ABSTRACT
Whether telbivudine (LdT) treatment to pregnant women with hepatitis B surface antigen (HBsAg) affects infant immune response to hepatitis B vaccine (HepB) has not been investigated. A total of 127 HBsAg positive mothers and their neonates were enrolled and followed up at 11–13 months. Mothers took LdT (LdT group) or did not receive antiviral therapy (control group). Infant anti-HBs, immune cells and cytokines were measured after HepB was administered according to 0-1-6 procedure. We performed a 1:3 propensity score matching (PSM). Immune indexes in the two groups were compared. Baseline characteristics of mother-baby pairs were comparable in LdT group and control group. Infant anti-HBs geometric mean concentration (GMC) did not differ significantly between the two groups [767.70 (745.35) vs. 711.90 (819.60), P = .599]. There was no difference between the two groups in infant positive rate of anti-HBs [97.8% (91/93) vs. 97.1% (33/34), P = .999] and strong positive rate of anti-HBs [40.9% (38/93) vs. 44.1% (15/34), P = .742]. Infants with negative, low, medium, and high anti-HBs levels were similarly distributed between the two groups (P = .511). No differences in proportion of helper T cells, cytotoxic T cells, B cells, myeloid dendritic cells, and plasmacytoid dendritic cells of infants (P > .05) were detected between the two groups. Children in the LdT and control group had comparable levels of interleukin-2, interleukin-4, interleukin-6, interleukin-10, interleukin-12, interferon-α, interferon-γ and tumor necrosis factor-α (P > .05). Intrauterine exposure to LdT was safe to infant immune response to HepB after birth.
1. Introduction
Chronic infection with hepatitis B virus (HBV) remains a serious global health problem, affecting approximately 296 million people worldwide and causing 820 000 deaths in 2019 due to Hepatitis B related diseases.Citation1 Among many routes of HBV transmission, HBV mother-to-child transmission (MTCT) is one of the most prominent route.Citation2
Management with hepatitis B immunoglobulin (HBIG) and hepatitis B vaccine (HepB) within 24 h after birth has achieved over 90% efficacy in preventing MTCT.Citation3,Citation4 Unfortunately, approximately 5–10% of infants born to highly viremic mothers are still infected with HBV despite immunoprophylaxis.Citation5,Citation6 Additional interventions to further decrease MTCT rate are in urgent need.
Peripartum antiviral prophylaxis is highly effective at further reducing the risk of HBV MTCT.Citation7 Telbivudine (LdT), as one of the nucleoside analogues, has been proven to be effective in reducing the viral load of women at delivery and the risk of HBV MTCT by 92% to 87%.Citation7,Citation8 However, LdT therapy during pregnancy remains a challenge for clinicians because the safety of fetal exposure to LdT is a primary concern.Citation9,Citation10
Several research teams have reported that taking LdT during pregnancy does not affect neonatal death, preterm birth, congenital abnormalities, infant growth, and neurocognitive development.Citation7,Citation11 Few data exist on the safety assessment of infant immune response to HepB after exposure to the LdT before birth.
One of the adverse effects of LdT on the persons taking the drug is neutropenia, which occurs in 2% of patients based on two clinical trials 007 GLOBE and NV-02B-015. Leukopenia and neutropenia were observed in a case report study when LdT was administered to a highly viremic patient with HBV.Citation12 In animal studies, LdT crossed the placenta in rats and rabbits.Citation13 LdT in the mother’s blood circulation may cross the placental barrier and cause fetal LdT exposure. We concern that fetal LdT exposure may affect development of the immune system and anti-HBs production in infants after vaccination. Given the increasing use of LdT in pregnant women with high HBV loads, data on the effects of fetal LdT exposure on infant immunity index and serologic response to HepB is urgently needed. The impact of LdT used for the management of mothers with high HBV DNA on the cellular and humoral immunity of infants has not been historically systematically studied.
Therefore, we designed a cohort study to evaluate anti-HBs, immune cells and cytokines of babies aged 11–13 months after LdT therapy to HBsAg positive pregnant women.
2. Materials and methods
2.1. Design
This cohort study was conducted at the Third People’s Hospital of Taiyuan in China. We recruited eligible HBV-infected pregnant women at delivery (within a window of ±10 days) and their neonates from June 2011 to July 2013. Mothers received LdT 600 mg once daily from gestational week 21–31 until delivery (LdT group) or did not receive antiviral therapy (control group) based on their willingness, which was compatible with recommendations at that time.Citation14 Participating infants received HBIG within 24 h after birth and HepB at 0, 1, and 6 months and were followed at 11–13 months of age to assess immune consequences. The study was approved by the Ethics Committee of Shanxi Medical University and informed consent was obtained from participants (approval number: 2015LL073).
2.2. Participants
The inclusion criteria were HBsAg positive mothers and their live births. The exclusion criteria were mothers coinfected with hepatitis C virus, hepatitis D virus, human immunodeficiency virus, syphilis and twins.
2.3. Exposure and baseline measurements
We acquired antiviral treatment data and baseline information including maternal gestational age, delivery mode, HBsAg, HBeAg, HBV DNA, neonatal gender and weight by questionnaires and medical records. Maternal quantitative HBsAg, HBeAg and HBV DNA at delivery was measured using electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics GmbH, Germany) and a real-time PCR-TaqMan kit (DAAN Gene Co. Ltd., Sun Yat-sen University, Guangdong, China), respectively.
2.4. Outcome assessments
Primary outcomes of this study are infant anti-HBs titers, helper T cells, cytotoxic T cells, B cells, dendritic cells, IL-2, IL-4, IL-6, IL-10, IL-12, INF-α, IFN-γ and TNF-α. Venous blood was collected from every infant aged 11–13 months. Anti-HBs was measured using ECLIA (Roche Diagnostics GmbH, Germany). Negative level, low level, medium level and high level of anti-HBs was defined as anti-HBs titers ~9.99 mIU/ml, 10 mIU/ml~99.99 mIU/ml, 100 mIU/ml~999.99 mIU/ml and 1000 mIU/ml ~, respectively. We applied Flow CytoMetry (FCM) to measure the proportion of CD3+CD4+T cells (Helper T cells, Th), CD3+CD8+T cells (Cytotoxic T cells, Tc), CD3-CD19+B cells, Lineage -HLADR+CD11c+myeloid dendritic cells (mDC) and Lineage -HLADR+CD123+plasmacytoid dendritic cells (pDC) in babies aged 11–13 months. Supplementary Table S1 shows antibodies used in FCM. Cytokines was tested by MultiSciences Biotech Co., Ltd., Hangzhou, China using MAGPIX (Luminex, US) and ProcartaPlex Human IL-2, IL-4, IL-6, IL-10, IL-12, INF-α, IFN-γ and TNF-α Simplex 96 tests (eBioscience, US).
2.5. Statistical methods
The characteristics of the mothers and neonates at baseline were reported using number with percentages (n, %), means with standard deviations (mean ± SD) and medians with interquartile ranges. Anti-HBs geometric mean concentration (GMC) was calculated.
To minimize group differences on covariates between the LdT group and control group, we used a 1:3 propensity score matching (PSM). PSM is a statistical method that balances the covariates of two groups and reduce confounding bias, enabling more accurate comparisons within observational studies by simulating randomized controlled trials (RCT).Citation15 Propensity scores were calculated with logistic regression. Matching on the propensity scores was done with a nearest-neighbor algorithm. The standardized mean difference (SMD) was calculated as the means divided by the square root of the half sum of the two variances to assess the balance of variables used for matching. After PSM, if SMD of each matched variable is less than 0.10, it indicates that these variables are comparable in both groups after PSM. The matched data in the two groups were compared using Mann-Whitney U test, Fisher’s exact test or Chi-Square test for babies’ anti-HBs and immune indexes. Sensitivity analysis was used to evaluate the robustness of the results by univariate and multivariate analyses on the complete dataset.
Statistical analyses were performed using R 4.0.3 and SPSS version 22.0 (SPSSInc., Chicago, IL, USA). All tests were two-tailed, and P < .05 was considered to indicate statistical significance.
3. Results
3.1. Enrollment and follow-up of the subjects
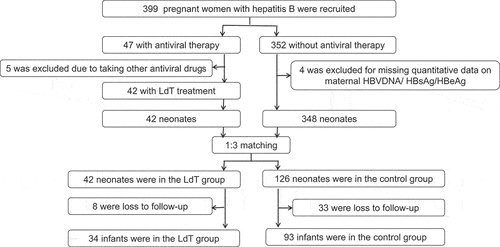
We recruited 399 HBsAg positive pregnant women. A total of 168 mother-baby pairs remained after PSM and 127 infants retained in the final analysis. depicts the enrollment and follow-up of the subjects. No infants had congenital malformations.
3.2. Descriptive data before and after PSM
lists the demographic and clinical information of participants before and after PSM. Maternal HBsAg, HBeAg, HBV DNA, infant gender and weight were not comparable between LdT and control group (P < .05 and/or SMD > 0.10) before PSM. The baseline characteristics of mother-baby pairs were comparable in the two groups after PSM (P > .05 and/or SMD < 0.10).
Table 1. Baseline characteristics of participants before and after PSM
3.3. Infant immune consequences
3.3.1. Infant anti-HBs level after hepatitis B vaccination
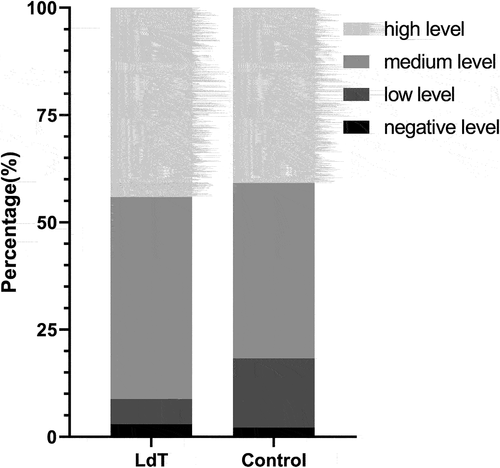
Maternal LdT administration during pregnancy did not reduce infant anti-HBs GMC after vaccination against hepatitis B [767.70 (745.35) vs. 711.90 (819.60), P = .599]. There was no difference between LdT and control group in infant positive rate of anti-HBs [97.8% (91/93) vs. 97.1% (33/34), P = .999] and strong positive rate of anti-HBs was [40.9% (38/93) vs. 44.1% (15/34), P = .742] (). Infants with negative, low, medium, and high anti-HBs levels were similarly distributed between the two groups (P = .511) ().
Table 2. Quantitative comparison of infant immune outcomes between LdT and control group after PSM
Figure 2. The distribution of infants with negative, low, medium and high level of anti-HBs between LdT and control group after PSM. The proportion of infants with negative level, low level, medium level and high level of anti-HBs is 2.94% (1/34), 5.88% (2/34), 47.06% (16/34) and 44.12% (15/34) in LdT group, respectively. The proportion of infants with negative level, low level, medium level and high level of anti-HBs is 2.15% (2/93), 16.13% (15/93), 40.86% (38/93) and 40.86% (38/93) in control group, respectively.
3.3.2. Infant immune cells and cytokines after hepatitis B vaccination
Maternal LdT therapy during pregnancy did not significantly reduce the proportion of Th, Tc, B cells, mDC and pDC among offspring (P > .05). Infant IL-2, IL-4, IL-6, IL-10, IL-12, INF-α, IFN-γ, TNF-α and Th1/Th2 levels were comparable between the two groups, despite slightly higher IFN-α, IFN-γ and Th1/Th2 of infants in LdT group (P > .05) ().
3.3.3. Sensitive analysis
Anti-HBs, immune cells and cytokines were comparable among babies in LdT group and control group on unmatched data in univariate analysis except for infant Th cells (Supplementary Table S2). In multivariate analysis, infant immune indicators in both groups were similar on the complete dataset (Supplementary Table S3).
3.4. The rate of HBV MTCT
The rate of HBV MTCT among 168 mother-baby pairs is 0.60% (1/168). The rate of HBV MTCT were 0% (0/42) in LdT group and 0.79% (1/126) in the control group, respectively (P = .750).
4. Disscussion
There are limited but much needed information concerning the adverse outcomes based on infant immunity after maternal LdT treatment during pregnancy. Our work showed children with prenatal exposure to LdT had comparable immune cells, cytokines and anti-HBs level at 11–13 months of age as those who were unexposed to LdT. We revealed intrauterine exposure to LdT did not affect infant immune response to HepB.
LdT is efficiently converted into the active triphosphate metabolite with a long intracellular halflife,Citation16 having potent antiviral activity against HBV.Citation17 In pre-clinical animal studies, LdT crossed the placenta in rats and rabbits, but not demonstrated any teratogenicity in rats at doses 6 times higher than therapeutic human doses.Citation13 Data on LdT transmission through the human placenta are not yet available,Citation18 but another nucleotide analogue, tenofovir disoproxil fumarate (TDF), have been confirmed to pass through human placenta barrier.Citation19 We are concerned that the adverse effects of LdT in adult patients will also occur in the offspring born to pregnant women taking LdT.
Our results uncovered the anti-HBs GMC was slightly higher in LdT group than control group and did not achieve a strong positive level (≥1000 mIU/mL) in both groups, which were similar to those of another study in Nanjing, China.Citation20 People with initial anti-HBs higher than 1000 mIU/mL were more likely to maintain long-term protection against HBV infection.Citation21 We anticipate future study to investigate additional intervention to increase the level of serological response to HepB in these infants.
We found no significant difference in infant positive rate of anti-HBs, strong positive rate of anti-HBs and distribution of infants with different intervals of anti-HBs whether pregnant women took LdT or not. One study have reported that the proportion of infants with detectable anti-HBs was significantly higher in the telbivudine group compared with the control group.Citation20 Our study, along with others, suggests that telbivudine therapy during pregnancy is safe in infants responding to HepB.
We disclosed maternal LdT therapy may have no significant effect on infant immune cells when the babies were 11–13 months old. In another study, proportion of CD4+CD25+Tregs was significantly decreased and proportion of CD8+T cells was significantly increased in neonates within 6 hours after birth, whose mothers were treated with LdT during the third trimester comparing with those without treatment.Citation22 The researchers believe that maternal use of LdT may be useful in regulating neonatal immune function. Their work provides short-term safety evidence on LdT. However, there has been no evidence of the long-term safety of LdT intrauterine exposure on immune cells in infants aged 11–13 months. Together with their study, our study indicates that fetal exposure to LdT is at least harmless to immune cells of children.
Our results manifest maternal use of LdT may have no significant effect on infant cytokines both at birth and at 11–13 months of age. LdT treatment for 11 months reduced IL-6, IL-27, and TNF-α levels in patients themselves, which observed by a retrospective study.Citation23 However, there has been no evidence of the effect of LdT administration during pregnancy on infant cytokines.
Our data were collected prospectively with longitudinal assessment from birth to 11–13 months of age. As participants in cohort study are not randomly assigned to the LdT group or control group, confounding covariates may be present. Propensity score adjustment is an increasingly popular statistical method used to balance these covariates and effectively control for the known selection bias and confounding bias. Propensity score analysis can simulate the randomization process of randomized controlled trials to minimize the limitations of cohort studies. Compared with conventional matching, propensity score matching can consider more matching variables and improve research efficiency. Previous studies have provided safety data related to infants’ physical development after exposure to LdT in utero.Citation24,Citation25,Citation26 Our work provides additional evidence on immune consequences after fetal exposure to LdT, contributing to the current safety profile of LdT use among pregnant women with HBsAg. This will be valuable first-hand information for clinicians, HBsAg positive pregnant women and their families.
We uncovered that intrauterine exposure to LdT in the second and third trimester did not reduce the infant immune response to HepB compared with those not exposed to LdT. Our work systematically manifestes the safety of LdT administration during the second and third trimester with respect to infant immune response to HepB. We provide evidence to support the use of LdT during the second and third trimester among HBsAg positive pregnant women, contributing to further interruption of HBV MTCT and global elimination of HBV. A future direction of this work is the effect of prenatal exposure to other anti-HBV drugs on the immune response to HepB in infants.
There are limitations to this study. First, this study was not a clinical trial. We began this study in 2011, when safety data for LdT in pregnant women with HBV infection were insufficient, making clinical trials in pregnant women virtually infeasible. However, we enhanced comparability between the two groups and minimized the confounding bias by propensity score matching and multivariate analysis. Second, not all mother-infant pairs enrolled in the baseline were willing to attend follow-up visits so that the sample size available for statistical analysis was small. We look forward to the randomized controlled clinical trials with larger sample size to validate our results.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical appproval
This study was approved by the Ethics Committee of Shanxi Medical University and informed consent was obtained from all parents of the children.
Author contributions
Suping Wang and Yongliang Feng designed research; Bo Wang and Shuying Feng provided patients to the study; Wenxin Chen, Cong Jin, Ting Wang and Tian Yao collected data; Yandi Li analyzed data and wrote the paper; Yandi Li had primary responsibility for final content. All authors read and approved the final manuscript. All authors have approved the final article.
Supplemental Material
Download MS Word (23.3 KB)Acknowledgments
We thank all mothers and their children participated the study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2029259.
Additional information
Funding
References
- Global progress report on HIV, viral hepatitis and sexually transmitted infections. World Health Organization; 2021.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis. 2004;11(2):97–6. doi:10.1046/j.1365-2893.2003.00487.x.
- Lee CGY, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunization in newborn infants of mothers positive for hepatitis B surface antigen: systemic review and meta-analysis. BMJ. 2006;332:332. doi:10.1136/bmj.38726.404120.7C.
- Chen H, Lin L, Hu F, Lee J, Lin W, Yang Y, Huang F, Wu S, Chen SC, Wen W, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142(4):773–781.e772. doi:10.1053/j.gastro.2011.12.035.
- Wen W, Chang M, Zhao L, Ni Y-H, Hsu H-Y, Wu J-F, Chen P-J, Chen D-S, Chen H-L. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59(1):24–30. doi:10.1016/j.jhep.2013.02.015.
- Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepatitis. 2012;19(2):e18–25. doi:10.1111/j.1365-2893.2011.01492.x.
- Funk A, Lu Y, Yoshida K, Zhao T, Boucheron P, van Holten J, Chou R, Bulterys M, Shimakawa Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(1):70–84. doi:10.1016/S1473-3099(20)30586-7.
- Terrault N, Lok A, McMahon B, Chang K-M, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99. doi:10.1002/hep.29800.
- Pan C, Duan Z, Bhamidimarri K, Zou H, Liang X, Li J, Tong MJ. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10(5):452–59. doi:10.1016/j.cgh.2011.10.041.
- Liver. EAftSot. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98. doi:10.1016/j.jhep.2017.03.021.
- Pan C, Li M, Zeng H, Zhang Y, Cao W-H, Wang Y, Zhou M-F, Hu Y-H, Wan G, Xie Y, et al. Developmental consequences of prenatal telbivudine exposure during the third trimester. Clin Gastroenterol Hepatol. 2021;19(5):1061–63. doi:10.1016/j.cgh.2020.04.056.
- Wang R, Nan Y. Telbivudine induced neutropenia in a patient with chronic hepatitis B: a case report. J Clin Hepatobiliary Dis. 2015;31:273–273.
- Bridges E, Selden J, Luo S. Nonclinical safety profile of telbivudine, a novel potent antiviral agent for treatment of hepatitis B. Antimicrob Agents Chemother. 2008;52(7):2521–28. doi:10.1128/aac.00029-08.
- Jia J, Li L. Guidelines for prevention and treatment of chronic hepatitis B (2010 China). J Pract Liver Dis. 2011;1:7–21.
- McDonald R, McDonald J, Kallmes D, CarterR, Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology. Radiology. 2013;269(3):640–645. doi:10.1148/radiol.13131465.
- Zoulim F, Locarnini S. Management of treatment failure in chronic hepatitis B. J Hepatol. 2012;56:S112–122. doi:10.1016/S0168-8278(12)60012-9.
- Peng C, Chien R, Liaw Y. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57(2):442–50. doi:10.1016/j.jhep.2012.02.033.
- Giles M, Visvanathan K, Sasadeusz J. Antiviral therapy for hepatitis B infection during pregnancy and breastfeeding. Antivir Ther. 2011;16(5):621–28. doi:10.3851/imp1813.
- Nachega J, Uthman O, Mofenson L, Anderson JR, Kanters S, Renaud F, Ford N, Essajee S, Doherty MC, Mills EJ, et al. Safety of tenofovir disoproxil fumarate-based antiretroviral therapy regimens in pregnancy for HIV-Infected women and their infants: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;76(1):1–12. doi:10.1097/qai.0000000000001359.
- Han G, Cao M, Zhao W, Jiang H-X, Wang C-M, Bai S-F, Yue X, Wang G-J, Tang X, Fang Z-X, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55(6):1215–21. doi:10.1016/j.jhep.2011.02.032.
- Yao T, Shao Z, Wu L, Dong S, Gao L, Wu Y, Shi X, Shi J, Liu G, Wang J, et al. Long-term persistent immunogenicity after successful standard and triple-dosed hepatitis B vaccine in hemodialysis patients: a 3-year follow-up study in China. Vaccine. 2021;39(18):2537–44. doi:10.1016/j.vaccine.2021.03.074.
- Liang X, Liu P, He Z, Chen X, and Xiao X. The effect of maternal use of telbivudine on neonatal CD4+CD25+ regulatory T cells for the prevention of mother-to-child transmission of hepatitis B virus. Clinics and Research in Hepatology and Gastroenterology. 2020; 44(2):195–203. doi:10.1016/j.clinre.2019.06.004
- Ho C, Chang T, Chien R. Telbivudine on IgG-associated hypergammaglobulinemia and TGF-β1 hyperactivity in hepatitis B virus-related liver cirrhosis. PloS One. 2019;14(11):e0225482. doi:10.1371/journal.pone.0225482.
- Zhang H, Pan C, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60(2):468–76. doi:10.1002/hep.27034.
- Pan C, Han G, Jiang H, Zhao W, Cao M, Wang C, Yue X, Wang G. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(5):520–26. doi:10.1016/j.cgh.2012.01.019.
- Han G, Jiang H, Wang C, Ding Y, Wang G-J, Yue X, Zhou L, Zhao W. Long-term safety and efficacy of telbivudine in infants born to mothers treated during the second or third trimesters of pregnancy. J Viral Hepat. 2017;24(6):514–21. doi:10.1111/jvh.12670.