ABSTRACT
A 60-year-old woman presented with a depressed lesion at the site of her first COVID-19 (Astra Zeneca) vaccine injection. The lesion was diagnosed as a case of injection related localized lipoatrophy as markers of autoimmune disease were negative and biopsy differentiated it from localized involutional lipoatrophy. This case of localized lipoatrophy was likely due to inadvertent subcutaneous injection of the COVID-19 vaccine with a 16 mm long needle.
Introduction
Localized lipoatrophy is a rare condition characterized by loss of subcutaneous adipose tissue in a particular area of the body.Citation1 It can be classifiedCitation1 as primary/idiopathic (localized involutional lipoatrophy—LIL) or secondary with particular host factors, connective tissue disease (lupus erythematosus, dermatomyositis, morphea, and T-cell lymphoma), and minor trauma and iatrogenesis (subcutaneous, intramuscular or dermal injection).
There are limited data on vaccine related localized lipoatrophy, with a female predominance and only with adjuvanted vaccines. Nine cases have been reported with DPT (diphtheria, pertussis, tetanus) vaccine (2 males, 7 females),Citation2,Citation3 two cases (2 female) with an adjuvanted influenza vaccine,Citation4,Citation5 and two cases with quadrivalent human papilloma virus (HPV) vaccine given only to females.Citation6,Citation7
In this presentation, a case of localized lipoatrophy is reported following a Covid-19 vaccination.
Patient presentation
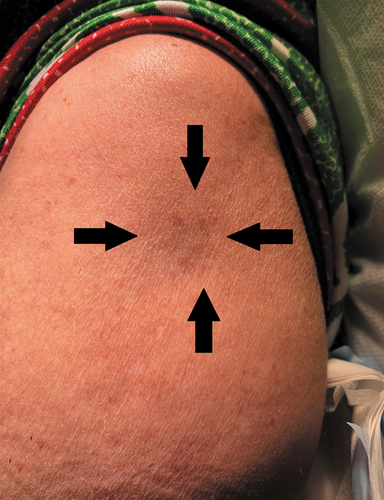
A fit 60-year-old woman (height 156.6cms, weight 76 kg, BMI 31.2) from Hay in NSW was given a first dose of Covid-19 vaccine (Astra Zeneca), on 25.5.2021, into the left deltoid muscle with a 16 mm long, orange hubbed needle. She reported no significant pain post vaccination but slowly developed an indentation shown as the arrowed area (). Routine blood tests (FBC, U/E, CR, LFT, CRP, TSH) were negative as were markers of autoimmune diseases (antinuclear antibodies—ANA, antinuclear cytoplasmic antibodies -ANCA and extractable nuclear antigens—ENA.
Ultrasound on 13/7/2021 revealed an area of subcutaneous fat necrosis at this site measuring 25 × 5 × 12 mm with no collection or hyperemia. Biopsy from its lateral border revealed a cuff of adipose tissue adjacent to a cavity which penetrated to the muscle.
Histology revealed a mild, predominantly perivascular lymphocytic infiltrate in the dermis with vascular ectasia. A second vaccination of COVID-19 (Astra Zeneca) with a 25 mm long needle gave no similar reaction.
Discussion
An extensive narrative reviewCitation8 has allowed a strong recommendation to be made for intramuscular compared with subcutaneous injection for all vaccine types (adjuvanted, live virus and non-adjuvanted [inactivated whole cell, split cell, and subunit]) where route comparative data have been published.
The recommendation that Covid-19 vaccines should be given intramuscularly is drawn from some of the data in this review.Citation9 The needle length for intramuscular injection of the deltoid muscleCitation10 has been shown to depend on sex and body mass indexCitation11 with a 16 mm long needle unlikely to penetrate muscle in a female BMI ≥ 30.
Therefore, the case of lipoatrophy reported here is likely due to inadvertent subcutaneous of the Covid-19 vaccine as the patient had a BMI of 31.2, and a 16 mm long needle was used to administer the vaccine. This case can be differentiatedCitation12 from localized involutional lipoatrophy (LIL) on the basis of histopathology, with LIL showing the presence of diminutive fat lobules composed of small adipocytes that resemble fetal fat tissue.
Other cases of inadvertent subcutaneous administration of a Covid-19 vaccine have been reported by NgCitation13 and Gyldenleve et al.Citation14 In the latter case, punch biopsy showed perivascular lymphocyte infiltration in the dermis as in the case reported here. Both studies stress the importance of correct intramuscular administration of Covid-19 vaccines.
It is not practical to measure the body mass index of all people presenting for Covid-19 vaccination, but vaccinators should be able to give all males and non-obese females injection with a 25 mm long needle and obese females injection with a 32-28 mm long needle to prevent subcutaneous injection.Citation11
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tanrikulu O, Yesilova Y, Aksoy M. A case of bilateral acquired localized lipoatrophy. Case Rep Dermatol. 2016;8(2):185–2. doi:10.1159/000447256.
- Sardana K, Garg VK, Bhushan P, Relhan V, Sharma S. DPT vaccine-induced lipoatrophy: an observational study. Int J Dermatol. 2007;46(10):1050–54. doi:10.1111/j.1365-4632.2007.03268.x.
- Rahul S. Localized lipoatrophy following DPT injection. J Case Rep. 2016;6(1):132–34. doi:10.17659/01.2016.0032.
- Mayet A, Ligier C, Gache K, Manet G, Nivoix P, Dia A. Adverse events following pandemic influenza vaccine Pandemrix® reported in the French military forces—2009–2010. Vaccine. 2011;29(14):2576–81. doi:10.1016/j.vaccine2011.01.056.
- Javelle E, Soulier B, Brosset C, Lorcy S, Simon F. Delayed focal lipoatrophy after AS03-adjuvanted influenza A (H1N1) 2009 vaccine. Vaccine. 2011;29(6):1123–25. doi:10.1016/j.vaccine2010.12.018.
- Stephan F, Korkomaz J, Abadjian G, Okais J, Tomb R. A case of lipoatrophy following quadrivalent human papilloma virus vaccine administration. J Am Acad Dermatol. 2014;70(6):e132–4. doi:10.1016/j.jaad.2013.09.038.
- Ojaimi S, Buttery JP, Korman TM. Quadrivalent human papillomavirus recombinant vaccine associated lipoatrophy. Vaccine. 2009;27(36):4876–78. doi:10.1016/j.vaccine.2009.06.026.
- Cook IF. Subcutaneous vaccine administration – an outmoded practice. Hum Vacc Immunother. 2021;17(5):1329–41. doi:10.1080/21645515.2020.1814094.
- Park JH, Lee HK. Delivery routes for Covid-19 vaccines. Vaccines (Basel). 2021;9(5):524. doi:10.3390/vaccines9050524.
- Poland GA, Borrud A, Jacobson RM. Determination of deltoid fat pad thickness: implications for needle length in adult immunization. Jama. 1997;277(21):1709–11. doi:10.1001/jama.1997.03540450065037.
- Cook IF, Williamson M, Pond D. Definition of needle length required for intramuscular deltoid injection in elderly adults: an ultrasonographic study. Vaccine. 2006;24(7):937–40. doi:10.1016/j.vaccine.2005.08.098.
- Hong KC, Noh TW, Baek JH, Kang YS, Lee UH, Park HS. Localized involutional lipoatrophy in a child. Ann Dermatol. 2013;25(1):124–26. doi:10.5021/ad.2013.25.1.124.
- Ng JY. Inadvertent subcutaneous injection of Covid-19 vaccine. Postgrad Med J. 2021;97(1148):400. doi:10.1136/postgradmedj-2021-139870.
- Gyldenlove M, Skov L, Hansen CB, Garred P. Recurrent injection-site reactions after incorrect subcutaneous administration of a Covid-19 vaccine. Jeadv. 2021;35(9):e545–6. doi:10.1111/jdv.17341.