ABSTRACT
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a relatively unknown autoimmune entity. Scant reports of post-infection/vaccination anti-NMDAR encephalitis exist. We, hereby, reviewed the relevant cases and added to the literature a possible case of anti-NMDAR encephalitis following COVID-19 vaccination with BBIBP-CorV (Sinopharm). A 50-year-old Persian woman with previously known rituximab-treated MS presented complaining of worsening neurological symptoms all gradually starting and worsening after receiving the second dose of BBVIP-CorV 2 weeks before. Notable findings in her physical examination included ataxic gait and Babinski sign. Considering an acute MS relapse, corticosteroid pulse therapy was initiated, and she was referred for MRI, which revealed multiple new plaques. Her serum sample interestingly tested positive for anti-NMDAR antibodies. CSF analysis was unfortunately not performed. She responded well to the corticosteroid pulse therapy and showed substantial resolution of the symptoms. Considering its relatively low cost of workup and the benefits of correct early diagnosis, clinicians are advised to consider autoimmune encephalitis encountering patients with progressive neurological symptoms after the administration of vaccines, including the ones for COVID-19 which are currently being used extensively.
Introduction
Many vaccines have been developed to accelerate the global efforts to bring the coronavirus disease 2019 (COVID-19) pandemic to an end. Among these has been the World Health Organization (WHO)-authorized BBIBP-CorV (Sinopharm, China), an inactivated virus vaccine utilizing the HB02 strain of the SARS-CoV-2. The BBIBP-CorV has shown reasonable efficacy and safety among the general adult population;Citation1 still, it remains to be tested among populations with special conditions such as people with autoimmunity and/or on immunosuppressive therapies. Sporadic adverse events following immunization (AEFIs) may be missed by phase I–III clinical trials of vaccines, as higher study powers are usually needed to document them. Hence, post-marketing surveillance of rare adverse events primarily relies on individual reports and documented observations. The aim is to provide a basis for future investigations and, more importantly, raise clinicians’ awareness of probable AEFIs; a large proportion of which may be managed with a reasonably low residual deficit in patients if identified and treated timely and appropriately. Anti-N-Methyl-d-Aspartate receptor (anti-NMDAR) encephalitis and multiple sclerosis (MS) relapses are no exception; correct and early diagnosis and treatment can improve the overall outcome significantly.
Anti-NMDAR encephalitis is a rare autoimmune entity, typically manifested through a prodromal phase of nonspecific symptoms, followed by psychiatric alterations, seizures, and movement abnormalities.Citation2 The detection of anti-NMDAR antibodies in serum or cerebrospinal fluid (CSF) samples, along with imaging and electroencephalogram (EEG) findings can confirm the diagnosis. The underlying etiology of this autoimmune entity is still unclear; paraneoplastic, infectious, and vaccination-induced pathomechanisms are suspected.Citation3 Interestingly, several reports of anti-NMDAR encephalitis following viral infections – including COVID-19,Citation4–6 and scant reports of anti-NMDAR encephalitis following vaccination exist, which may hint toward future research directions into its unclear pathophysiology. We aimed to review these cases, and add to the literature the case of a middle-aged woman with previously known MS, who presented with manifestations of an acute MS relapse and tested positive for anti-NMDAR antibodies after receiving the second dose of the BBIBP-CorV COVID-19 vaccine.
Case presentation
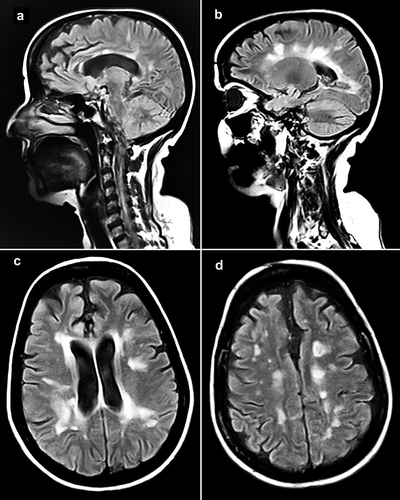
The presented case was a 50-year-old woman diagnosed with MS in 2014 after presenting with ataxia and numerous periventricular lesions in MRI. She was put on teriflunomide, and later in April 2020, on rituximab (500 mg every 6 months). She contracted COVID-19 in September 2020 and recovered without complications. On her last pre-vaccination visit on April 4th 2021 – for receiving her third rituximab infusion – she had an expanded disability status scale (EDSS) score of 1.5, and her symptoms were well controlled. She did not disclose any other remarkable detail in her past medical, social, and familial histories. She received her first dose of the BBIBP-CorV vaccine on June 2nd, and her second dose on June 28th, 2021. On July 18th, 2021, she presented to our clinic complaining of worsening behavioral changes, myalgia, precipitation, vomiting, leg weaknesses, ataxia, dizziness, and fatigue, all gradually starting and worsening after receiving the second dose of BBVIP-CorV. She was mildly agitated with an ataxic gait, loss of force in lower extremities, Babinski sign, but no sign of fever/infections. Primarily considering an acute MS relapse, a venous blood sample was obtained and sent for infectious/serological studies, methylprednisolone pulse therapy was initiated immediately, and she was referred for MRI. CSF analysis was not performed, as she did not convey consent for a lumbar puncture. Serum analysis was unremarkable except for elevated C-reactive protein, positive anti-NMDAR IgG, and positive anti-SARS-CoV-2 Spike IgG – both detected using enzyme-linked immunosorbent assay (ELISA). MRI later revealed multiple new plaques in periventricular, juxtacortical, and cortical areas (). The patient continued to receive methylprednisolone pulse therapy (500 mg/day for five days), resulting in partial resolution of the symptoms. She was then discharged with an EDSS score of 2.5, and a prescription of oral steroid tapering. She returned for a follow-up visit on August 10th, 2021, in which she had an EDSS score of 2 and showed substantial resolution of the symptoms. As CSF analysis was not performed and the new lesions were only detectable in white matter (), we could not establish whether the clinical picture was linked to the anti-NMDAR antibodies, rather than an acute MS relapse. Hence, the diagnosis of the case might have been either anti-NMDAR encephalitis, acute MS relapse, or a combination of both.
Discussion and review of literature
We hereby reported the case of a serious AEFI, which might have been either anti-NMDAR encephalitis or an MS flare. Detection of anti-NMDAR antibodies in this case is especially intriguing considering the anti-CD20 therapy, which is believed to prevent B-cell maturation and therefore, formation of new antibodies. A similar noteworthy point in our case was the successful seroconversion after BBVIP-CorV vaccination, despite rituximab infusion only a month before the first dose of the vaccine. The prior SARS-CoV-2 infection and the low-dose regimen of rituximab both might have played a role in the observed seroconversion. As most people on anti-CD20 therapies fail to elicit humoral immunization following both COVID-19 contraction and vaccination,Citation7,Citation8 similar to another presented case,Citation9 this case may be interpreted as a highlight of the importance of booster doses of COVID-19 vaccines among these patients.
The BBIBP-CorV has appeared promising regarding its safety, with its phase 3 trial reporting serious adverse events occurring in about 0.4% of individuals receiving it.Citation1 Among the documented AEFIs, only 0.9% were deemed serious (n = 49); three of which being demyelination and one being meningitis, but none being autoimmune encephalitis. In two recent population-based studies among the people with MS (pwMS) receiving the BBIBP-CorV – one of which being contributed by the present authors – although MS disease activity was not shown to be increased, acute MS relapses, and an acute ulcerative colitis flare were among the reported serious AEFIs,Citation10,Citation11 however, no cases of autoimmune encephalitis were reported.
We searched MEDLINE and Scopus using proper Boolean operators and the keywords “Autoimmune encephalitis,” “Anti-N-Methyl-d-Aspartate receptor antibodies,” “COVID-19,” and “vaccination,” and reviewed the previous relevant cases. Anti-NMDAR encephalitis was reported in two cases following H1N1 influenza vaccines.Citation12 A 15-year-old girl developed anti-NMDAR encephalitis after receiving a booster vaccination dose against tetanus-diphtheria-pertussis and poliomyelitis.Citation13 Vaccination against Japanese encephalitis in a 2-year-old girl has also been reported to be associated with anti-NMDAR encephalitis.Citation3 A possible association between anti-NMDAR encephalitis and yellow fever vaccination has also been highlighted.Citation14 Regarding the COVID-19 vaccines, one case of post-vaccination immune-mediated encephalitis after immunization with ChAdOx1 nCov-19 (AstraZeneca) has been reported, with proper resolution following immunosuppressive therapies.Citation15 Another case of acute encephalitis and myoclonus has been reported in temporal association with the first dose of the mRNA-1273 (Moderna) vaccine in a 77-year-old man with background cardiovascular disease and hypothyroidism.Citation16 A somewhat relevant case of a 56-year-old lady with a history of post-infectious rhombencephalitis has been documented as well; she presented with symptoms of CNS involvement shortly after receiving the first dose of BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine, eventually being diagnosed with acute disseminated encephalomyelitis.Citation17
Considering its relatively-low work-up cost and the benefits of correct early diagnosis, it may be reasonable to consider anti-NMDAR encephalitis upon encountering progressive neurological symptoms following vaccination. Nevertheless, no causality should be assumed at this stage, regardless of the mentioned sporadic reports of CNS pathologies in temporal correlation with vaccines, including COVID-19 vaccines. Large-scale observational studies are warranted to provide a reliable estimate of the incidence of such probable AEFIs. Until so, clinicians should be vigilant in detecting and identifying potentially serious AEFIs and take immediate therapeutic measures to prevent unfortunate outcomes in patients after immunization against COVID-19.
Consent to participate statement
Not applicable based on local/national guidelines.
Consent to publish statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying unidentifiable images.
Study approval statement
Ethical approval was not required for this study in accordance with local/ national guidelines.
Author contributions
ME and MS identified and managed the presented case, and critically revised the initial draft. NS and HN gathered the presented data and drafted the initial manuscript. All of the mentioned authors have approved the final manuscript and agreed to be accountable for all aspects of the work including its accuracy and integrity.
Acknowledgments
We acknowledge the cooperation of the reported patient enabling us to report her case.
Disclosure statement
The authors have no conflicts of interest to declare.
Data availability statement
To prevent de-anonymization of the reported patient, additional data is not openly available. All available additional data will be shared with qualified investigators only upon reasonable request, unless it could be used to de-anonymize the presented case.
Additional information
Funding
References
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–4. doi:10.1001/jama.2021.8565.
- Etemadifar M, Aghababaei A, Nouri H, Kargaran PK, Mohammadi S, Salari M. Autoimmune encephalitis: the first observational study from Iran. Neurol Sci. 2021. doi:10.1007/s10072-021-05400-1.
- Wang H. Anti-NMDA receptor encephalitis and vaccination. Int J Mol Sci 18 1 . 2017;193.
- Álvarez Bravo G, Ramió ITL. Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurologia (Engl Ed). 2020;35:699–700. doi:10.1016/j.nrl.2020.07.013.
- Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-Methyl-d-Aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. 2021;114:75–76. doi:10.1016/j.pediatrneurol.2020.10.002.
- Hou R, Wu J, He D, Yan Y, Li L. Anti-N-methyl-D-aspartate receptor encephalitis associated with reactivated Epstein-Barr virus infection in pediatric patients: three case reports. Medicine (Baltimore). 2019;98:e15726. doi:10.1097/MD.0000000000015726.
- Sormani MP, Schiavetti I, Landi D, Carmisciano L, De Rossi N, Cordioli C, Moiola L, Radaelli M, Immovilli P, Capobianco M, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: an international cohort study. Multiple Sclerosis J. 2021;13524585211035318. doi:10.1177/13524585211035318.
- Etemadifar M, Sedaghat N, Nouri H, Lotfi N, Chitsaz A, Khorvash R, Zolfaghari H, Ghasemi Movaghar A, Pourabbas M, Salari M, et al. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination: same story, different vaccine. Mult Scler Relat Disord. 2021;103417. doi:10.1016/j.msard.2021.103417.
- Etemadifar M, Nouri H, Salari M, Sedaghat N. Successful seroconversion following mild COVID-19 contraction in a double-vaccinated B-cell-depleted person with multiple sclerosis: a hint towards booster efficacy? Neuroimmunol Rep. 2021;1:100047. doi:10.1016/j.nerep.2021.100047.
- Etemadifar M, Abhari AP, Nouri H, Akhavan Sigari A, Piran Daliyeh SM, Maracy MR, Salari M, Maleki S, Sedaghat N. Self-reported safety of the BBIBP-CorV (Sinopharm) COVID-19 vaccine among Iranian people with multiple sclerosis. medRxiv. 2021;2021.
- Ali Sahraian M, Ghadiri F, Azimi A, Naser Moghadasi A. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP-CorV. Vaccine. 2021;39:6347–50. doi:10.1016/j.vaccine.2021.09.030.
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi:10.1016/S1474-4422(10)70253-2.
- Hofmann C, Baur MO, Schroten H. Anti-NMDA receptor encephalitis after TdaP-IPV booster vaccination: cause or coincidence? J Neurol. 2011;258:500–01. doi:10.1007/s00415-010-5757-3.
- Coeckelbergh E, Reynders T. Anti-NMDA receptor encephalitis after yellow fever vaccination: a case report. Acta Neurol Belg. 2021. doi:10.1007/s13760-021-01673-7.
- Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–11. doi:10.1002/ana.26182.
- Torrealba-Acosta G, Martin JC, Huttenbach Y, Garcia CR, Sohail MR, Agarwal SK, Wasko C, Bershad EM, and Hirzallah MI. Acute encephalitis, myoclonus and sweet syndrome after mRNA-1273 vaccine. BMJ Case Rep 14 7 . 2021;e243173.
- Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D’Agostini S, Fabris M, Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi:10.1016/j.clineuro.2021.106839.