ABSTRACT
The current scenario of typhoid fever warrants early prevention with typhoid conjugate vaccines in susceptible populations to provide lifelong protection. We conducted a multicenter, single-blind, randomized, Phase 2/3 study to assess the immunogenicity and safety of Biological E’s Typhoid Vi-CRM197 conjugate vaccine (TyphiBEVTM) compared to Vi-TT conjugate vaccine manufactured by Bharat Biotech International Limited (Typbar-TCV; licensed comparator) in healthy infants, children, and adults from India. The study’s primary objective was to assess the non-inferiority of TyphiBEVTM in terms of the difference in the proportion of subjects seroconverted with a seroconversion threshold value of ≥2.0 µg/mL against Typbar-TCV. A total of 622 healthy subjects (311 each in both vaccine groups) were randomized and received the single dose of the study vaccine. The TyphiBEVTM group demonstrated noninferiority compared to the Typbar-TCV group at Day 42. The lower 2-sided 95% confidence interval limit of the group difference was −.34%, which met the non-inferiority criteria of ≥10.0%. The geometric mean concentration (24.79 µg/mL vs. 26.58 µg/mL) and proportion of subjects who achieved ≥4-fold increase in antiVi IgG antibody concentrations (96.95% vs. 97.64%) at Day 42 were comparable between the TyphiBEVTM and Typbar-TCV vaccine groups. No apparent difference was observed in the safety profile between both vaccine groups. All adverse events reported were mild or moderate in intensity in all age subsets. This data demonstrates that TyphiBEVTM is non-inferior to TypbarTCV in terms of immunogenicity, and the overall safety and reactogenicity in healthy infants, children, and adults studied from India was comparable.
Introduction
Typhoid fever is a devastating systemic infectious disease in humans. Enteric fever is the collective term used in describing the systemic infection caused by Salmonella enterica subspecies serovars Typhi and Paratyphi A, B, and C causing typhoid and paratyphoid fevers.Citation1,Citation2 Humans are the only host for Salmonella. Typhi (S. Typhi). The bacteria are acquired through the ingestion of contaminated food and water. Once inside the body, via the intestinal mucosa, it enters the liver, spleen, bone marrow, and in some instances, even the gall bladder.Citation3 Typhoid and paratyphoid infections primarily cause bacteremic febrile illnesses, with classic signs, such as prolonged high fever, headache, and malaise.Citation2,Citation4
The disease poses a significant risk to human health in low- and middle-income countries, especially due to extensively drug resistant strains of S. Typhi.Citation3 An estimated 14.3 million cases of typhoid and paratyphoid fever and 135.9 thousand deaths due to the disease have occurred globally in 2017.Citation2 Enteric fever is a major health problem in developing countries like India.Citation5,Citation6 India had reported 24, 45,611 cases of enteric fever in the year 2019.Citation7 Large-scale community studies conducted in an urban slum setting in India have reported the incidence of enteric fever as high as 2/1000 population/year in those under 5 years and 5.1/1000 population/year in under 10 years of age.Citation6 Children are affected disproportionately by typhoid fever. A prospective surveillance study of a community-based cohort has reported the incidence rate of typhoid per 1000 person-years as 27·3 for children under 5 years, 11·7 for those between 5 and 19 years and 1.1 for those between 19 and 40 years.Citation8 Based on hospital-based estimates, another study from India suggested the disease incidence in children in the 1–4 age group to be between 1 and 5 per 1000 child years.Citation9 A study from Bangladesh has also reported the incidence of typhoid fever to be highest among preschool children. The study reported 18.7 episodes/1,000 person-years typhoid fever incidence among preschool children compared to 2.1 episodes/1,000 person-years among older participants.Citation10 Another study from sub-Saharan Africa reported the adjusted incidence rate of typhoid fever significantly higher in children aged 2–14 years than those above 15 years.Citation11 Higher incidences of typhoid fever are also reported in children between the age group of 5–15 years from South Asian countries, including India, Indonesia, and Pakistan.Citation12
The main cause of this disease’s higher incidence is the lack of safe drinking water and lack of improved sanitation. In addition, lower social class, overcrowding, illiteracy, irregular latrine uses, and failure to wash hands are associated with an increase in cases of enteric fever.Citation13 The use of vaccines against S. Typhi will reduce the dependence on antibiotics in treatment of the disease and subsequently will help reduce antibiotic resistance.Citation14 World Health Organization (WHO) has recommended the usage of vaccines against typhoid since 1999.Citation15 The Indian Academy of Pediatrics has also recommended typhoid conjugate vaccine be included in the national immunization schedule and to vaccinate children at 6-9 months of age.Citation16 Typhoid vaccine is also recommended for travelers to endemic areas for enteric fever.Citation17 Currently, there are three typhoid vaccines approved for use namely, the newer generation typhoid conjugate vaccine (TCV) consisting of Vi polysaccharide (Vi-PS) antigen linked to tetanus toxoid protein,Citation18 the unconjugated Vi-PS vaccine,Citation19 and the live attenuated Ty21a vaccine.Citation20 The surface polysaccharides of bacteria which are widely used in vaccine are T independent antigens that are poorly immunogenic.Citation21 Hence, the unconjugated Vi-PS confers protection against typhoid fever through the formation of serum immunoglobulin G (IgG) antiVi, and it does not develop immunological memory due to which even a booster dose is ineffective. A Cochrane review has found the efficacy of Vi-PS vaccine to be 69% at year 1 and 59% at year 2.Citation22 Also, the immune response generated is of short duration with poor immunogenicity in infants.Citation23,Citation24 The Ty21a vaccine produces humoral and cell-mediated immune responses.Citation6 A randomized placebo-controlled field trial in school children between ages of 6–19 in Chile, using three every other day doses of Ty21 vaccine, have reported the enteric-coated capsule format to provide 67% protection over 3 years and 62% protection over 7 years of follow-up. The same study also reported a liquid vaccine formulation of Ty21 using every other day schedule to elicit 77% protection over 3 years and 78% over 5 years of follow-up.Citation25 WHO recommends the use of TCV in all age groups in view of its improved immunological properties, usage in younger children, and longer duration of protection. TCV is recommended to be administered as a .5 mL single dose intramuscularly for infants and children from 6 months of age and in adults up to 45 years in typhoid endemic regions.Citation24 Peda-Typh™ and Typbar-TCV are reported to provide protection anywhere between 2 and 5 years respectivelyCitation26,Citation27
The conjugate Vi vaccine, where the Vi-PS is covalently linked to a carrier protein, is safe and immunogenic in infants and younger children and induces protective antiVi antibodies, stimulates memory cells, and produces an immune response.Citation26,Citation28 An injectable subunit Vi-capsular polysaccharide vaccine is a second-generation typhoid vaccine.Citation28 Vi—CRM197 conjugate vaccine contains the Vi—CRM197 conjugate in which Vi-PS from Citrobacter freundii WR7011 is conjugated to CRM197, a nontoxic mutant of diphtheria toxoid.Citation21 Studies using the Vi—CRM197 conjugate vaccine have been reported to be safe and more immunogenic compared to licensed Vi-PS in European adults.Citation29 It has also been reported to be safe and immunogenic in infants, children and adults from south and southeast Asia.Citation30
Biological E’s Typhoid Vi-CRM197 conjugate vaccine (TyphiBEVTM) is a glyco-conjugated vaccine developed based on the conjugation of the Vi polysaccharide separately with the CRM197 carrier protein. Due to the T-cell-dependent immunological properties of glycol-conjugates, this conjugate vaccine is expected to overcome the limitations of the currently available polysaccharide vaccines and offer an effective solution for immunizing not only young children ≥2 years but also in infants and toddlers <2 years of age with a long-lasting immune response against S. Typhi infection. Peda-TyphTM from Biomed India and the Vi-TT conjugate vaccine from Bharat Biotech International Limited (Typbar-TCV) were the only two typhoid vaccines available in India at the time of conduct of this study. The present study was conducted to demonstrate safety and immunogenic non-inferiority in terms of difference in the seroconversion rates after a single intramuscular (IM) dose of Biological E’s Vi-CRM197 conjugate vaccine (TyphiBEVTM) in ≥6 months to <64-year-old healthy infants, children, adolescents, and adults in comparison with the TypbarTCV (licensed comparator) at Day 42.
Methods
Study population and study design
Study population
Healthy subjects of either gender between ≥6 months to <64 years of age were included in the study. Subjects with a history of typhoid infection, vaccination against typhoid, contact to an individual with laboratory confirmed S. Typhi infection, any serious or chronic disease, bleeding disorder, autoimmune disease, use of immunosuppressants, allergic reaction to vaccine-related components, body temperature ≥38.0°C within 3 days prior to the day of vaccination, or unwillingness or inability to understand and follow study procedures were excluded from the study recruitment.
Of the 10 study sites chosen, the study protocol was approved by the respective Institutional Ethics Committees (IEC) of 9 study sites where the study was carried out. One of the selected sites could not take up the protocol for ethics review as the IEC was not active during the period of the study. The study was registered prospectively with Clinical Trial Registry of India (CTRI) [Regd. No. CTRI/2018/11/016419]. The study was conducted in accordance with the ethical principles defined in the Declaration of Helsinki, International Council for Harmonization Good Clinical Practices (ICH-GCP) guidelines, and applicable regulatory requirements. Written informed consent and/or assent was obtained from each subject, or from their parents/legally authorized representatives of all children involved in the study before the enrollment.
Study design
This was a multicenter, single-blind, randomized, Phase 2/3 clinical study to assess the overall safety and immunogenic non-inferiority of TyphiBEVTM vaccine compared to Typbar-TCV available in India in healthy infants, children, and adults. A total of 622 subjects were enrolled in the study. The total duration of the study was 42 days, with 2 visits, that is, Visit 1 (Day 0) and Visit 2 (Day 42) with +7-day time window. Post-screening, all eligible healthy subjects of either gender were block randomized into one of the 2 treatment groups viz., the TyphiBEVTM group or the Typbar-TCV group in 1:1 ratio using Interactive Web Response System (IWRS) using Statistical Analysis System (SAS) program. A stratified block randomization (Proc Plan) was used for treatment allocation in this study.
Both vaccine groups comprised of three age subsets: ≥6 months to <2 years, ≥2 years to <18 years, and ≥18 years to <64 years. The subjects in both vaccine groups received a single .5 mL dose of study vaccine intramuscularly.
Immunogenicity evaluations
Immunogenicity was the primary objective of this study. Immunogenicity analysis was based on anti-Vi IgG serum antibodies measured at approximately 42 days following completion of single-dose immunization. The subject sera samples (collected pre-vaccination and 42 days after vaccination) were tested for anti-Vi IgG antibodies using the Vacczyme kit (Vacczyme Human Anti-S Typhi Vi IgG Enzyme Immunoassay Kit manufactured by the Binding Site Group UK). Reference standard for anti-Vi IgG (expressed in microgram/mL) provided by Center for Biologics Evaluation and Research (CBER)/National Institutes of Health (NIH) and National Institute for Biological Standards and Control (NIBSC) (expressed in IU/mL) were also tested on the same kit to establish the conversion factors for reporting of anti-Vi IgG concentration in Vacczyme units/mL to µg/mL and IU/mL.
The immunogenicity measurements included:
Assessment of seroconversion rates in both vaccine groups was measured by the number and percentage of subjects who achieved anti-Vi IgG antibody concentration ≥2.0 µg/mL in the post-vaccination blood sample (Day 42).
The comparison of the geometric mean concentration (GMC) of antiVi IgG antibodies as determined by enzyme-linked immunosorbent assay (ELISA) in both vaccine groups during pre (Day 0) and post vaccination (Day 42) period.
The proportion of subjects who achieved ≥4-fold rise in antiVi IgG antibodies at Day 42 from baseline was assessed in both vaccine groups. Geometric mean fold rise (GMFR) in antiVi IgG antibody concentrations was assessed along with their 2-sided 95% confidence interval (CI) from baseline at Day 42.
Immunogenicity assessment in terms of percentage of subjects equal to or above the suggested protective threshold value of ≥4.3 μg/mL (measured by ELISA) was also assessed in the present study on an exploratory basis. As per the WHO TRS No.1030, 2021, Annex.2, this threshold value was found to be associated with a high level of sustained protection lasting 4 years after vaccination.Citation31
Safety evaluations
The evaluation of the safety and tolerability of the investigational vaccine was the secondary objective of this study. Following the vaccination, the subjects were observed for 30 minutes for any adverse reactions. During the 7-day follow-up (Day 0 to Day 6), the solicited local and systemic adverse events (AEs), were recorded in a diary by the subjects’ parents, or legally acceptable representative. The proportion of subjects with unsolicited AEs during the follow-up period until Day 42 of post vaccination in both study groups was also reported. Medically attended and serious adverse events (SAEs), if any, during the post-vaccination 42-day follow-up period, was also recorded in both vaccine groups. Vital signs, physical examination, recording of body temperature, and clinical symptomatology were also evaluated during the study.
Statistical methods
Sample size determination
As per published data, the licensed comparator vaccine is shown to offer a seroconversion rate of 98.05% in 6–23 month old healthy infants and toddlers and 97.29% in 2 – 45-year-old healthy subjects after single dose.Citation32 For a formal power of 80% and for a significance level of 5%, a sample size of 282 infants and toddlers in age subset 1 (n = 141/group) and a sample size of 340 children, adolescents, and adults in age subset 2 (n = 170/group), was needed to demonstrate immunogenic noninferiority against licensed comparator. The non-inferiority margin was set at minus 10% points that was based on statistical considerations and clinical judgment. The sample size included a 10% dropout allocation at each age subset.
Analysis sets
The intent-to-treat analysis (ITT) population included all subjects randomized to one of the treatment groups. The per protocol (PP) population included all evaluable subjects who met all the eligibility criteria and followed the procedures defined in the protocol for whom data concerning immunogenicity endpoint measures were available. The safety population included all subjects who entered the study and received the vaccination.
Statistical analyses
Demographic and baseline characteristics were summarized descriptively. For continuous variables n, mean, standard deviation (SD), median, minimum, and maximum were presented. For categorical data, frequencies, and relative frequencies were computed.
The primary objective of the study was to show non-inferiority, in terms of the difference in the proportion of subjects who achieved anti-Vi IgG antibody concentration ≥2.0 μg/mL after administration of TyphiBEVTM compared with Typbar-TCV at Day 42. A 2-sided 95% CI for the difference in the proportion of subjects between both vaccine groups, achieving seroconversion ≥2.0 μg/mL threshold, was calculated by pooled Z-test. Non-inferiority was established if the lower limit of the 2-sided 95% CI for difference in proportions was on the positive side of the minus 10% points NI margin set.
Geometric mean concentrations of anti-Vi IgG antibodies, pre (Day 0) and post-vaccination (Day 42), as determined by ELISA, the proportion of subjects who achieved ≥4-fold rise in antiVi IgG antibodies at Day 42, and GMFR in anti-Vi IgG antibody concentrations along with their 2-sided 95% CI at Day 42 were summarized descriptively. Summary analysis of data was also presented in terms of using NIBSC and by both standard (IU/mL) and by Vacczyme units (U/mL) for GMC evaluations.
The AEs were recorded throughout the study duration (42 + 7 days). All reported AEs during the entire study period were summarized by calculating frequencies and relative frequencies by treatment group and were listed, including severity, relationship to the vaccine (causality) and action taken. Number, percentage, and 95% CI for percentage of subjects with AEs (solicited local and systemic AEs, and unsolicited AEs) and SAEs, and cumulative incidence rates were presented overall and by body system/preferred term and by treatment group. The 2-sided 95% CIs were calculated by Clopper–Pearson method for all the occurrence rates of reported AEs and SAEs during the study. All SAEs and medically attended AEs reported during the study were analyzed for expectedness and causality. All SAEs and/or AEs leading to withdrawal were also listed by subject and treatment group. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v22.0.
Comparative statistics for the safety variables were calculated, the study was designed to detect differences in the incidence of local and systemic reactions between vaccination groups descriptively. Systemic tolerability was assessed through recording of body temperature and clinical symptomatology. Changes in body temperature, from study start to end of study was analyzed descriptively by treatment group and was part of study evaluation, only in case of clinically significant changes.
Results
Study population
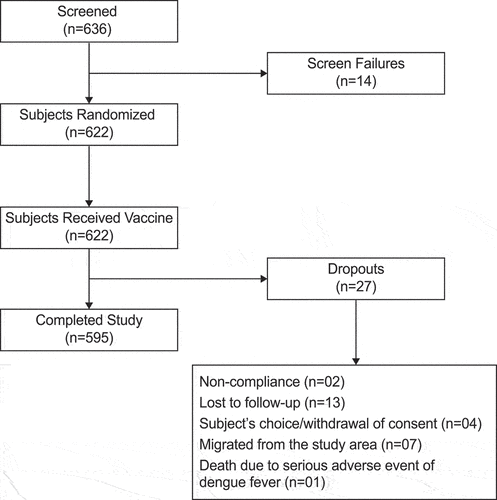
This study was carried out at 9 sites in India. Of the 636 subjects screened, 622 subjects (311 in TyphiBEVTM group and 311 in TypbarTCV group) were randomized into the study and received the single dose of the study vaccine. Both vaccine groups were further divided into three age subsets: age subset 1 consisting of healthy infants and toddlers aged ≥6 months to <2 years (n = 141), age subset 2 consisting of healthy children and adolescents aged ≥2 years to <18 years (n = 85) and age subset 3 consisting of old healthy adults aged ≥18 years to <64 years (n = 85) in each treatment group.
Of the 622 subjects, 595 (95.66%) subjects completed the study (296 in TyphiBEVTM group and 299 in TypbarTCV group respectively), and 27 (4.34%) subjects did not complete the study (15 in TyphiBEVTM group and 12 in Typbar-TCV group) due to the following reasons: lost to follow-up (13 subjects), migration from the study area (7 subjects), subjects’ choice/withdrawal of consent (4 subjects), noncompliance to study protocol (2 subjects), and death due to SAE (dengue fever) in 1 subject (from Typbar-TCV group) which was considered unrelated the study vaccine. ().
Demographics and baseline characteristics
The detailed demographic and baseline characteristics are described in . Demographics characteristics were analyzed in the ITT population in both TyphiBEVTM (n = 311) and Typbar-TCV (n = 311) groups. The range for the subject’s age at the time of vaccination was 6 months to 53.9 years. The male/female ratio was 1.02. The range for subject’s height and weight at the time of vaccination was 50.0 to 187.9 Cms and 6.2 to 98.0 Kgs, respectively. The demographic profile of the three age subsets was comparable between both vaccine groups for mean age, gender, weight, and height.
Table 1. Demographics and baseline characteristics-ITT population
Analgesics were the major class of concomitant medication taken during the 42 day post-vaccination period. The incidence of analgesics administration during the 42-day post-vaccination period was 2.84% and 4.26% in subjects aged ≥6 months to <2 years; 2.35% and 2.35% in subjects aged ≥2 years to <18 years and 1.18% and 2.35% in subjects with ≥18 years to <64 years in the TyphiBEVTM and Typbar-TCV group, respectively.
Immunogenicity findings
Immunogenicity assessments were primarily based on the PP population that consisted of 591/622 (95%) subjects. Overall, 295/311 (94.86%) subjects in the TyphiBEVTM group and 296/311 (95.18%) subjects in the Typbar-TCV group were included in the immunogenicity analysis. The proportion of subjects with anti-Vi IgG antibody concentrations above the seroconversion threshold ≥2.0 µg/mL (primary) were 98.98% (292/295 subjects) and 99.32% (294/296 subjects) in the TyphiBEVTM and Typbar-TCV groups, respectively . In the present study, the difference in seroconversion rates between the treatment groups at Day 42 was −0.34%, with its lower limit of 95% CI −1.82% and upper limit of 1.14% ().
Table 2. Summary of seroconversion (≥2 µg/ml) rates for Anti-Vi IgG antibody concentration by three age subsets and treatment groups-PP population
In the age subsets of ≥6 months to <2 years, ≥2 years to <18 years, and ≥18 years to <64 years, the difference (95% CI) in seroconversion rates between the treatment groups at Day 42 was −0.78%, 1.22%, and −1.19%, respectively, with a lower and upper confidence limit at (2.29%, 0.74%) for ≥6 months to <2 years, (1.16%, 3.60%) for ≥2 years to <18 years, and (5.19%, 2.81%) for ≥18 years to <64 years. Hence, the TyphiBEVTM demonstrated non‑inferiority compared to TypbarTCV as the lower limit of 95% CI of the difference in seroconversion rate was above the pre-defined noninferiority limit of −10%. In all three age subset analyses, the proportion of subjects seroconverted was similar in the TyphiBEVTM and Typbar-TCV groups.
Overall, the GMC at Day 42 was 24.79 µg/mL in the TyphiBEVTM group and 26.58 µg/mL in the Typbar-TCV groups . The geometric mean ratio between the TyphiBEVTM group and the Typbar-TCV group was .93. The difference in GMCs between the treatment groups was considered not clinically meaningful. The GMCs at Day 42 in the TyphiBEVTM vs TypbarTCV groups were 20.66 vs 29.66 µg/mL, 27.24 vs 32.69 µg/mL, and 29.92 vs 18.34 µg/mL, respectively, in the age subset of ≥6 months to <2 years, ≥2 years to <18 years, and ≥18 years to <64 years, respectively ().
Table 3. Summary of geometric mean concentrations for three age subsets by treatment groups-PP population
The proportion of subjects who achieved ≥4-fold increase in anti-Vi IgG antibody concentrations at Day 42 from baseline were 96.95% (286/295 subjects) with a GMFR of 223.38 in the TyphiBEVTM group against 97.64% (289/296 subjects) with a GMFR of 260.81 in the Typbar-TCV group ().
Table 4. Fold increase of IgG antibody concentrations from baseline to Day 42 by vaccine group-PP population
In the additional exploratory immunogenicity analysis, a threshold value of 4.3 μg/mL anti-Vi antibody measured by ELISA was used as mentioned in Annex-2 of WHO TRS No.1030, 2021, which was found to be associated with a high level of sustained protection lasting 4 years after vaccination.Citation28
On using this threshold of ≥4.3 µg/mL of anti-Vi IgG antibody concentrations, it was observed that the seroconversion rate was 95.59% (282/295 subjects) and 96.28% (285/296 subjects) in the TyphiBEVTM group and Typbar-TCV group, respectively. The seroconversion rate corresponding to sustained protection threshold in the TyphiBEVTM group vs Typbar-TCV group was 96.90% vs 99.23%, 95.12% vs 97.56%, and 94.05% vs 90.48%, respectively, in the age subset of ≥6 months to <2 years, ≥2 years to <18 years, and ≥18 years to <64 years.
The testing was performed using Vacczyme kit from Binding Site Group Ltd., and initial number was reported in Vacczyme units (VU/mL). These values were then converted to µg/mL based on CBER standard and into IU/mL based on NIBSC standard.
Safety findings
Safety analysis was performed on the safety cohort, which included 622 subjects (311 subject each in the TyphiBEVTM and Typbar-TCV groups) who received the vaccination. Overall, 113 AEs (35 in the TyphiBEVTM group and 78 in the Typbar-TCV group) were reported in the study by 26/311 (8.36%) subjects in the TyphiBEVTM group and 47/311 (15.11%) subjects in the Typbar-TCV group (Supplementary Table S1). The incidence rate of AEs was lower in the TyphiBEVTM group compared with the Typbar-TCV group in all age subsets; 9.93% vs 15.60% in the subset of ≥6 months to <2 years, 10.59% vs 15.29% in the subset of ≥2 years to <18 years, and 3.53% vs 14.12% in the subset of ≥18 years to <64 years.
The majority of AEs were mild or moderate in severity. The most commonly reported AEs (>2% of subjects in any treatment group) in the TyphiBEVTM and Typbar-TCV groups were injection site pain (14/311 [4.50%] subjects vs 29/311 [9.32%] subjects), pyrexia (7/311 [2.25%] subjects vs 13/311 [4.18%] subjects), injection site erythema (2/311 [.64%] subjects vs 10/311 [3.22%] subjects), and injection site swelling (1/311 [.32%] subjects vs 8/311 [2.57%] subjects) (Supplementary Table S2). The incidence of local AEs was 5.14% (16/311 subjects) in the TyphiBEVTM group and 11.90% (37/311 subjects) in the Typbar-TCV group. The most commonly reported local AEs (>2% of subjects in any treatment group) in the TyphiBEVTM and Typbar-TCV groups were injection site pain (14/311 [4.50%] subjects vs 29/311 [9.32%] subjects), injection site erythema (2/311 [0.64%] subjects vs 10/311 [3.22%] subjects), and injection site swelling (1/311 [0.32%] subjects vs 8/311 [2.57%] subjects) ().
Figure 2. Local and systemic adverse events reported. TyphiBEVTM =Biological E’s Typhoid Vi-CRM197conjugate vaccine, Typbar-TCV=Bharat Biotech’s Typbar-Typhoid Vi-TTconjugate vaccine.
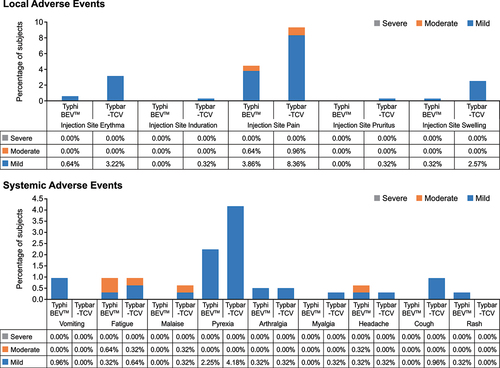
The incidence of systemic AEs was 3.86% (12/311 subjects) in the TyphiBEVTM group and 5.47% (17/311 subjects) in the Typbar-TCV group. The most commonly reported systemic AE (>2% of subjects in any treatment group) in the TyphiBEVTM and TypbarTCV groups was pyrexia (7/311 [2.25%] subjects vs 13/311 [4.18%] subjects) (). There were no AEs reported in the first 30-min post-vaccination period. One subject from the Typbar-TCV group reported an SAE of dengue fever of Grade 3 severity on Day 29. On Day 38, the subject died due to dengue shock syndrome with multiple organ dysfunction syndrome. The investigator considered the event unrelated to the study medication.
The incidence of medically attended AEs was 2.25% (7/311 subjects) in the TyphiBEVTM group and 4.50% (14/311 subjects) in the Typbar-TCV group. The most commonly reported medically attended AE (>2% of subjects in any treatment group) in the TyphiBEVTM and TypbarTCV groups was pyrexia (7/311 [2.25%] subjects vs 9/311 [2.89%] subjects). All the medically attended AEs resolved without sequel: the incidence of AEs within 7 days post-vaccination period was lower in the TyphiBEVTM (26/311 [8.36%] subjects) group compared with the Typbar-TCV (46/311 [14.79%] subjects) group. Three unsolicited AEs were reported in the study by three subjects from the TypbarTCV group. All three AEs (dengue fever [SAE led to the death of subject], Varicella zoster [considered to be mild in intensity by the investigator], and wheezing) were considered unrelated to the study medication by the investigator. No subject was withdrawn from the study due to any adverse event. No marked clinically significant changes over time were observed in the vital signs recorded.
Discussion
The present study was a multicentric, single-blind, randomized, controlled, Phase 2/3 study to evaluate immunogenicity and safety of single IM dose of TyphiBEVTM in infants and toddlers ≥6 months to <2 years, in children and adolescents ≥2 years to <18 years, and in adults ≥18 years to 64 years of age, in comparison with a licensed comparator TypbarTCV. Studies have reported the Vi conjugate vaccine to be superior to unconjugated vaccines in offering better and longer protection, involving memory cells in addition to the production of Vi antibodies. The Vi conjugate vaccine is reported to be safe and immunogenic in infants and children.Citation6,Citation23,Citation29 The results of present study are in line with these findings.
Animal studies have shown that glycoconjugate vaccine Vi-CRM197 stimulates the production of Vi-specific serum IgG1 titers that persisted for more than 60 days post vaccination. The intestinal washes of the animals studied also had Vi-specific IgG antibodies. The study also reported the Vi-CRM197 to stimulating T cell response that augmented the Vi-specific B cells to produce antibodies.Citation33 In the present study, a single-dose Vi-CRM197 conjugate vaccine induced a strong anti-Vi immune response as demonstrated with the overall proportion of subjects seroconverted (99%) in the age group of ≥6 to <64 years. This finding was in line with the findings of another study that reported a single dose of Vi-CRM197 conjugate vaccine to induce a strong anti-Vi immune response in adults, children, and infants aged 9–11 months with a seroconversion rate of 100%.Citation30 A Phase 1 study comparing EuTCV, a Vi-CRM197 conjugate vaccine to Typbar-TCV and Vi Polysaccharide vaccine Typhim Vi® in Filipino adults has also reported anti-Vi IgG antibody titer to be higher in Vi-CRM197 vaccine than Typbar-TCV and Typhim Vi.Citation34 The proportion of subjects seroconverted in EuTCV and Typbar-TCV was 100%, compared to 84% in Typhim Vi. In line with the study, the proportion of subjects seroconverted was comparable across all 3 age subsets in both vaccine groups in the present study. There was also no significant difference in terms of proportion of subjects achieving ≥4-fold increase in anti-Vi IgG antibody concentrations at Day 42 from baseline between treatment groups. The seroconversion rates corresponding to the sustained protection threshold were also comparable in the TyphiBEVTM group vs. Typbar-TCV group in all age subsets in the present study. The primary objective of demonstrating immunogenic non-inferiority of TyphiBEV™ compared with the TypbarTCV was also achieved with the lower limit of 95% CI (−1.82%, 1.14%) for the difference in proportion (−0.34%) of subjects seroconverted (≥2.0 µg/mL) was above the minus 10% points.
In contrast to the previous study, which reported comparable rates of adverse events in Vi-CRM197 vaccine and other vaccines,Citation30 the overall incidence of AEs (including local, systemic, medically attended, and unsolicited AEs) reported during the present study was lower in the TyphiBEV™ group (8.36%; 26/311 subjects) compared with Typbar-TCV group (15.11%; 47/311 subjects). The incidence of AEs was similar in all three age subsets studied in the TyphiBEV™ group. The most commonly reported AEs in both vaccine groups were injection site pain, pyrexia, injection site erythema, and injection site swelling. The majority of AEs in both vaccine groups were mild in their intensity. The present data clearly shows that the TyphiBEV™ vaccine is well tolerated in all age groups tested, and its’ safety profile is consistent with that of licensed Typbar-TCV used as a comparator in the present study.
Study limitations: The present study is not double-blinded. In this study, we presented immunogenicity and safety data only until Day 42 as the follow-up of a Phase 3 study is ongoing, assessing immunological persistence of anti‑Vi IgG antibodies at 12, 24, and 36 months. The Phase 3 study also will assess the immune response to a booster dose of conjugate typhoid vaccine in both treatment groups administered at 36 months in subjects (6 months to 45‑year-old) who participated in the present study.
In conclusion, this study demonstrated the primary immunogenicity objective of noninferiority of TyphiBEVTM in terms of the difference in the proportion of subjects seroconverted (protective threshold value ≥2.0 µg/mL) against TypbarTCV. In addition, the non-inferior immunogenicity was also shown by the exploratory analysis using WHO referred correlate of protection threshold value of ≥4.3 µg/mL. The safety and tolerability profile of the TyphiBEVTM was comparable to the TypbarTCV in healthy infants, children, adolescents, and adults from India.
Authors’ contribution
All authors met the ICMJE criteria for authorship and were responsible for conception and design of the research, acquisition of data, and its analysis. All the authors were involved in revising the manuscript critically for important intellectual content and approved the final manuscript.
Supplemental Material
Download MS Word (17.1 KB)Supplemental Material
Download MS Word (17.6 KB)Acknowledgments
The authors appreciate the study participants, the investigators, and study coordinators for their contributions to this study. The authors would like to thank Scientific Advisory Board (SAB) and management of Biological E Limited for their support and valuable guidance. The writing support for the manuscript was provided by Pavithran Purushothaman, Shivanika Sharma, and Vivek Rane, of Kinapse (a Syneos Health Company).
Disclosure statement
Subhash Thuluva, Vikram Paradkar, Ramesh Matur, Kishore Turaga, and Subba Reddy G. V. are employees of Biological E Limited.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2043103.
Additional information
Funding
References
- Azmatullah A, Qamar FN, Thaver D, Zaidi AK, Bhutta ZA. Systematic review of the global epidemiology, clinical and laboratory profile of enteric fever. J Glob Health. 2015;5(2). doi:10.7189/jogh.05.020407.
- Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE. GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of disease study 2017. Lancet Infect Dis. 2019;19:369–11. doi:10.1016/s1473-3099(18)30685-6.
- Yang Y-A, Chong A, Song J. Why is eradicating typhoid fever so challenging: implications for vaccine and therapeutic design. Vaccines. 2018;6:45. doi:10.3390/vaccines6030045.
- Khan K. Recent trends in typhoid research- A review. Int J Biosci. 2012;2:110–20.
- Makkar A, Gupta S, Khan ID, Gupta RM, Rajmohan KS, Chopra H, Gupta M, Bansal S, Poonia B, Malik M, et al. Epidemiological profile and antimicrobial resistance pattern of enteric fever in a tertiary care hospital of North India - a seven year ambispective study. Acta Medica (Hradec Kralove). 2018;61:125–30. doi:10.14712/18059694.2018.130.
- Mukhopadhyay B, Sur D, Gupta SS, Ganguly NK. Typhoid fever: control & challenges in India. Indian J Med Res. 2019;150:437–47. doi:10.4103/ijmr.IJMR_411_18.
- National Health Profile India: Central Bureau of Health Intelligence. Directorate general of health services. India: Ministry of Health & Family Welfare, Government of India; 2020.
- Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, and Bhan MK. Typhoid fever in children aged less than 5 years. Lancet. 1999;354:734–37. doi:10.1016/s0140-6736(98)09001-1.
- John J, Bavdekar A, Rongsen-Chandola T, Dutta S, Kang G. Estimating the incidence of enteric fever in children in India: a multi-site, active fever surveillance of pediatric cohorts. BMC Public Health. 2018;18:18. doi:10.1186/s12889-018-5498-2.
- Brooks WA, Hossain A, Goswami D, Sharmeen AT, Nahar K, Alam K, Ahmed N, Naheed A, Nair GB, and Luby S, et al. Bacteremic typhoid fever in children in an Urban Slum, Bangladesh. Emerg Infect Dis. 2005;11:326–29. doi:10.3201/eid1102.040422.
- Marks F, Von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Global Health. 2017;5:e310–e23. doi:10.1016/s2214-109x(17)30022-0.
- Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh DG, Ali M, and Shin S, et al. Domi Typhoid Study Group. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86:260–68. doi:10.2471/blt.06.039818.
- Divyashree S, Nabarro LEB, Veeraraghavan B, Rupali P. Enteric fever in India: current scenario and future directions. Trop Med Int Health. 2016;21:1255–62. doi:10.1111/tmi.12762.
- Andrews JR, Baker S, Marks F, Alsan M, Garrett D, Gellin BG, Saha SK, Qamar FN, Yousafzai MT, and Bogoch I, et al. Typhoid conjugate vaccines: a new tool in the fight against antimicrobial resistance. Lancet Infect Dis. 2019;19:e26–e30. doi:10.1016/s1473-3099(18)30350-5.
- Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. The Lancet. 2015;385:1136–45. doi:10.1016/s0140-6736(13)62708-7.
- Kasi SG, Shivananda S, Marathe S, Chatterjee K, Agarwalla S, Dhir SK, Verma S, Shah AK, Srirampur S, and Kalyani S, et al. Indian Academy of Pediatrics (IAP) advisory committee on vaccines and immunization practices (ACVIP): recommended immunization schedule (2020-21) and update on immunization for children aged 0 through 18 years. Indian Pediatr. 2021;58:44–53. doi:10.1007/s13312-021-2096-7.
- Beran J, Goad J. Routine travel vaccines. Travel medicine. London: Elsevier; 2019. pp. 89–100. doi:10.1016/b978-0-323-54696-6.00011-2.
- Szu SC. Development of Vi conjugate – a new generation of typhoid vaccine. Expert Rev Vaccines. 2013;12:1273–86. doi:10.1586/14760584.2013.845529.
- Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, Dutta S, Donner A, Kanungo S, and Park JK, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–44. doi:10.1056/nejmoa0807521.
- World Health O. Surveillance standards for vaccine-preventable diseases. Geneva: World Health Organization; 2018.
- Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, Rappuoli R, Szu S, Saul A, and Martin LB. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011;29:712–20. doi:10.1016/j.vaccine.2010.11.022.
- Milligan R, Paul M, Richardson M, Neuberger A. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2018. doi:10.1002/14651858.cd001261.pub4.
- Verma R, Bairwa M, Chawla S, Prinja S, Rajput M. New generation typhoid vaccines: an effective preventive strategy to control typhoid fever in developing countries. Hum Vaccin. 2011;7:883–85. doi:10.4161/hv.7.8.16282.
- World Health O. Typhoid vaccines: WHO position paper, March 2018 - recommendations. Vaccine. 2019;37:214–16. doi:10.1016/j.vaccine.2018.04.022.
- Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17:S22–S7. doi:10.1016/S0264-410X(99)00231-5.
- Vashishtha VM, Kalra A. The need & the issues related to new-generation typhoid conjugate vaccines in India. Indian J Med Res. 2020;151:22–34. doi:10.4103/ijmr.IJMR_1890_17.
- Balaji Chinnasami K, Vivekanandhan A, Arunachalam P, Pasupathy S. A study on longevity of immune response after vaccination with Salmonella Typhi Vi conjugate vaccine (Pedatyph™) in children. J Clin Diagn Res. 2015;9:SC01–SC3. doi:10.7860/jcdr/2015/.5903.
- Syed KA, Saluja T, Cho H, Hsiao A, Shaikh H, Wartel TA, Mogasale V, Lynch J, Kim JH, and Excler JL, et al. Review on the recent advances on typhoid vaccine development and challenges ahead. Clin Infect Dis. 2020;71:S141–S50. doi:10.1093/cid/ciaa504.
- van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, Rondini S, Micoli F, Qasim Khan RM, and Marchetti E, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM(1)(9)(7) conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One. 2011;6:e25398. doi:10.1371/journal.pone.0025398.
- Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, and Juvekar S, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14:119–29. doi:10.1016/S1473-3099(13)70241-X.
- World Health Organization. Recommendations to assure the quality, safety and efficacy of typhoid conjugate vaccines, Annex 2, TRS No 1030. WHO Expert Committee on Biological Standardization Report of the seventy-second and seventy-third meetings. Geneva: World Health Organisation, 2020.
- Typbar-TCV (Typhoid Vi Capsular Polysaccharide-Tetanus Toxoid Conjugate Vaccine). Summary of product charactristics. India: CDSCO; 2018. pp. 1–7.
- Fiorino F, Ciabattini A, Rondini S, Pozzi G, Martin LB, Medaglini D. Immunization with the conjugate vaccine Vi-CRM197 against Salmonella Typhi induces Vi-specific mucosal and systemic immune responses in mice. Vaccine. 2012;30:6111–14. doi:10.1016/j.vaccine.2012.05.081.
- Choi SK, Baik YO, Kim CW, Kim SK, Oh IN, Yoon H, Yu D, and Lee C . An open-label, comparative, single dose, clinical phase study to assess the safety and immunogenicity of typhoid conjugate vaccine (Vi-CRM197) in healthy Filipino adults. Vaccine. 2021;39:2620–27. doi:10.1016/j.vaccine.2021.03.089.