ABSTRACT
Rotavirus (RV) is a leading cause of gastroenteritis in children. In Japan, Rotarix (RV1; GlaxoSmithKline), which is a monovalent vaccine derived from human RV (G1P[8]), has been introduced since November 2011, and RotaTeq (RV5; MSD) which is an pentavalent, human-bovine mono-reassortant vaccine (G1, G2, G3, G4, and P1A[8]), has been introduced since July 2012. Long-term follow-up on vaccine efficacy and RV genotypical change should be carried out in order to control RV infection. The RV gastroenteritis (RVGE) outbreak occurred during the 2018/2019 season in Aichi prefecture, Japan. Therefore, the molecular epidemiology of RV among three different groups of RVGE, which were outpatients who received RV1, those who received RV5, and those without vaccination, was explored. Clinical features of RVGE patients were compared among the three patient groups. Children less than 15 years of age with gastroenteritis who visited any of seven pediatric practices between January and June 2019 were enrolled in the study. G, P, and E genotypes were determined by direct sequencing of reverse transcription–polymerase chain reaction products amplified from stool samples. Among 110 patients, there were 27, 28, and 55 in the RV1-vaccinated, RV5-vaccinated, and unvaccinated groups, respectively. The most frequent genotype was G8P[8] (92/110 patients, 83.6%). Genotype distributions did not significantly differ among the three patient groups (P = .125). Mean Vesikari score was significantly lower among RV1-vaccinated (7.1) and RV5-vaccinated patients (6.4) than among unvaccinated patients (10.2) (P < .001). Even in RVGE patients treated in an outpatient clinic, RV vaccine reduced the severity of the disease in this cohort.
Introduction
Rotavirus (RV), a leading cause of gastroenteritis in children, causes substantial morbidity and mortality worldwide.Citation1 In order to decrease the huge disease burden, two live attenuated RV vaccines have been introduced: Rotarix (RV1; GlaxoSmithKline) is an orally administered monovalent attenuated live vaccine derived from human RV, and RotaTeq (RV5; MSD) is an orally administered pentavalent, live vaccine with a backbone derived from bovine RV. Although the concept for developing the vaccines differed, both can prevent severe RV gastroenteritis (RVGE) caused by common RV genotypes, including G1–G4, G9, P[4], P[6], and P[8].Citation2–5 Although the vaccines are expected to cross-protect against other RV genotypes, several large-scale molecular epidemiological studies have demonstrated genotypic changes of RV after introduction of the vaccine in several countries,Citation6–8 suggesting the possibility of escape from vaccine-induced immune pressure or vaccine-independent genotype variation over time. These observations emphasize the importance of long-term follow-up on vaccine efficacy and RV genotypical change in order to control RV infection.Citation9 Furthermore, because different vaccines have been introduced in each region, molecular epidemiology and vaccine efficacy should be elucidated locally.
RV vaccination has been available in Japan since November 2011. Although RV vaccine was initially administered on a voluntary basis, it has been included in the national immunization program starting in October 2020. In order to increase vaccine coverage, several local governments have supported the cost of the vaccination before starting the national immunization program. Consequently, vaccine coverage has gradually increased, and that has resulted in a remarkable decrease in the numbers of RVGE patients.Citation10–13 Support of vaccination costs by Nagoya, the largest city in Aichi prefecture, has resulted in a remarkable impact on the numbers of RVGE-related hospitalizations and outpatient visits in the city.Citation12 Because vaccine administered varies across clinics between two different vaccines, RVGE patients fall into three different groups: patients who received RV1, those who received RV5, and unvaccinated patients. Because the RVGE outbreak occurred during the 2018/2019 season in several areas in Japan, including Aichi prefecture, we sought to compare the molecular epidemiology of RV among the three different groups of RVGE patients. In addition, we compared the clinical features of RVGE patients among the three patient groups.
Methods
Subjects and sampling
Children less than 15 years of age with acute gastroenteritis who visited any of seven pediatric practices around our institution between January and June 2019 were eligible for the study. Eligible children were invited and study staff administered written informed consent to parents of those who enrolled. Rotavirus infection was confirmed by each doctor at the time of clinic visit using an immunochromatographic test (Dipstick ‘Eiken’ Rota kit: Eiken Chemical, Tokyo, Japan), which detects RV antigen in stool. Demographic and clinical information, including age, gender, maximum number of diarrheal and vomiting episodes per day, duration of diarrhea and vomiting, presence of fever, maximum fever level, and presence of drip infusion were collected by each practitioner; this clinical information was collected retrospectively from the medical record. Vesikari scores were calculated based on the collected clinical information.Citation14 This study was approved by the review board of our university (No. 19–046).
RNA extraction and G, P, and E genotyping
Ten percent suspensions (1 mL) of each stool sample were prepared in physiological saline solution; alternatively, swab samples were immersed in 200 μL of physiological saline solution.Citation15 Suspensions were clarified by centrifugation for 20 min at 4000 g, and 140 μL of the supernatant was used for RNA extraction. RNA was extracted from samples using the QIAamp viral RNA Mini kit (QIAGEN, Chatsworth, CA, USA). In order to determine the G, P, and E genotypes, we performed reverse transcription–polymerase chain reaction (RT-PCR) using the SuperScript III One-Step RT-PCR System with Platinum Taq High-Fidelity DNA Polymerase Kit (Invitrogen, Carlsbad, CA, USA). Primers for the target genes were designed to amplify the common sequences of RVA strains.Citation16–18 The BigDye Terminator V3.1 Cycle Sequencing Reaction Kit (Applied Biosystems, Foster City, CA, USA) was used for Sanger sequencing of each PCR product. Sequencing products were analyzed on an ABI 3130 Genetic Analyzer (Applied Biosystems). Finally, G, P, and E genotyping of the obtained sequences was performed using the Rota C v2.0 automated genotyping tool (http://rotac.regatools.be/). It is out of order. The ViPR-tool ((https://www.viprbrc.org/brc/rvaGenotyper.spg?method=ShowCleanInputPage&decorator=reo) can be used as an alternative.).Citation19
Statistical analysis
Patientsʻ characteristics (age and gender) and clinical symptoms (fever, maximum fever level, maximum number of diarrheal or vomiting episodes per day, duration of diarrhea or vomiting, drip infusion, hospitalization, and Vesikari score) were compared between each pair of groups (RV1-vaccinated patients, RV5-vaccinated patients, and unvaccinated patients) using the Steel–Dwass test or Pearsonʻs chi-square test with Bonferroni correction. Distributions of genotypes were compared among the three groups using Pearsonʻs chi-square test. P < .05 was considered to be statistically significant. Statistical analysis was performed with JMP version 12.2.0 software (SAS Institute, Cary, NC, USA).
Results
Study subjects and age distribution of RVGE patients
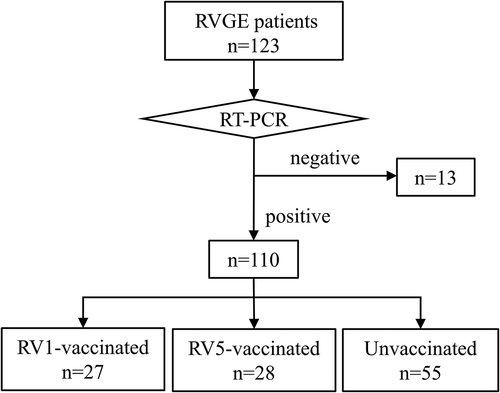
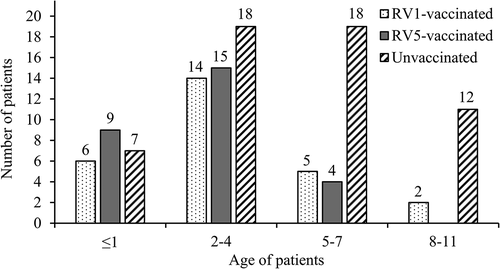
A total of 123 RVGE patients were enrolled in this study (). Thirteen patients were excluded because RV genomes were not detected in rectal swabs by RT-PCR. Among the remaining 110 patients, 27 were RV1-vaccinated, 28 were RV5-vaccinated, and 55 were unvaccinated. Distribution of RVGE patient by age and vaccine status is shown in The median age of the patients was 4.8 years (range, 4 months to 11 years). Patients aged 1 year old or younger, 2–4 years, 5–7 years, and 8–11 years accounted for 18%, 44%, 25%, and 12% of all patients, respectively. In patients 4 years or younger (n = 69), the total number of vaccinated patients (n = 44) was almost double the number of unvaccinated patients (n = 25). By contrast, the number of unvaccinated patients (n = 30) was much higher than vaccinated patients (n = 11) among patients 5 years and older. The two vaccinated patients older than 8 years had been vaccinated abroad.
RV genotypes determined by direct sequencing analysis
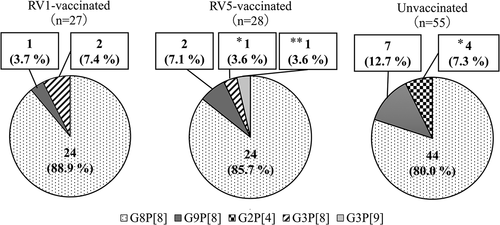
Distribution of G/P genotype of the 110 patients is shown in The most frequent genotype was G8P[8] (92/110 patients, 83.6%), followed by G9P[8] (10/110 patients, 9.1%), G2P[4] (4/110 patients, 3.6%), G3P[8] (3/110 patients, 2.7%), and G3P[9] (1/110 patients, .9%). The frequencies of the G8P[8] genotype in RV1-vaccinated, RV5-vaccinated, and unvaccinated patients were 88.9% (24/27), 85.7% (24/28), and 80.0% (44/55 patients), respectively; no significant difference in genotype distribution was observed among the three groups (P = .125). We also determined E genotype. All but 3 of the RVs in the 110 patients (97.3%) had the E2 genotype (DS-1–like). One of the 10 G9P[8] and 1 of the 3 G3P[8] RVs had the E1 genotype (Wa-like), and one G3P[9] RV had the E3 (AU-1–like) genotype. The G9P[8]-E1 strain was detected in one unvaccinated patient, and the G3P[8]-E1 and G3P[9]-E3 strains were detected in RV5-vaccinated patients.
Comparison of patientʻs backgrounds and clinical features of RVGE
Patientʻs background and clinical characteristics, including disease severity of RVGE based on vaccine status, are shown in . Both RV1-vaccinated (P = .001) and RV5-vaccinated (P = .017) patients were significantly younger than the unvaccinated patients. There was no significant gender difference between RV1-vaccinated (P = .101) or RV5-vaccinated (P = .916) and unvaccinated patients. Duration of diarrhea was significantly lower in RV1-vaccinated (P = .005) and RV5-vaccinated patients (P < .001) than in unvaccinated patients. Maximum number of diarrheal episodes per day was significantly lower in RV5-vaccinated patients than in unvaccinated patients (P = .027), but no significant difference was observed between RV1-vaccinated and unvaccinated patients (P = .149). Maximum number of vomiting episodes per day was significantly lower in RV1-vaccinated (P = .005) and RV5-vaccinated patients (P < .001) than in unvaccinated patients. Duration of vomiting was significantly shorter in RV5-vaccinated than in unvaccinated patients (P < .001), but no significant difference was observed between RV1-vaccinated and unvaccinated patients (P = .077). Frequency of fever and maximum body temperature did not significantly differ among the three groups. The percentage of patients requiring drip infusion was significantly higher in unvaccinated patients than in RV1-vaccinated (P = .011) and RV5-vaccinated patients (P < .001). Finally, mean Vesikari score was significantly lower in RV1-vaccinated (P < .001) and RV5-vaccinated patients (P < .001) than in unvaccinated patients.
Table 1. Comparison of demographic characteristics and clinical features, including disease severity of RVGE, based on vaccine status
Next, we compared background and clinical features between 27 RV1 vaccinated and 28 RV5 vaccinated patients. Neither age nor gender differed significantly between the two groups. Maximum number (P = .007) and duration (P = .010) of vomiting were significantly higher in RV1-vaccinated patients than in RV5-vaccinated patients. However, severity of diarrhea (maximum number; P = .947, duration; P = .992) and fever (P = .092; maximum body temperature, P = .699) did not significantly differ between RV1-vaccinated and RV5-vaccinated patients. Finally, Vesikari score did not significantly differ between these two groups (P = .473).
Discussion
Unlike other vaccines such as those for measles, current formulations of RV vaccine do not protect the host against viral infection but can prevent the disease from becoming severe. Therefore, although implementation of a population-wide RV vaccination program can decrease the numbers of RVGE patients, sporadic outbreaks of RVGE still sometimes occur.Citation20,Citation21 Biennial RVGE outbreaks have occurred in the United States after introduction of the RV vaccine.Citation6 We previously reported a remarkable reduction in the numbers of RVGE-related hospital admissions and outpatient visits after increases in RV vaccine coverage and re-increase in number of RVGE patients in 2016 in Japan.Citation12 Subsequently, the numbers of RVGE patients were extremely low in 2017 and 2018, but as mentioned above, in 2019, sporadic RVGE outbreaks occurred again in several areas in Japan, including Aichi prefecture. Because data comparing the molecular epidemiology and clinical features of RV infection based on the vaccination status are sparse, we thought that this RVGE outbreak represented a good opportunity to elucidate the issue.
The age of RVGE patients has gradually increased after RV vaccination was implemented.Citation6,Citation21,Citation22 In this study, only 18% of patients were 1 year old or younger, and those aged 2–4 years and 5–7 years accounted for 44% and 25% of all patients, respectively. This finding is consistent with the idea that the age of RVGE patients in Japan is increasing, as in other countries. Furthermore, as patients get older, the proportion of unvaccinated patients increases, as shown in The cost of RV vaccination has been supported by several local governments since 2013; over the intervening time, vaccine coverage has increased from 40% to 80%.Citation12 Therefore, lower vaccination rates among children age 6 and older without local governmentʻs support is likely to be the main reason for the low proportion of RV vaccinated RVGE patients in the 5–7 years old group in this study.
Monitoring of RV genotype diversity, which can be induced by vaccine-related immune pressure, is important for controlling RVGE outbreaks. Like many other countries, in Japan, the main rotavirus strains circulating in pediatric patients were G1P[8], G2P[4], G3P[8], and G9P[8] before RV vaccine introduction.Citation23 Among the five RV genotypes that were common before vaccine introduction, only G2P[4] belongs to the DS-1–like constellation. By contrast, G1P[8], the original strain of RV1, belongs to the Wa-like constellation, similar to three other common RV genotypes (G3P[8], G4P[8], and G9P[8]). Therefore, there was a concern that the G2P[4] would become predominant after implementing universal immunization using the RV1 vaccine.Citation24–26 In fact, G2P[4] has become prevalent in Brazil, where RV1 is used for universal immunization.Citation22 On the other hand, as G2P[4] also increased even in neighboring Brazil, where the RV vaccine program was not implemented, it remains controversial that the increase in G2P[4] RV strains in Brazil was solely due to immune pressure caused by the introduction of the RV1. Meanwhile, a state-based vaccine choice strategy was adopted for the national immunization program in Australia. The increased diversity and differences in genotype predominance have been demonstrated in states using RV1 (G2P[4] and equine-like G3P[8]) and RV5 (G12P[8]), suggesting that different types of immune pressures are induced by different RV vaccines.Citation8,Citation27 In addition, the genotype distribution differs between vaccinated and unvaccinated patients in the United States, where both RV1 and RV5 are used for the national immunization program.Citation6 However, that study did not compare RV genotypes among RV1-vaccinated, RV5-vaccinated, and unvaccinated patients. Although the present study analyzed a limited number of samples and the number of non-G8P [8] infected patients may have been too small for meaning statistical analysis, the proportions of RV genotypes did not significantly differ between vaccinated and unvaccinated patients (P = .125). Long-term RV genotyping is needed to determine whether vaccine type–specific genotypic changes occur in regions using both RV1 and RV5 simultaneously. It is also important for the development of the RV vaccine that are highly effective against newly emerged RV genotypes.
In this study, the most prevalent genotype was DS-1–like G8P[8], irrespective of vaccine status and vaccine type. Previously, the G8 genotype was rarely detected outside of Africa. Subsequently, DS-1–like G8P[8] has emerged in several southeastern Asian countries, including Japan.Citation28–30 Furthermore, in 2017 in Australia, G8P[8] was the second most common RV genotype for all ages except in infants less than 6 months of age.Citation21 Vaccine-related immune pressure is thought to play an important role in expanding DS-1–like G8P[8] in these countries. Meanwhile, a systematic review and meta-analysis revealed that no specific emerging RV genotype has been continuously detected over the years.Citation31 Therefore, long-term monitoring of RV genotype distribution should be carried out to determine whether DS-1–like G8P[8] continues to predominate in Japan.
As shown in , the severity of RVGE symptoms and Vesikari score were significantly lower in RV1- and RV5-vaccinated patients than in unvaccinated patients. Although vaccinated patients were significantly younger than unvaccinated patients, it is well known that older children generally have milder symptoms due to repeated natural RV infection.Citation32 Unvaccinated older children might not have experienced repeated RV infections during infancy and early childhood due to the suppression of RVGE outbreaks after vaccine introduction. Therefore, the increase in number of RVGE patients in older children may be due to the attenuating herd immunity of them induced by RV vaccination. In any case, findings in the present study corroborates the large body of evidence for high vaccine effectiveness, even in patients with mild RVGE treated with outpatient clinics. To date, many studies have assessed efficacy of RV vaccine, and reported a marked reduction in the number of RVGE patientsCitation33,Citation34 and the RV detection rate in stool specimens collected from acute gastroenteritis patients.Citation6,Citation35,Citation36 In addition, a clinical studyCitation37 and a systematic review of vaccine effectivenessCitation31 demonstrated that RV1 and RV5 exert similar vaccine effectiveness against homotypic and heterotypic RV genotypes. Thus, this cohort study conducted on a regional RVGE outbreak supports the results of previous studies,Citation31, Citation37 demonstrating a clear impact of RV vaccines regardless of vaccine types.
Although Vesikari score did not significantly differ between RV1-vaccinated and RV5-vaccinated patients, severity of vomiting was significantly higher in the former group. The reason for this difference is unclear, but it is important to determine whether it is real. Therefore, a large number of RVGE patients should be analyzed in future studies to answer common questions asking which RV vaccine is better.
In conclusion, we investigated the molecular epidemiology and clinical characteristics of patients during a local RVGE outbreak after RV vaccine national immunization program. The proportion of RV genotypes did not differ depending on the presence or absence of vaccination or the type of vaccine used. Even though this cohort was in an outpatient setting, we clearly observed that vaccination decreased disease severity. The RV vaccine reduced RV gastroenteritis. However, RV outbreaks may have occurred due to attenuated herd immunity mainly on older children in the future. It is necessary to analyze clinical outcomes and distribution of RV genotypes in as in the present study.
Authorsʻ contribution
Dr. Kei Kozawa and Dr. Yoshiki Kawamura carried out conceptualization, data collection, data analysis, and literature research, and wrote and edited the manuscript. Dr. Fumihiko Hattori carried out data analysis and edited the manuscript. Dr. Yuki Higashimoto carried out data collection and edited the manuscript. Dr. Satoshi Komoto and Dr. Koki Taniguchi discussed and edited the manuscript. Dr. Tetsushi Yoshikawa contributed conceptualization, discussion, editing, and supervision.
Acknowledgement
Sampling and treatment of patients were graciously performed by Dr. Susumu Kawai, Dr. Naoko Itakura, Dr. Hiroshi Tanaka, Dr. Toshiko Maeda, Dr. Masato Hibi, Dr. Masahiro Ohashi, and Dr. Kyoko Suzuki. In addition, the authors are grateful for the technical support of Akiko Yoshikawa, Chieko Mori, and Yoko Osakabe and for helpful suggestions on statistical analysis by Hiroshi Yatsuya.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–7. doi:10.1016/S1473-3099(11)70253-5.
- Matthijnssens J, Joelsson DB, Warakomski DJ, Zhou T, Mathis PK, van Maanen MH, Ranheim TS, Ciarlet M. Molecular and biological characterization of the 5 human-bovine rotavirus (WC3)-based reassortant strains of the pentavalent rotavirus vaccine, RotaTeq®. Virology. 2010;403(2):111–27. doi:10.1016/j.virol.2010.04.004.
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58:1–25.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi:10.1056/NEJMoa052434.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a eentavalent Human–Bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi:10.1056/NEJMoa052664.
- Staat MA, Payne DC, Halasa N, Weinberg GA, Donauer S, Wikswo M, McNeal M, Edwards KM, Szilagyi PG, Bernstein DI, et al. Continued evidence of the impact of rotavirus vaccine in children less than 3 years of age from the United States new vaccine surveillance network: a multisite active surveillance program, 2006-2016. Clin Infect Dis. 2020;71(9):e421–e429. doi:10.1093/cid/ciaa150.
- Hungerford D, Allen DJ, Nawaz S, Collins S, Ladhani S, Vivancos R, Iturriza-Gómara M. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Euro Surveill. 2019;24(6):1700774. doi:10.2807/1560-7917.ES.2019.24.6.1700774.
- Roczo-Farkas S, Kirkwood CD, Cowley D, Barnes GL, Bishop RF, Bogdanovic-Sakran N, Boniface K, Donato CM, Bines JE. The impact of rotavirus vaccines on genotype diversity: a comprehensive analysis of 2 decades of Australian surveillance data. J Infect Dis. 2018;218(4):546–54. doi:10.1093/infdis/jiy197.
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Bányai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4(10):1303–16. doi:10.2217/fmb.09.96.
- Oishi T, Matsunaga M, Nakano T, Sudo S, Kuwajima H, Tokuriki S, Study SR. Letter from the editor. Hum Vaccin Immunother. 2020;16(1):1–7. doi:10.1080/21645515.2020.1704580.
- Kobayashi M, Miyazaki M, Ogawa A, Tatsumi M. Sustained reduction in rotavirus-coded hospitalizations in children aged <5 years after introduction of self-financed rotavirus vaccines in Japan. Hum Vaccin Immunother. 2020;16(1):132–37. doi:10.1080/21645515.2019.1638204.
- Yoshikawa T, Matsuki T, Sato K, Mizuno M, Shibata M, Hasegawa S, Morita M, Iwasa M, Gopala K, Holl K. Impact of rotavirus vaccination on the burden of acute gastroenteritis in Nagoya city, Japan. Vaccine. 2018;36:527–34. doi:10.1016/j.vaccine.2017.12.006.
- Asada K, Kamiya H, Suga S, Nagao M, Ichimi R, Fujisawa T, Umemoto M, Tanaka T, Ito H, Tanaka S, et al. Rotavirus vaccine and health-care utilization for rotavirus gastroenteritis in Tsu City, Japan. Western Pac Surveill Response J. 2016;7(4):28–36. doi:10.5365/wpsar.2016.7.3.005.
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–67.
- Miura H, Kawamura Y, Sugata K, Koshiyama N, Yoshikawa A, Komoto S, Taniguchi K, Ihira M, Yoshikawa T. Rotavirus vaccine strain transmission by vaccinated infants in the foster home. J Med Virol. 2017;89(1):79–84. doi:10.1002/jmv.24613.
- Wu H, Taniguchi K, Wakasugi F, Ukae S, Chiba S, Ohseto M, Hasegawa A, Urasawa T, Urasawa S. Survey on the distribution of the gene 4 alleles of human rotaviruses by polymerase chain reaction. Epidemiol Infect. 1994;112(3):615–22. doi:10.1017/S0950268800051311.
- Taniguchi K, Wakasugi F, Pongsuwanna Y, Urasawa T, Ukae S, Chiba S, Urasawa S. Identification of human and bovine rotavirus serotypes by polymerase chain reaction. Epidemiol Infect. 1992;109(2):303–12. doi:10.1017/S0950268800050263.
- Cunliffe NA, Woods PA, Leite JP, Das BK, Ramachandran M, Bhan MK, Hart CA, Glass RI, Gentsch JR. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J Med Virol. 1997;53(1):41–50. doi:10.1002/(SICI)1096-9071(199709)53:1<41:AID-JMV8>3.0.CO;2-Q.
- Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009;9(1):238. doi:10.1186/1471-2180-9-238.
- Hoque SA, Kobayashi M, Takanashi S, Anwar KS, Watanabe T, Khamrin P, Okitsu S, Hayakawa S, Ushijima H. Role of rotavirus vaccination on an emerging G8P[8] rotavirus strain causing an outbreak in central Japan. Vaccine. 2018;36:43–49. doi:10.1016/j.vaccine.2017.11.056.
- Maguire JE, Glasgow K, Glass K, Roczo-Farkas S, Bines JE, Sheppeard V, Macartney K, Quinn HE. Rotavirus epidemiology and monovalent rotavirus vaccine effectiveness in Australia: 2010-2017. Pediatrics. 2019;144(4):144. doi:10.1542/peds.2019-1024.
- Carvalho-Costa FA, de Assis RMS, Fialho AM, Araújo IT, Silva MF, Gómez MM, Andrade JS, Rose TL, Fumian TM, Volotão EM, et al. The evolving epidemiology of rotavirus A infection in Brazil a decade after the introduction of universal vaccination with Rotarix®. BMC Pediatr. 2019;19(1):42. doi:10.1186/s12887-019-1415-9.
- Thongprachum A, Khamrin P, Maneekarn N, Hayakawa S, Ushijima H. Epidemiology of gastroenteritis viruses in Japan: prevalence, seasonality, and outbreak. J Med Virol. 2016;88(4):551–70. doi:10.1002/jmv.24387.
- de Oliveira LH, Camacho LA, Coutinho ES, Ruiz-Matus C, Leite JP. Rotavirus vaccine effectiveness in Latin American and Caribbean countries: A systematic review and meta-analysis. Vaccine. 2015;33(Suppl 1):A248–54. doi:10.1016/j.vaccine.2014.11.060.
- Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, López P, MacíMacíAs-Parra M, Ortega-BarríBarríA E, Rivera-Medina DM, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–89. doi:10.1016/S0140-6736(08)60524-3.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–63. doi:10.1016/S0140-6736(07)61744-9.
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australiaʻs National Childhood vaccine schedule. Pediatr Infect Dis J. 2011;30(1):S25–9. doi:10.1097/INF.0b013e3181fefdee.
- Haque W, Haque J, Barai D, Rahman S, Moni S, Hossain ME, Faruque ASG, Ahmed S, Zaman K, Rahman M. Distribution of rotavirus genotypes in Dhaka, Bangladesh, 2012–2016: re-emergence of G3P[8] after over a decade of interval. Vaccine. 2018;36(43):6393–400. doi:10.1016/j.vaccine.2018.08.081.
- Guntapong R, Tacharoenmuang R, Singchai P, Upachai S, Sutthiwarakom K, Komoto S, Tsuji T, Tharmaphornpilas P, Yoshikawa T, Sangkitporn S, et al. Predominant prevalence of human rotaviruses with the G1P[8] and G8P[8] genotypes with a short RNA profile in 2013 and 2014 in Sukhothai and Phetchaboon provinces, Thailand. J Med Virol. 2017;89(4):615–20. doi:10.1002/jmv.24669.
- Kondo K, Tsugawa T, Ono M, Ohara T, Fujibayashi S, Tahara Y, Kubo N, Nakata S, Higashidate Y, Fujii Y, et al. Clinical and molecular characteristics of human rotavirus G8P[8] outbreak strain, Japan, 2014. Emerg Infect Dis. 2017;23(6):968–72. doi:10.3201/eid2306.160038.
- Leshem E, Lopman B, Glass R, Gentsch J, Bányai K, Parashar U, Patel M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(9):847–56. doi:10.1016/S1473-3099(14)70832-1.
- Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–28. doi:10.1056/NEJM199610033351404.
- Baker JM, Dahl RM, Cubilo J, Parashar UD, Lopman BA. Effects of the rotavirus vaccine program across age groups in the United States: analysis of national claims data, 2001–2016. BMC Infect Dis. 2019;19(1):186. doi:10.1186/s12879-019-3816-7.
- Pitzer VE, Bilcke J, Heylen E, Crawford FW, Callens M, De Smet F, Van Ranst M, Zeller M, Matthijnssens J. Did large-scale vaccination drive changes in the circulating rotavirus population in Belgium? Sci Rep. 2015;5(1):18585. doi:10.1038/srep18585.
- Hallowell BD, Parashar UD, Curns A, DeGroote NP, Tate JE. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination — United States, 2000–2018. MMWR Morb Mortal Wkly Rep. 2019;68(24):539–43. doi:10.15585/mmwr.mm6824a2.
- Enweronu-Laryea CC, Armah G, Sagoe KW, Ansong D, Addo-Yobo E, Diamenu SK, Mwenda JM, Parashar UD, Tate JE. Sustained impact of rotavirus vaccine introduction on rotavirus gastroenteritis hospitalizations in children <5 years of age, ghana, 2009-2016. Vaccine. 2018;36(47):7131–34. doi:10.1016/j.vaccine.2018.02.058.
- Payne DC, Boom JA, Staat MA, Edwards KM, Szilagyi PG, Klein EJ, Selvarangan R, Azimi PH, Harrison C, Moffatt M, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009-2011. Clin Infect Dis. 2013;57(1):13–20. doi:10.1093/cid/cit164.