverage of early vaccination in girls who are naïve to HPV infection is of vital importance to reduce the prevalence of HPV. Our study was focused on a high-risk population for HPV infection; therefore, the data obtained can be used for the design of strategies aimed to target this population.
Consent to participate
Written informed consent was obtained from each patient before blood and tissue donation.
Consent for publication
This work has not been published before; it is not under consideration for publication anywhere else, and its publication has been approved by all co-authors.
Ethics approval
All procedures performed in studies involving human participants were following the ethical standards of the institutional research committee of the FES Iztacala and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Supplemental Material
Download MS Word (23.7 KB)Acknowledgments
We thank Estanislao Antonio Calixto for the technical assistant.
Data availability statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2028514
Additional information
Funding
References
- Bravo IG, Felez-Sanchez M. Papillomaviruses: viral evolution, cancer and evolutionary medicine. Evol Med Public Health. 2015;2015:32–12. doi:10.1093/emph/eov003.
- Aguilar-Lemarroy A, Vallejo-Ruiz V, Cortes-Gutierrez EI, Salgado-Bernabe ME, Ramos-Gonzalez NP, Ortega-Cervantes L, Arias‐Flores R, Medina‐Díaz IM, Hernández‐Garza F, Santos‐López G, Piña‐Sánchez P. Human papillomavirus infections in Mexican women with normal cytology, precancerous lesions, and cervical cancer: type-specific prevalence and HPV coinfections. J Med Virol. 2015;87:871–84. doi:10.1002/jmv.24099.
- Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, et al. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int J Oncol. 2018;52:637–55. doi:10.3892/ijo.2018.4256.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F.
- Handler NS, Handler MZ, Majewski S, Schwartz RA. Human papillomavirus vaccine trials and tribulations: vaccine efficacy. J Am Acad Dermatol. 2015;73:759–67. quiz 67-8. doi:10.1016/j.jaad.2015.05.041.
- Human Papillomaviruses. 90 IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer. 2007;689. ISBN:978-92-832-1290-4 .
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi:10.1016/j.vaccine.2008.05.064.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC information centre on HPV and cancer (HPV information centre). Human papillomavirus and related diseases in Mexico. Summary Rep. 2019 Jun;17:1–171 .
- Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, Anh P, Ferreccio C, Hieu NT, Matos E, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–98. doi:10.1016/S0140-6736(05)67069-9.
- Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi:10.1038/sj.bjc.6600688.
- de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, de Sanjosé S, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–59. doi:10.1016/S1473-3099(07)70158-5.
- Giuliano AR, Papenfuss M, Abrahamsen M, Denman C, de Zapien JG, Henze JL, Ortega L, Brown de Galaz EM, Stephan J, Feng J, et al. Human papillomavirus infection at the United States-Mexico border: implications for cervical cancer prevention and control. Cancer Epidemiol Biomarkers Prev. 2001;10:1129–36.
- Gonzalez-Losa Mdel R, Rosado-Lopez I, Valdez-Gonzalez N, Puerto-Solis M. High prevalence of human papillomavirus type 58 in Mexican colposcopy patients. J Clin Virol. 2004;29:202–05. doi:10.1016/S1386-6532(03)00138-0.
- Jacome-Galarza I, Ito-Nakashimada MA, Figueroa-Aguilar G, Garcia-Latorre E, Salazar MI, Lopez-Orduna E, Camacho AD, Valdez-Alarcón JJ, Hernández JM, León-Avila G, et al. Prevalence of human papillomavirus in women from the state of Michoacan, Mexico, showed high frequency of unusual virus genotypes. Rev Invest Clin. 2017;69:262–69. doi:10.24875/ric.17002065.
- Velazquez-Marquez N, Paredes-Tello MA, Perez-Terron H, Santos-Lopez G, Reyes-Leyva J, Vallejo-Ruiz V. Prevalence of human papillomavirus genotypes in women from a rural region of Puebla, Mexico. Int J Infect Dis. 2009;13:690–95. doi:10.1016/j.ijid.2008.10.010.
- Drolet M, Benard E, Perez N, Brisson M, Ali H, Boily M-C, Baldo V, Brassard P, Brotherton JML, Callander D. Group HPVVIS. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi:10.1016/S0140-6736(19)30298-3.
- Kjaer SK, Nygard M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339–45. doi:10.1093/cid/cix797.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4:e453–63.
- World Health Organization estimates of human papillomavirus immunization coverage. 2019. [accessed 2020 Dec 14]. https://www.who.int/immunization/monitoring_surveillance/data/HPV_estimates.xls.
- Censo de población y vivienda 2020 . Mexico: Instituto Nacional de Estadística, Geografía e Informática (INEGI). [accessed 2020 Oct 22].https://www.inegi.org.mx/programas/ccpv/2020/
- Dilley S, Miller KM, Huh WK. Human papillomavirus vaccination: ongoing challenges and future directions. Gynecol Oncol. 2020;156:498–502. doi:10.1016/j.ygyno.2019.10.018.
- Kotloff KL, Wasserman SS, Russ K, Shapiro S, Daniel R, Brown W, Frost A, Tabara SO, Shah K. Detection of genital human papillomavirus and associated cytological abnormalities among college women. Sex Transm Dis. 1998;25:243–50. doi:10.1097/00007435-199805000-00005.
- Revzina NV, Diclemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS. 2005;16:528–37. doi:10.1258/0956462054679214.
- Herrera-Ortiz A, Conde-Glez CJ, Olamendi-Portugal ML, Garcia-Cisneros S, Plett-Torres T, Sanchez-Aleman MA. College women, HPV genotyping and sexual behavior before HPV vaccination: results from samples stored for a long time. J Infect Public Health. 2018;11:286–89. doi:10.1016/j.jiph.2017.08.014.
- World Health Organisation. Human papillomavirus laboratory manual. Geneva: World Health Organization; 2010.
- Ramanakumar AV, Thomann P, Candeias JM, Ferreira S, Villa LL, Franco EL. Use of the normalized absorbance ratio as an internal standardization approach to minimize measurement error in enzyme-linked immunosorbent assays for diagnosis of human papillomavirus infection. J Clin Microbiol. 2010;48:791–96. doi:10.1128/JCM.00844-09.
- Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–53. doi:10.1093/nar/gkw419.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi:10.1016/S1470-2045(10)70230-8.
- Yang J, Wang W, Wang Z, Wang Z, Wang Y, Wang J, Zhao, W, Li, D, Liu, H, Hao, M, . Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: a population-based survey of 10086 women. Hum Vaccines Immunother. 2019;16(7):1–8.
- Pierce Campbell CM, Curado MP, Harlow SD, Soliman AS. Variation of cervical cancer incidence in Latin America and the Caribbean. Rev Panam Salud Publica. 2012;31:492–98. doi:10.1590/S1020-49892012000600007.
- Dominguez Bauta SR, Trujillo Perdomo T, Aguilar Fabré K, Hernandez Menendez M. Infeccion por el virus del papiloma humano en adolescentes y adultas jovenes. Rev Cub Obst Y Ginecol. 2018;44:1–13.
- Gallegos-Bolanos J, Rivera-Dominguez JA, Presno-Bernal JM, Cervantes-Villagrana RD. High prevalence of co-infection between human papillomavirus (HPV) 51 and 52 in Mexican population. BMC Cancer. 2017;17:531. doi:10.1186/s12885-017-3519-7.
- Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, Clifford, GM, Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–59. doi:10.1002/ijc.27485.
- Burk RD, Ho GY, Beardsley L, Lempa M, Peters M, Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis. 1996;174:679–89. doi:10.1093/infdis/174.4.679.
- Itarat Y, Kietpeerakool C, Jampathong N, Chumworathayi B, Kleebkaow P, Aue-Aungkul A, Nhokaew W. Sexual behavior and infection with cervical human papillomavirus types 16 and 18. Int J Womens Health. 2019;11:489–94. doi:10.2147/IJWH.S218441.
- Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Sankaranarayanan R, Verma Y, Esmy PO, Poli URR, Shah A, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 2021;22(11):1518–29. doi:10.1016/S1470-2045(21)00453-8.
- Whitworth HS, Gallagher KE, Howard N, Mounier-Jack S, Mbwanji G, Kreimer AR, Basu P, Kelly H, Drolet M, Brisson M, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. 2020;38:1302–14. doi:10.1016/j.vaccine.2019.12.017.
- Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, Schiffman, M, Rodriguez, AC, Chanock, S et al, . Evidence for single-dose protection by the bivalent HPV vaccine-review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36:4774–82. doi:10.1016/j.vaccine.2017.12.078.
- Schwarz TF, Galaj A, Spaczynski M, Wysocki J, Kaufmann AM, Poncelet S, Suryakiran, PV, Folschweiller, N, Thomas, F, Lin, L, Struyf, F, . Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017;6:2723–31. doi:10.1002/cam4.1155.
- Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli URR, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi:10.1016/S1470-2045(15)00414-3.
- Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway D. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–19. doi:10.1086/315498.
- Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, Kiviat N, Galloway DA. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–36. doi:10.1093/infdis/174.5.927.
- Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, Lowy DR, Porras C, Schiller JT, Quint W, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst. 2016;108:djv302. doi:10.1093/jnci/djv302.
- Prata TT, Bonin CM, Ferreira AM, Padovani CT, Fernandes CE, Machado AP, Tozetti IA. Local immunosuppression induced by high viral load of human papillomavirus: characterization of cellular phenotypes producing interleukin-10 in cervical neoplastic lesions. Immunology. 2015;146:113–21. doi:10.1111/imm.12487.
- Molling JW, de Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, van den Eertwegh, JM, Scheper, RJ, von Blomberg, ME, Bontkes, HJ, . CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121:1749–55. doi:10.1002/ijc.22894.
- Enerly E, Flingtorp R, Christiansen IK, Campbell S, Hansen M, Myklebust TA, Weiderpass, E, Nygård, M, . An observational study comparing HPV prevalence and type distribution between HPV-vaccinated and -unvaccinated girls after introduction of school-based HPV vaccination in Norway. PLoS One. 2019;14:e0223612. doi:10.1371/journal.pone.0223612.
- Feiring B, Laake I, Christiansen IK, Hansen M, Stalcrantz J, Ambur OH, Magnus P, Jonassen CM, Trogstad L. Substantial decline in prevalence of vaccine-type and nonvaccine-type Human papillomavirus (HPV) in vaccinated and unvaccinated girls 5 years after implementing HPV vaccine in Norway. J Infect Dis. 2018;218:1900–10. doi:10.1093/infdis/jiy432.
- Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20-26 years). Hum Vaccines Immunother. 2015;11:2337–44. doi:10.1080/21645515.2015.1066948.
- Lin SW, Ghosh A, Porras C, Markt SC, Rodriguez AC, Schiffman M, Wacholder S, Kemp TJ, Pinto LA, Gonzalez P, Wentzensen N. HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One. 2013;8:e53067. doi:10.1371/journal.pone.0053067.
- Mariz FC, Bender N, Anantharaman D, Basu P, Bhatla N, Pillai MR, Prabhu PR, Sankaranarayanan R, Eriksson T, Pawlita M, Prager K. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. NPJ Vaccines. 2020;5:14. doi:10.1038/s41541-020-0165-x.
- Bonanni P, Boccalini S, Bechini A. Efficacy, duration of immunity and cross protection after HPV vaccination: a review of the evidence. Vaccine. 2009;27(Suppl 1):A46–53. doi:10.1016/j.vaccine.2008.10.085.
- Scherpenisse M, Schepp RM, Mollers M, Meijer CJ, Berbers GA, van der Klis FR, Gray C. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS One. 2013;8:e74797. doi:10.1371/journal.pone.0074797.
