ABSTRACT
While current live, oral rotavirus vaccines (LORVs) are reducing severe diarrhea everywhere, their effectiveness is lower in high burden settings. Alternative approaches are in advanced stages of clinical development, including injectable next-generation rotavirus vaccine (iNGRV) candidates, which have the potential to better protect children, be combined with existing routine immunizations and be more affordable than current LORVs. In an effort to better understand the real public health value of iNGRVs and to help inform decisions by international agencies, funders, and vaccine manufacturers, we conducted an impact and cost-effectiveness analysis examining 20 rotavirus vaccine use cases. We evaluated several currently licensed LORVs, one neonatal oral NGRV (oNGRV), one iNGRV, and one iNGRV-DTP (iNGRV comprising part of a DTP-containing combination) over a ten-year timeframe in 137 low- and middle-income countries. The most promising use case identified was a high efficacy iNGRV-DTP, predicted to have the lowest vaccine program cost (US$1.4 billion), the highest vaccine benefit (750,000 rotavirus deaths averted, 13 million rotavirus hospital admissions averted, US$ 2.7 billion health-care cost averted), and most favorable cost-effectiveness (cost-saving). iNGRV-DTP vaccine remained the most affordable, safe, and cost-effective option even when it was assumed to have equivalent efficacy to the current LORVs. This study shows that while the development of iNGRVs with superior efficacy to currently licensed LORVs would be ideal, iNGRVs with similar efficacy to LORVs would offer substantial public health value. It also highlights the economic value of accelerating the development of DTP-based combination vaccines that include iNGRV to provide rotavirus protection.
Introduction
Rotavirus remains the leading cause of diarrhea deaths globally despite the availability of vaccines. In 2019, rotavirus caused approximately 150,000 deaths in children younger than 5 years of age.Citation1,Citation2 Most of the rotavirus burden is found in low- and middle-income countries (LMICs). Live oral rotavirus vaccines (LORVs) have been available for more than a decade and are currently used in the national immunization programs of 110 countries worldwide.Citation3 LORVs have contributed to reducing the global rotavirus burden and are cost-effective interventions in most contexts.Citation4–9 However, clinical trials of LORVs have reported lower efficacy in LMICs where the rotavirus mortality burden is highest.Citation10 In addition, several studies of LORVs have reported an elevated risk of intussusception, a rare but serious bowel disorder, in some settings, occurring shortly after administration of the first and second dose.Citation11,Citation12 While other studies have reported no elevated risk of intussusceptionCitation13–15 and modeling studies have shown that the benefits would strongly outweigh any potential risk,Citation16 it is possible that concerns about vaccine safety may still be a barrier to adoption of LORVs in some countries.
One possible pathway to enhanced rotavirus vaccine impact and safety is the development of injectable next-generation rotavirus vaccines (iNGRVs).Citation17–20 Several iNGRVs are in development, with the most advanced in the pipeline being the trivalent P2-VP8 candidate (SK chemicals, Seongnam, South Korea).Citation20,Citation21 The intramuscular administration of iNGRVs offers three potential benefits. First, it is possible that iNGRVs could offer higher vaccine protection than LORVs in LMICs. Second, avoiding the oral route of administration may eliminate any safety concerns associated with intussusception. Third, iNGRVs could be combined with other injectable vaccines in the future, such as diphtheria, tetanus, pertussis (DTP)-containing vaccines like DTP pentavalent (diphtheria, tetanus, pertussis, haemophilus influenzae type B, hepatitis B).Citation18,Citation20
In parallel to clinical development, efforts to better understand the real public health value of iNGRVs are underway. This modeling study contributes to this effort, using recently updated information about the burden of rotavirus disease and the different rotavirus vaccine products, including the trivalent P2-VP8 candidate.Citation16,Citation22 In this analysis, we explored the potential benefits, risks, and cost-effectiveness of different use cases of an iNGRV i.e. different iNGRV schedules and co-administration scenarios. Because there are no clinical efficacy trial data yet available for iNGRVs, we compared scenarios in which iNGRV had efficacy equivalent to that of licensed LORVs, as well as scenarios assuming iNGRV would have substantially higher efficacy. We also modeled scenarios of the potential impact and cost-effectiveness of oral next-generation rotavirus vaccines (oNGRVs) because some are close to licensure and may exhibit higher efficacy, most notably the neonatal vaccine RV3-BB (Biofarma, Bandung, Indonesia).Citation23 We aimed to identify the most promising use cases and understand scenarios in which iNGRV could represent a viable alternative to oral rotavirus vaccines. More broadly, this analysis can help inform vaccine development and testing, policy, and investment decisions by international agencies, funders, and vaccine manufacturers.
Methods
Study design
We modeled the potential benefits, risks, and cost-effectiveness of a series of rotavirus vaccine use cases and product profiles for infants in 137 LMICs, from the societal perspective, from 2025 to 2034. We chose 2025 as our start year as this is the earliest expected World Health Organization prequalification year for the leading candidate, trivalent P2-VP8. We evaluated several currently licensed LORVs (ROTAVAC®, ROTASIIL®, and ROTARIX®), one neonatal oNGRV (e.g. RV3-BB), one iNGRV (e.g. trivalent P2-VP8) and one iNGRV-DTP (e.g. trivalent P2-VP8 comprising part of a DTP-containing combination vaccine). In the absence of available evidence about the feasibility of combining a rotavirus component with DTP-pentavalent or DTP-hexavalent, we assumed that such a combination would not create any physicochemical or immunological interference. We compared each option to no vaccination and to each other.
We used UNIVAC version 1.4.16, a proportionate outcomes model developed at the London School of Hygiene and Tropical Medicine, which has been described in detail elsewhere.Citation6,Citation16,Citation24–30 In brief, the model generates estimates of rotavirus disease events (cases, clinic visits, hospital admissions, deaths), intussusception disease events (cases, hospital admissions, deaths), and costs (vaccine program costs, healthcare costs) with or without rotavirus vaccination. UNIVAC is a static model and does not account for indirect effects of vaccination. The primary outcome measure is the cost (US$) per Disability Adjusted Life Year (DALY) averted. We interpret results using a range of thresholds covering .25 to 1 times each country’s Gross Domestic Product per capita (GDP p.c.), examining the percentage of countries where vaccines and vaccine use cases would be considered cost-effective under these benchmarks.Citation31 We used a 3% discount rate on costs and outcomes and all costs in the analysis are reported in 2018 US$.
Rotavirus disease burden
We assume that when infected with rotavirus, children under five will experience either a non-severe or a severe rotavirus gastroenteritis (RVGE) episode. We assume non-severe episodes resolve with or without seeking care in an outpatient setting while severe episodes resolve or are fatal, after seeking care in outpatient or inpatient settings or without seeking care. This study uses similar methods and data from previously published studies, including country-specific rates for non-severe cases, severe cases, clinic visits, hospital admissions and deaths aged <5 years caused by RVGE in the absence of vaccination (Table S1). In the event a country has already introduced an RV vaccine, disease burden estimates in the absence of vaccination are estimated from the most recent pre-vaccine year. All RVGE events were distributed into weeks of age <5 years.Citation6,Citation16
We included intussusception as a severe adverse event (SAE) in the analysis for LORVs and oNGRVs. We assumed a lower risk for oNGRV than for LORVs, and no elevated risk associated with iNGRVs and iNGRV-DTP. We estimated the number of excess intussusception cases, hospitalizations and deaths using previously described methods (Table S2).Citation9,Citation16 The relative risks of intussusception in the 1–7- and 8–21-day periods after the first and second doses were based on a global meta-analysis of self-controlled case series studies.Citation16
Vaccination scenarios and efficacy assumptions
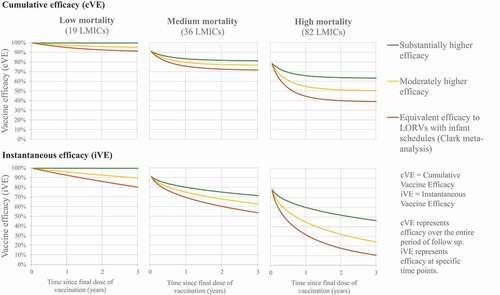
We evaluated 20 vaccine use case scenarios for different combinations of LORVs, oNGRV, iNGRV, and iNGRV-DTP. Because it is theoretically possible that increased overall rotavirus vaccine efficacy would occur if LORVs were administered alongside iNGRVs, we also evaluated various co-administration scenarios of these options (). Licensed LORVs are modeled using a base efficacy scenario informed by existing pooled estimates of vaccine efficacy by duration of follow-up from all published LORV randomized controlled trials as compiled by Clark et al. for low-, medium-, and high-mortality settings,Citation32 and assuming all licensed LORVs (ROTARIX, ROTAVAC, ROTASIIL) confer similar protection (, ). The scenario for oNGRV is evaluated using a moderately higher efficacy than used for licensed LORVs based on data from the RV3-BB clinical trial in Indonesia (, ).Citation23 Finally, the base cases for iNGRV and iNGRV-DTP assume a hypothetical substantially higher efficacy than used for licensed LORVs (, ). In the high mortality countries (n = 82), the moderately higher and substantially higher efficacy scenarios represent approximately a relative increase of 25% and 50% in cumulative efficacy after 12–18 months of follow-up, respectively, compared to the licensed LORVs. For medium mortality countries (n = 36), the increases were 5% and 10%, respectively. For low mortality countries (n = 19), the increases were 2% and 5%, respectively (, ). However, because there is substantial uncertainty about the potential efficacy of NGRVs, we also evaluated iNGRV and iNGRV-DTP with an efficacy comparable to licensed LORVs, and oNGRV with a substantially higher efficacy (). We present both the cumulative vaccine efficacy (cVE) and instantaneous vaccine efficacy (iVE) in and . The cVE corresponds to the main outcome reported in randomized controlled trials, i.e. the VE over a period of many weeks. But if there is evidence of vaccine waning, the cVE over the entire follow-up period might be different to the iVE at different times within that period of follow-up. For example, the VE after 12 months of follow-up could be 60%, but if there is waning, then the VE between 0–3 months of follow-up could be much higher (e.g. 80%) than the efficacy between 9–11 months of follow-up (e.g. 40%). Reporting the cumulative efficacy between 0 and 12 months of follow-up gives an accurate picture of the overall/average efficacy for the entire period, but masks differences in efficacy at specific times within that period of follow-up.Citation32
Table 1. Vaccination scenarios evaluated
For all scenarios, we assume that efficacy of any vaccine against non-severe RVGE was 85% of the efficacy value against severe RVGE.Citation33
Vaccine coverage and timeliness
We used infant DTP coverage rates as a proxy for rotavirus vaccine coverage as reported by WUENIC.Citation34 The schedule is therefore DTP1, DTP2, and DTP3 accounting for potential country-specific delays based on DHS and MICS data.Citation35 The only exception is for oNGRV for which the study uses a neonatal schedule where the first dose is given at birth, accounting for earlier protection. Vaccine coverage proxies for oNGRV in the main scenario are therefore BCG, DTP1, and DTP2 and associated delays. For three countries that do not report BCG coverage rate (Grenada, Lebanon, and Suriname) we used HEP B birth dose coverage rates instead of BCG, reported by the same source of data. Vaccine coverage rates per country are available in the supplementary files.
We assumed that rotavirus vaccines would achieve the same timeliness (coverage by week of age) as reported for other vaccines administered at the same visit, e.g., we assumed BGG timeliness for a neonatal rotavirus vaccine dose, and DTP1, DTP2, and DTP3 for rotavirus vaccine doses given later in infancy. We did not apply the manufacturer’s recommended age restrictions for rotavirus vaccine administration.Citation36
For countries already using rotavirus vaccines, we did not use estimates of rotavirus vaccine coverage as the indicator reported by WUENIC (rotaC) provides coverage rate of the last dose of rotavirus vaccine, without specifying if this is a second or a third dose, or which vaccine is in use.
Vaccine price
Vaccine prices are defined for each country depending on whether they can access Gavi, the Vaccine Alliance prices or the PAHO revolving fund, or if they do not benefit from any pricing agreements. For licensed LORVs, prices have been extracted from Gavi’s detailed product profile documents and the vaccine purchase database.Citation37,Citation38 Based on informal communications with vaccine developers and public health officials, we made assumptions for iNGRV, iNGRV-DTP, and oNGRV, the products not yet available on the market. The analysis uses full prices and does not account for Gavi co-financing to countries as the level and evolution of support to countries is difficult to project so far into the future. below provides each vaccine price by country group. Data on country-specific vaccine prices are available in the supplementary files.
Table 2. Vaccine characteristics, price per dose, and other inputs
Other vaccine parameters include wastage, syringe and safety box cost as applicable, international handling and transportation, and immunization delivery cost per dose. Wastage is dependent on each vaccine’s presentation. Presentations included in the analysis are the five-tube liquid presentations for ROTARIX and ROTASIIL, a 5-dose vial liquid presentation for ROTAVAC, a 2-dose vial liquid presentation for oNGRV and iNGRV, and finally bulk for iNGRV-DTP. For vaccine wastage, we used rates provided by Gavi’s detailed product profile documents, allocating 4% wastage rates to single-dose presentations and 10% to multi-dose presentations.Citation36 No wastage is associated with the bulk presentation. Safety box and syringe cost, when applicable, as well as handling and international transportation costs are derived from UNICEF. Though some countries in this analysis may not procure through UNICEF, we assume they would still have to support similar handling charges.Citation39,Citation40 Immunization delivery costs per dose came from the Immunization Delivery Cost Catalog.Citation41 Syringe costs are excluded for any oral vaccine option. Syringe, safety box cost, and immunization delivery costs are excluded for the iNGRV-DTP use case as they are already supported by immunization programs delivering DTP-containing combination vaccines. For all concomitant delivery use cases, we accounted for the incremental delivery cost for each dose given. All vaccines characteristics, price, and other vaccine-related inputs are available in .
Healthcare costs
RVGE-related clinic visit and hospitalization costs are taken from recently published estimates of the cost of diarrhea in children in LMICs.Citation42 Intussusception treatment costs are estimated for each country based on previously reported regional treatment patterns, estimated surgery costs, and assumptions on costs for non-operative management.Citation9 For both RVGE and intussusception, this study accounts for direct medical, direct non-medical, and indirect costs. Country-specific healthcare costs for RVGE and intussusception-related events are available in the supplementary files.
Probabilistic uncertainty analysis
We ran probabilistic sensitivity analysis (PSA) for all standalone vaccine use cases. For each PSA, we ran 1,000 iterations, randomly selecting inputs from their parameter distributions. The parameters included in the analysis as well as the statistical distributions used are available in the supplementary files.
Results
Over the 2025–2034 period, the estimated rotavirus vaccination program costs for all LMICs combined ranged from US$1.4 billion for iNGRV-DTP to US$32.3 billion for the co-administration of iNGRV with ROTARIX. The estimated number of rotavirus deaths aged <5 years without vaccination was ~1.5 million. In scenarios with vaccination, rotavirus deaths <5 years were estimated to be ~ 750,000 for all scenarios involving a high efficacy iNGRV, ~870,000 for the moderately high efficacy oNGRV scenario, and ~950,000 for currently licensed LORVs. The numbers of averted cases, visits, hospitalizations, and deaths are available in the supplementary files. The number of intussusception SAE cases, hospitalizations and deaths are also available from supplementary files. Average cost-effectiveness ratios (ACERs), where each scenario was compared to no vaccination, ranged from cost-saving (iNGRV-DTP) to US$1,510 (iNGRV with ROTARIX). Country-specific results for each use case are available in the supplementary files. For nearly all countries already using rotavirus vaccine in 2021, iNGRV and iNGRV-DTP were cost saving compared to the vaccine currently in use (Table S12).
Considering all the modeled criteria (cost, benefit, risk, cost-effectiveness), the most promising vaccine use case scenario was iNGRV-DTP (). This option was predicted to have the lowest vaccine program cost (US$1.4 billion), the highest vaccine benefit—750,000 rotavirus deaths averted, 13 million rotavirus hospital admissions averted, US$2.7 billion healthcare cost averted), the fewest excess cases of intussusception (zero), and the most favorable cost-effectiveness estimate (cost-saving). Furthermore, we estimate that a high efficacy iNGRV-DTP vaccine would dominate all other options i.e., provide the same or greater benefit at lower cost and with fewer excess cases of intussusception. Probabilistic uncertainty analyses confirm the superiority of iNGRV-DTP over iNGRV and LORVs ().
Figure 2. Deterministic cost-effectiveness and probabilistic uncertainty analysis results for all LMICs over 10 years starting in 2025.
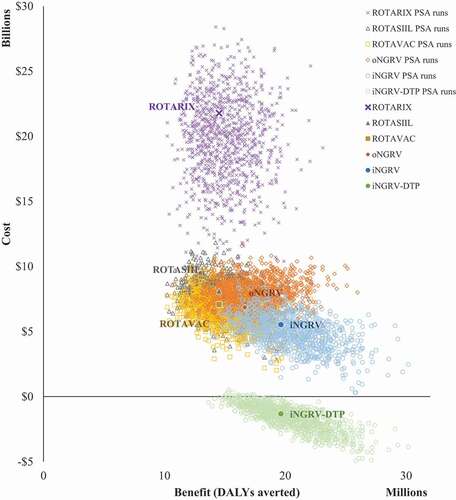
Table 3. Impact and cost-effectiveness results per vaccination scenario for all LMICs over 10 years starting in 2025
Even in a more conservative scenario where iNGRV-DTP was assumed to have equivalent efficacy to the current LORVs, such a moderate efficacy iNGRV-DTP vaccine was still estimated to be the most affordable, safe, and cost-effective option (Figure S1). However, in this scenario, a high efficacy neonatal oNGRV was estimated to offer greater benefits at a higher cost, and concomitant delivery of moderate efficacy iNGRV-DTP and LORV or oNGRV could also be considered (Figure S1).
We estimate that a high efficacy iNGRV-DTP vaccine would be cost-effective in all LMICs based on a WTP (willingness-to-pay) threshold as low as .25 times the national GDP per capita in each country (). In the absence of a high efficacy iNGRV-DTP vaccine, we estimate that a high efficacy iNGRV could provide the same benefit, albeit at a much higher cost (US$ 8.3 billion versus US$1.4 billion) and would be cost-effective in 84% of LMICs at .5 times the GDP per capita in each country. Probabilistic uncertainty analyses confirm the superiority of iNGRV over oNGRV with the majority of PSA runs for the high efficacy iNGRV generating results preferable to the PSA runs for oNGRV (). In the absence of any iNGRVs, a neonatal oNGRV would be the preferred option and would be cost-effective in 75% of LMICs at .5 times the GDP per capita threshold. Finally, if no NGRVs were available/licensed, then ROTAVAC would be the preferred option and would be cost-effective in 67% of LMICs at the same WTP threshold.
Table 4. Percentage of LMICs and Gavi countries with deterministic cost-effectiveness results below alternative national GDP per capita thresholds
Discussion
If iNGRVs are substantially more efficacious than licensed LORVs in clinical trials, an additional 200,000 rotavirus deaths could be prevented over a 10-year period compared to currently licensed LORVs. Our analysis shows that while currently available LORVs remain a good investment for countries and donors today, an iNGRV with comparable or superior efficacy to LORVs is likely to be cost-effective in the majority of LMICs and could provide increased impact and safety. A future vaccine that combines iNGRV with a DTP-containing vaccine into one formulation could be particularly favorable. Such a vaccine could provide the same or greater health benefit at lower cost. If countries using LORVs switched to iNGRV or to iNGRV-DTP, billions of dollars could be saved.
Concomitant delivery use cases involving entire courses of LORVs and iNGRVs to yield superior efficacy compared to either alone, however, are not as cost-effective. This is due to the cost of procuring and delivering multiple doses of multiple vaccines (up to 6). However, concomitant delivery use cases involving an iNGRV-DTP are more favorable in a large share of LMICs.
One of the most striking findings of our analyses is that inclusion of iNGRV within DTP-containing combinations was by far the most cost-effective of all the rotavirus vaccine options assessed—even when the efficacy of the iNGRV was no better than that of current LORVs. These and other results shown here lend support to the idea that, while development of an iNGRV with superior efficacy to currently licensed LORVs would be ideal, the development of an iNGRV with efficacy similar to that of LORVs would possess substantial public health value. In addition, these results highlight the economic value of accelerating the development of DTP-containing vaccines combined with iNGRV to provide rotavirus protection.
This analysis accounts solely for full vaccine prices. As such, results should be considered as potentially conservative for Gavi countries. Assuming Gavi financial support to countries for rotavirus vaccines is similar in 2025 and beyond, ICER values for supported vaccines would likely be more favorable, making most options even more cost-effective.
We applied the same immunization delivery cost per dose to each vaccine and country, based on their income group. There may be differences in the cost to deliver different vaccines, or from one country to another. We assumed that the incremental delivery cost per dose covers a range of expenses including potential switch costs for countries changing vaccines and introduction costs for countries that are not currently using rotavirus vaccines. Though a basic way to account for immunization program-related costs, we were not able to make better informed assumptions due to lack of data. We accounted for a wide range of uncertainty of that parameter in our uncertainty analyses.
Similarly, we used the ‘no vaccination’ comparator to maintain a consistent comparator across all countries included in the study and generate results for all LMICs, despite each country’s status regarding use of rotavirus vaccination.
For simplicity, we assumed no age restrictions in this analysis. Removing age restrictions favors rotavirus vaccines in terms of estimated benefits, but not in terms of cost-effectiveness as it involves spending more money on doses given later in age when they are less effective, i.e., after the peak of rotavirus disease. Running our analysis without age restrictions also emphasized the potential safety benefits of iNGRVs in comparison to oral vaccine options, as it provided an estimate of the maximum potential excess intussusception burden that could be caused by LORVs or oNGRVs, and therefore avoided by iNGRVs.Citation16
The methods used to develop the higher efficacy scenarios led to a starting efficacy value of 100% for low <5 mortality settings. While this is likely unrealistic, we elected to maintain it for completeness and consistency of methods applied to other settings and explore a lower range of starting efficacy in uncertainty analysis. It is important to note that cumulative vaccine efficacy falls below 100% in these settings.
We used a static model that does not account for any indirect effects of vaccination, such as herd effects. This is likely to make our results conservative. Recent studies in India and Niger showed that the direct impact of rotavirus vaccine predicted by static models gave results very similar to the overall impact predicted by transmission models.Citation43,Citation44 Impact studies in different countries have shown different results, with some countries witnessing short-term herd effects and some none.Citation16
These results contribute to better understanding the full public health value of iNGRVs. Our findings may inform the decision-making of donors and vaccine developers who wish to better understand the rotavirus vaccine use scenarios likely to have the most promising results in LMICs. Our analysis suggests there could be great value in accelerating the development of iNGRV vaccines, particularly those administered in combination with existing DTP-containing vaccines. These results should be considered alongside those from a recent feasibility and acceptability study conducted among national stakeholders and healthcare workers in several LMICs that showed substantial interest in iNGRVs, especially if they were part of a larger DTP combination.Citation45 Taken together, these analyses required us to make several assumptions yet to be confirmed by clinical studies but would support a positive public health value proposition for the next generation of injectable rotavirus vaccines in LMICs.
Supplemental Material
Download MS Word (654.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The model used is available online at https://www.paho.org/en/provac-toolkit
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2040329.
Additional information
Funding
References
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, Fullman N, Thompson RL, Abajobir A, Ahmed M, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909–10. doi:10.1016/S1473-3099(17)30276-1.
- GBD Results Tool. Institute for health metrics and evaluation; [accessed 2021 Apr 7]. http://ghdx.healthdata.org/gbd-results-tool.
- ViewHub. International Vaccine Access Center (IVAC); [accessed 2021 Mar 25]. https://view-hub.org/ .
- Haider S, Chaikledkaew U, Thavorncharoensap M, Youngkong S, Islam MA, Thakkinstian A. Systematic review and meta-analysis of cost-effectiveness of rotavirus vaccine in low-income and lower-middle-income countries. Open Forum Infect Dis. 2019;6(4):ofz117. doi:10.1093/ofid/ofz117.
- Kotirum S, Vutipongsatorn N, Kongpakwattana K, Hutubessy R, Chaiyakunapruk N. Global economic evaluations of rotavirus vaccines: A systematic review. Vaccine. 2017;35(26):3364–86. doi:10.1016/j.vaccine.2017.04.051.
- Debellut F, Clark A, Pecenka C, Tate J, Baral R, Sanderson C, Parashar U, Kallen L, Atherly D. Re-Evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. Lancet Global Health. 2019;7(12):e1664–e1674. doi:10.1016/S2214-109X(19)30439-5.
- Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. J Infect Dis. 2020;222(10):1731–39. doi:10.1093/infdis/jiaa081.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Global Health. 2019;7(7):e893–e903. doi:10.1016/S2214-109X(19)30207-4.
- Debellut F, Clark A, Pecenka C, Tate J, Baral R, Sanderson C, Parashar U, Atherly D. Evaluating the potential economic and health impact of rotavirus vaccination in 63 middle-income countries not eligible for Gavi funding: a modelling study. Lancet Global Health. 2021;9(7):e942–e956. doi:10.1016/S2214-109X(21)00167-4.
- Carvalho MF, Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Human Vaccin Immunother. 2018;15(6):1237–50. doi:10.1080/21645515.2018.1520583.
- Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation. Vaccine. 2016;34(32):3684–89. doi:10.1016/j.vaccine.2016.04.050.
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014;370(6):503–12. doi:10.1056/NEJMoa1303164.
- Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin Microbiol Infect. 2016;22:S128–S135. doi:10.1016/j.cmi.2016.03.007.
- Apte A, Roy S, Bavdekar A, Juvekar S, Hirve S. Facilitators and barriers for use of rotavirus vaccine amongst various stakeholders and its implications for Indian context – a systematic review. Human Vaccin Immunother. 2018;18:1–8. doi:10.1080/21645515.2018.1489190.
- Groome MJ, Tate JE, Arnold M, Chitnis M, Cox S, de Vos C, Kirsten M, le Grange SM, Loveland J, Machaea S, et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clin Infect Dis. 2020;70(8):1606–12. doi:10.1093/cid/ciz431.
- Clark A, Tate J, Parashar U, Jit M, Hasso-Agopsowicz M, Henschke N, Lopman B, Van Zandvoort K, Pecenka C, Fine P, et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modelling analysis of current and alternative schedules. Lancet Global Health. 2019;7(11):e1541–e1552. doi:10.1016/S2214-109X(19)30412-7.
- Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gómara M, Prendergast AJ, Grassly NC. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13(1):97–118. doi:10.2217/fmb-2017-0128.
- Lee B. Update on rotavirus vaccine underperformance in low- to middle-income countries and next-generation vaccines. Human Vaccin Immunother. 2021;17(6):1787–802. doi:10.1080/21645515.2020.1844525.
- Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, Diaz Z, Parashar UD, Kirkwood CD. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Human Vaccin Immunother. 2019;15(6):1215–27. doi:10.1080/21645515.2018.1553593.
- Fix A, Kirkwood CD, Steele D, Flores J. Next-Generation rotavirus vaccine developers meeting: summary of a meeting sponsored by PATH and the Bill & Melinda Gates Foundation (19–20 June 2019, Geneva). Vaccine. 2020;38(52):8247–54. doi:10.1016/j.vaccine.2020.11.034.
- Kirkwood CD, Ma L-F, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine. 2019;37(50):7328–35. doi:10.1016/j.vaccine.2017.03.076.
- Groome MJ, Fairlie L, Morrison J, Fix A, Koen A, Masenya M, Jose L, Madhi SA, Page N, McNeal M, et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: a multisite, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2020;20(7):851–63. doi:10.1016/S1473-3099(20)30001-3.
- Bines JE, Thobari JA, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, et al. Human neonatal rotavirus vaccine (RV3-BB) to target rotavirus from birth. N Engl J Med. 2018;378(8):719–30; [accessed 2021 Sep 15]. 10.1056/NEJMoa1706804.
- Anwari P, Debellut F, Pecenka C, Parwiz SM, Clark A, Groman D, Safi N. Potential impact and cost-effectiveness of rotavirus vaccination in Afghanistan. Vaccine. 2018;36(51):7769–74. doi:10.1016/j.vaccine.2017.10.058.
- Lusvan M-E, Debellut F, Clark A, Demberelsuren S, Otgonbayar D, Batjargal T, Purevsuren S, Groman D, Tate J, Pecenka C. Projected impact, cost-effectiveness, and budget implications of rotavirus vaccination in Mongolia. Vaccine. 2019;37(6):798–807. doi:10.1016/j.vaccine.2018.12.056.
- Lee H, Park SY, Clark A, Debellut F, Pecenka C, Kim DS, Kim HM, Kim JH, Cho H, Kim A-Y, et al. Cost-Effectiveness analysis of the implementation of a National Immunization Program for rotavirus vaccination in a country with a low rotavirus gastroenteritis-related mortality: a South Korean study. Vaccine. 2019;37(35):4987–95. doi:10.1016/j.vaccine.2019.07.030.
- Krishnamoorthy Y, Eliyas SK, Nair NP, Sakthivel M, Sarveswaran G, Chinnakali P. Impact and cost effectiveness of pneumococcal conjugate vaccine in India. Vaccine. 2019;37(4):623–30. doi:10.1016/j.vaccine.2018.12.004.
- Anwari P, Debellut F, Vodicka E, Clark A, Farewar F, Zhwak ZA, Nazary D, Pecenka C, Scott LaMontagne D, Safi N. Potential health impact and cost-effectiveness of bivalent human papillomavirus (HPV) vaccination in Afghanistan. Vaccine. 2020;38(6):1352–62. doi:10.1016/j.vaccine.2019.12.013.
- Pempa LA, Luangasanatip N, Kingkaew P, Adhikari D, Isaranuwatchai W, Choiphel D, Pecenka C, Debellut F. Economic evaluation of rotavirus vaccination in children of Bhutan. Vaccine. 2020;38(32):5049–59. doi:10.1016/j.vaccine.2020.05.035.
- Debellut F, Jaber S, Bouzya Y, Sabbah J, Barham M, Abu-Awwad F, Hjaija D, Ramlawi A, Pecenka C, Clark A, et al. Introduction of rotavirus vaccination in Palestine: an evaluation of the costs, impact, and cost-effectiveness of ROTARIX and ROTAVAC. PloS One. 2020;15(2):e0228506. doi:10.1371/journal.pone.0228506.
- 2019 growth domestic product per capita (current US$). The World Bank; [accessed 2021 Jan 15]. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- Clark A, van Zandvoort K, Flasche S, Sanderson C, Bines J, Tate J, Parashar U, Jit M. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis. 2019;19(7):717–27. doi:10.1016/S1473-3099(19)30126-4.
- Rogawski ET, Platts-Mills JA, Ross Colgate E, Haque R, Zaman K, Petri WA, et al. Quantifying the impact of natural immunity on rotavirus vaccine efficacy estimates: a clinical trial in Dhaka, Bangladesh (PROVIDE) and a simulation study. J Infect Dis Nat Immun Vaccine Effic. JID. 2018:861. doi:10.1093/infdis/jix668.
- WHO UNICEF coverage estimates WHO World Health Organization: immunization, vaccines and biologicals. Vaccine preventable diseases vaccines monitoring system 2020 Global Summary Reference Time Series: DTP3; [accessed 2021 Sep 15]. https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html .
- Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–49. doi:10.1016/S0140-6736(09)60317-2 .
- Rotavirus vaccines WHO position paper. World Health Organization; 2013. p. 49–64. Weekly Epidemiological Record Report No.: 5. https://www.who.int/wer/2013/wer8805.pdf?ua=1 .
- Product information for vaccines and cold chain equipment. Gavi The Vaccine Alliance; [accessed 2020 Apr 16]. https://www.gavi.org/our-alliance/market-shaping/product-information-vaccines-cold-chain-equipment .
- Market Information for Access to Vaccines (MI4A). World Health Organization; [accessed 2020 Apr 16]. https://www.who.int/teams/immunization-vaccines-and-biologicals/vaccine-access/mi4a/mi4a-vaccine-purchase-data#:~:text=MI4A%20Vaccine%20Purchase%20Database,database%20is%20updated%20every%20year .
- Auto-Disable AD syringes and Safety Boxes price data. UNICEF; [accessed 2020 Jul 14]. https://www.unicef.org/supply/reports/auto-disable-ad-and-re-use-prevention-rup-syringes-and-safety-boxes-price-data .
- Handling Fees. Supplies and Logistics. UNICEF; [accessed 2020 Jul 14]. https://www.unicef.org/supply/handling-fees .
- Immunization Costing Action Network. ImmunizationEconomics.Org; [accessed 2020 Apr 3]. https://immunizationeconomics.org/ican-home .
- Baral R, Nonvignon J, Debellut F, Agyemang SA, Clark A, Pecenka C. Cost of illness for childhood diarrhea in low- and middle-income countries: a systematic review of evidence and modelled estimates. BMC Public Health. 2020;20(1):619. doi:10.1186/s12889-020-08595-8 .
- Park J, Goldstein J, Haran M, Ferrari M. An ensemble approach to predicting the impact of vaccination on rotavirus disease in Niger. Vaccine. 2017;35(43):5835–41. doi:10.1016/2Fj.vaccine.2017.09.020 .
- Rose J, Homa L, Meropol SB, et al. Health impact and cost-effectiveness of a domestically-produced rotavirus vaccine in India: a model based analysis. PloS One. 2017:12e0187446. doi:10.1371/journal.pone.0187446 .
- Price J, Mooney J, Bain C, et al. National stakeholder preferences for next-generation rotavirus vaccines: results from a six-country study. Vaccine. 2022;40(2):370–79. doi:10.1016/j.vaccine.2021.11.009 .