ABSTRACT
Dengue (DENV) is a mosquito-borne virus with four serotypes causing substantial morbidity in tropical and subtropical areas worldwide. V181 is an investigational, live, attenuated, quadrivalent dengue vaccine. In this phase 1 double-blind, placebo-controlled study, the safety, tolerability, and immunogenicity of V181 in baseline flavivirus-naïve (BFN) and flavivirus-experienced (BFE) healthy adults were evaluated in two formulations: TV003 and TV005. TV005 contains a 10-fold higher DENV2 level than TV003. Two-hundred adults were randomized 2:2:1 to receive TV003, TV005, or placebo on Days 1 and 180. Immunogenicity against the 4 DENV serotypes was measured using a Virus Reduction Neutralization Test (VRNT60) after each vaccination and out to 1 year after the second dose. There were no discontinuations due to adverse events (AE) or serious vaccine-related AEs in the study. Most common AEs after TV003 or TV005 were headache, rash, fatigue, and myalgia. Tri- or tetravalent vaccine-viremia was detected in 63.9% and 25.6% of BFN TV003 and TV005 participants, respectively, post-dose 1 (PD1). Tri- or tetravalent dengue VRNT60 seropositivity was demonstrated in 92.6% of BFN TV003, 74.2% of BFN TV005, and 100% of BFE TV003 and TV005 participants PD1. Increases in VRNT60 GMTs were observed after the first vaccination with TV003 and TV005 in both flavivirus subgroups for all dengue serotypes, and minimal increases were measured PD2. GMTs in the TV003 and TV005 BFE and BFN groups remained above the respective baselines and placebo through 1-year PD2. These data support further development of V181 as a single-dose vaccine for the prevention of dengue disease.
Introduction
Dengue, a member of the Flaviviridae family, is a prevalent vector-borne virus transmitted to humans by mosquitos of the Aedes genus. Dengue disease is caused by four closely related dengue virus (DENV) serotypes, DENV1, DENV2, DENV3, and DENV4, which cocirculate over time in endemic areas putting an estimated 3.9 billion people at risk of infection.Citation1 Transmission primarily occurs in tropical and subtropical regions including South America, South Asia, Southeast Asia, the Caribbean, the Pacific Islands, and Africa.Citation1,Citation2 The endemic areas and associated disease burden are increasing due to travel and trade, rural to urban migration, population growth, and an increasing distribution of the Aedes mosquito vector.Citation3 Approximately 50 million of these infections manifest clinicallyCitation4 causing substantial morbidity and an estimated 8,000–20,000 deaths per year.Citation5,Citation6 In recognition of the rising burden of dengue, the World Health Organization (WHO) highlighted dengue as one of the top threats to global health in 2019.
Recovery from infection from one DENV serotype provides durable homotypic protectionCitation7and short-term heterotypic cross protection against symptomatic infection with other serotypes.Citation8–10 Once this short-term heterotypic immunity wanes, there is an increased risk of developing severe dengue from subsequent heterologous infection. This increase in severe disease in secondary infection is hypothesized to be immune-related through antibody-dependent enhancement (ADE).Citation11 According to this hypothesis, preexisting antibodies from a primary DENV infection may bind, but not neutralize an infecting DENV during a subsequent infection with a different dengue serotype. The antibody-viral complex then binds to Fcγ receptors (FcγR) on monocytes and may increase the overall replication of the virus, increasing the risk of severe disease. Therefore, current vaccine research and development efforts are focused on candidates that simultaneously generate protective and durable immunity against all four serotypes. Since preexisting dengue serostatus impacts response to dengue vaccination, it is important to evaluate immunogenicity and safety in both flavivirus-experienced and flavivirus-naïve populations.
Currently, Dengvaxia ® [CYD-TDV] (Sanofi Pasteur, Lyon, France), is the only licensed vaccine for dengue prophylaxis. CYD-TDV is a chimeric dengue—yellow fever vaccine shown to be efficacious for the prevention of dengue disease in seropositive individuals. In contrast, however, clinical studies demonstrated an increase in the incidence of hospitalization and severe illness in vaccinated individuals who had no previous dengue infections and children under the age of 9 years.Citation12–14 Studies later demonstrated that the younger children who were vaccinated and later hospitalized, were more frequently dengue seronegative as compared to the older participants.Citation12 Thus, age was a surrogate for dengue-seronegative status. This resulted in a WHO recommendation for pre-vaccination screening and vaccination only in people with a confirmed history of infection, or where screening is not feasible, to limit vaccination to endemic areas with seroprevalence rates over 80% by the age of 9 years,Citation15 and also within a limited age range (in most countries 9–45 years). Further, the U.S. Advisory Committee on Immunization Practices (ACIP) recently recommended the vaccine in children 9 to 16 with laboratory confirmed previous DENV infection living in dengue endemic areas. The development of a vaccine which is safe and effective in both children and adults regardless of baseline dengue serostatus would overcome barriers to widespread vaccine uptake.
V181 is a live-attenuated, quadrivalent (rDENV1∆30, rDENV2/4∆30(ME), rDENV3∆30/31, and rDENV4∆30) investigational dengue virus vaccine. In the current study, the vaccine was evaluated in two formulations, TV003 and TV005, which are identical with the exception that the DENV2 component was given at a 10-fold higher dose in TV005. Studies of the analogous live-attenuated DENV components evaluated by the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the National Institutes of Health (NIH),Citation16–21 and Instituto ButantanCitation22 showed the vaccine to be well tolerated and demonstrated robust immunogenicity, supporting further evaluations of the vaccine candidate. The purpose of the current study was to assess the safety, tolerability, and immunogenicity of V181 in flavivirus-experienced and flavivirus-naïve healthy adults.
Methods
Study design
This was a phase 1, 3-arm, randomized, placebo-controlled, multi-center, blinded trial of a live, attenuated quadrivalent dengue vaccine V181 (protocol #V181–001) in flavivirus-naïve and flavivirus-experienced 18- to 50-year-old healthy adults. The study was conducted to evaluate the safety, tolerability, and immunogenicity of V181; participants were enrolled at 5 study sites (3 in the continental United States and 2 in Puerto Rico) and participated from January 2018 through November 2019. Eligible participants were stratified by geography (continental United States and Puerto Rico) using an interactive response technology. Participants were randomized in a 2:2:1 ratio to receive 2 doses of a .5 mL subcutaneous injection of either active vaccine (TV003 or TV005 formulations of V181) or placebo. Dose 1 was administered at Day 1 and dose 2 at Month 6. Each volunteer participated in the study for approximately 1.5 years, from the time of signing the informed consent form until the final study visit.
Dose selection and timing for the second dose were based upon data from previous phase I studies with the TV003 and TV005 formulations conducted by NIAID. The sample size of the study provided 97.5% confidence that if no vaccine-related serious adverse events (SAE) were observed among the 80 participants in each V181 vaccination group, that the underlying percentage of participants with vaccine-related serious adverse events is <4.6% in each of the V181 vaccination groups.
The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional ethical review boards and regulatory agencies. In addition to the routine safety monitoring in the trial, a standing internal Data Monitoring Committee (siDMC) monitored safety, including checking for pre-specified trial pause and stopping rules. Written informed consent was obtained from each participant prior to any study procedure.
Objectives
The primary objective of this study was to assess the safety and tolerability of V181. The secondary objective was to determine the immunogenicity of V181 by evaluating the percentage of individuals who were seropositive by Virus Reduction Neutralization Test at 60% neutralization (VRNT60) at 28 days PD1 for each dengue serotype. Exploratory objectives included evaluating 1) the percentage of individual who were seropositive for each dengue serotype by VRNT60 at each immunogenicity time point, 2) the geometric mean titers (GMT) of VRNT60 for each dengue serotype at each immunogenicity assessment time point, 3) the geometric mean fold-rise (GMFR) of VRNT60 for each dengue serotype at 28 days post each vaccination compared to the relative pre-vaccination, and 4) the percentage of individuals with detectable viremia for each dengue serotype after each vaccination.
Participants
Adult males and females in generally good health, between 18 and 50 years of age, inclusive, were eligible for enrollment in this study. Male and female participants were either of non-reproductive potential or agreed to avoid becoming pregnant or impregnating a partner for 4 weeks following the last study vaccination via abstinence or protocol defined contraception. Race and ethnicity were self-identified by the participants as two independent variables in this study. Good general health was determined by physical examination, laboratory screening, and a review of medical history. Participants were excluded if they had a history of receiving any investigational flavivirus vaccine or licensed dengue vaccine, had a known history of hypersensitivity to any component of the dengue vaccine, were pregnant, breastfeeding, or planning to conceive during the time of the study. Other key exclusion criteria included a history of being immunocompromised or on immunosuppressive therapy, positive serum result for HIV, Hep B or C, poorly controlled diabetes mellitus, recent receipt of blood products, febrile illness ≤72 h before vaccination, or receipt of any licensed non-live or live vaccine within 14 or 28 days, respectively, prior to each vaccination. For the purpose of the subgroup analysis, a participant was considered flavivirus-experienced before any vaccination by fulfilling any one of the following criteria 1) a history of flavivirus vaccination, 2) a history of natural flavivirus infection, 3) a positive dengue IgG enzyme-linked immunoassay result, or 4) a positive dengue VRNT60 titer.
Vaccines
V181 is a live, attenuated, quadrivalent vaccine comprising four vaccine viral strains representing each of the four dengue serotypes. Attenuation of all four dengue strains was achieved by deletion of a stem-loop structure comprising approximately 30 nucleotides in the 3' noncoding region of the dengue genome. The DENV3 component has an additional 31-nucleotide deletion in the 3' noncoding region. The DENV2 component is a chimeric virus with the prM and E proteins from DENV2 inserted into an attenuated DENV4 backbone. The viral vaccine components are designated rDENV1∆30, rDENV2/4∆30(ME), rDENV3∆30/31, and rDENV4∆30.Citation23 The vaccine viruses were licensed from the NIAID, manufactured from a master virus seed stock and formulated by Merck and Co., Inc., Kenilworth, NJ, USA. Two formulations of V181 (TV003 and TV005) were evaluated. TV003 and TV005 are admixtures of the same four recombinant monovalent DENV vaccines. Each component of the vaccine was administered at a targeted dose of 103 Plaque Forming Units (PFUs) with the exception of the rDENV2/4Δ30(ME) component in TV005, which was administered at a targeted dose of 104 PFU. A higher dose of DENV2 in the TV005 formulation was evaluated due to previous studies of TV003 conducted by the NIAID demonstrating lower DENV2 titers as compared to the other serotypes.Citation16,Citation19 Leibovitz L-15 medium was used for the placebo. All products were prepared, packaged, and labeled according to Good Manufacturing Practice (GMP) guidelines from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), and applicable local laws and regulations.
Safety assessments
Adverse events (AEs) were recorded using a standardized electronic Vaccination Report Card (eVRC) for 28 days after each vaccination. Specific injection-site adverse events (injection-site pain, injection-site erythema, and injection-site swelling) were solicited Day 1 through Day 5 following each vaccination. Solicited systemic AEs (fatigue, myalgia, headache, malaise, rash) were collected from Days 1 to 28 following each vaccination. The participants were additionally surveyed from Day 1 to 28 postvaccination for the following: body temperature, unsolicited systemic and injection site AEs. If the participant developed a rash within 28 days post each vaccination, the rash location and description were documented by the investigator on a rash-specific case report form. In addition, all participants were followed for dengue-related adverse events (regardless of seriousness) including laboratory-confirmed dengue fever (DF), dengue hemorrhagic fever (DHF), or dengue shock syndrome (DSS), all SAEs, and deaths due to any cause, from the time of informed consent for the entire duration of the study (~1 year after the second vaccination). The severity of AEs and laboratory abnormalities were categorized by the investigator as mild (Grade 1), moderate (Grade 2), severe (Grade 3), or possibly life-threatening (Grade 4) using prespecified criteria according to the clinical protocol, as adapted from FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials September 2007.Citation24 For solicited injection-site erythema and injection-site swelling, mild events were defined as those measuring >0 to <5.1cm, moderate events measured ≥5.1 to ≤10cm and severe are defined as >10.0cm . Maximum size injection-site swelling was designated as unknown in cases where the participant did not report a maximum size. Maximum size injection-site erythema was designated as unknown due to an eVRC programming error that prevented capture of maximum size.
All injection-site events were considered to be vaccine related. Relatedness to study vaccine for systemic AEs was assessed by the investigator.
Vital signs and physical examinations were taken periodically from the beginning of study through 1-year PD2. Clinical laboratory assessments including urinalysis were taken at screening and Days 1, 7, 12, 28, 180, 187, 192, and 208. Predetermined criteria were defined for 2 laboratory tests of interest and also assessed as ≥Grade 3: absolute neutrophil counts <1.0 Standard International Units (SI), and Alanine Aminotransferase (ALT) ≥149 SI.
Immunogenicity assessments
Serum neutralizing antibody titers to DENV1–4 were measured at Days 1, 28, 180, 208, 360, and 530 using a VRNT60.Citation25 In the assay, serum is serially diluted from 1:10 to 1:5120 (two-fold) and mixed with equal volume of dengue wild-type virus (DENV1, DENV2, DENV3, or DENV4) to allow for neutralization. The serum/virus mixture is then used to inoculate Vero cells. Following adsorption, the infected cells are incubated for 20–22 h at which time the cells are fixed. Fluorescent signal is detected using a serotype-specific anti-dengue primary antibody and a secondary antibody labeled with Alexa Fluor 488. The number of individual infected cells is counted using the BioTek Cytation 5 cell imaging system. Sixty-percent neutralizing titers (VRNT60) are determined based upon percent reduction of the virus control and are calculated using a four-parameter logistic regression equation. The 60% cutoff was derived from assay validation and selected on the basis of a stringent cutoff for low assay false-positive rates. A virus control, cell control, negative and positive human serum control, and a cell monolayer staining control are included on each plate to monitor the performance of the assay.
Vaccine viremia was evaluated using RT-PCR at Days 7, 12, 28, 187, 192, and 208 (corresponding to 7, 12, and 28 days after each vaccination) using fluorescent probe and primers specific to each dengue serotype.
Data analysis
There was no formal hypothesis testing in this study. The overall safety and tolerability of the tested vaccine formulations were assessed and reported as a summary of serious and non-serious solicited and unsolicited adverse events using the All Subjects as Treated (ASaT) population. The ASaT population consisted of all randomized participants who received at least 1 dose of the study vaccine. Clinical laboratory test results, vital sign measurements, and physical examination data were presented in data listings.
The immunogenicity data was analyzed using the Per-Protocol (PP) population. The PP population excluded participants with major deviations from the protocol that could substantially affect the results of the primary immunogenicity endpoints. Seropositivity was defined as a titer greater than or equal to the lower limit of quantitation (LLOQ) of the VRNT60 for each dengue serotype (DENV1 titer ≥14; DENV2 titer ≥16; DENV3 titer: ≥20 and DENV4 titer ≥15). The Geometric Mean Titer (GMT) was calculated using ½ the LLOQ of the VRNT60 for each serotype as the lowest value. The seropositivity frequencies with 95% confidence intervals (CIs), and GMT with 95% CIs were plotted by treatment group and time point. In addition, a subgroup analysis based on baseline flavivirus status was also conducted. The 95% CIs were calculated based on the Clopper Pearson method. All analyses were conducted using SAS® software (SAS Institute Inc., Cary, North Carolina) Version 9.3 or higher.
Results
Participants
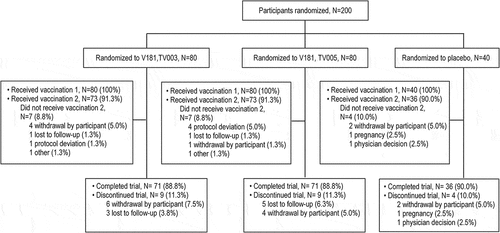
The study enrolled 80 participants each in the TV003 and TV005 arms and 40 participants in the placebo arm. For both the TV003 and TV005 groups, 71 participants (88.8%) completed the trial. The most common reasons for trial discontinuations were withdrawal by participant or loss to follow-up ().
Participant baseline characteristics were generally consistent across vaccination groups (). The mean age was 35.0, 34.9, and 34.7 years for the TV003, TV005, and placebo groups, respectively. Overall, the majority of participants were white (72.5%, 71.3%, and 70.0% for TV003, TV005, and placebo, respectively), female (62.5% 65.0%, and 57.5% for TV003, TV005, and placebo respectively, and identified as Hispanic or Latino (70.0%, 71.3%, and 72.5% for TV003, TV005, and placebo, respectively). The study enrollment achieved the target of approximately 50% each flavivirus-experienced and flavivirus-naïve target in each group (55.0%, 51.2%, and 57.5% flavivirus-experienced participants in TV003, TV005, and placebo groups, respectively)
Table 1. Participant baseline characteristics
Safety
Overall, 70 (87%), 67 (84.8%), and 26 (66.7%) of participants reported at least one AE in TV003, TV005, or placebo groups, respectively (Supplemental Table 1). There were no discontinuations due to AEs or deaths during the trial. The frequency and intensity of solicited injection-site (Day 1–5) and solicited systemic AEs (Day 1–28) after each vaccination are shown in . Rashes were reported from participants vaccinated with TV003 or TV005, and all occurred after the first vaccination with the exception of 1 participant in TV003 (1.4%) and 1 in TV005 (1.4%) reporting rash after the second vaccination. The majority of all solicited adverse events were mild. One TV005 participant (1.3%) reported severe event of fatigue PD1. The most commonly reported overall AEs (i.e. inclusive of solicited and unsolicited) Days 1–28 post any vaccination for TV003 and TV005 were headache, rash, fatigue, and myalgia (Supplemental Table 2).
Figure 2. Percentage of participants with solicited adverse events after each vaccination, by severity.
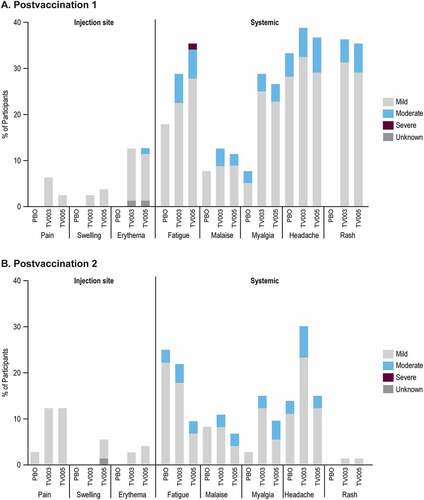
The solicited systemic AEs were also evaluated by baseline flavivirus serostatus (Supplemental Figure 1). The percentage of participants reporting the systemic solicited AEs of fatigue, malaise, myalgia, and headache in the BFE and BFN subgroups after vaccination with TV003 or TV005 were generally comparable overall, or within the variability observed between the placebo BFE and BFN subgroups. There were a higher percentage BFN of participants reporting rashes after the first vaccination, with 47.2% of the TV003 BFN and 52.6% of the TV005 BFN subgroups reporting rash, compared with 27.3% of the TV003 BFE and 19.5% of the TV005 BFE.
No SAEs were reported in the first 28 days after either vaccination. Eight SAEs and 1 infant SAE were reported outside the 28-day postvaccination follow-up period: none were considered to be related to study vaccine and all were resolved.
There were 9 laboratory AEs deemed related to the vaccination in this study. In the TV003 group: 1 alanine aminotransferase (ALT) increase, 1 ALT and aspartate aminotransferase (AST) increase, in TV005: 2 ALT increase, 2 AST increase, 1 blood urea increase, 1 blood creatine increase, and in placebo: 1 ALT and AST increase. Two participants met protocol-defined criteria for specific laboratory results; 1 participant in TV003 had an AE of neutropenia (≥ Grade 3) with onset prior to dose 1 which had not resolved, and 1 participant in TV005 had an AE of ALT (≥ Grade 3) with onset prior to dose 1 which resolved.
Body temperature was measured daily from Day 1–28 post each vaccination. The percentage of participants who reported a maximum temperature that qualified as a fever (≥38.0C [≥100.4°F]) after any vaccination was comparable across TV003, TV005, and placebo groups (6.3%, 5.1%, and 5.1%, respectively). There were no virologically confirmed cases of dengue in any participants during the study as measured by RT-PCR.
Although not considered an AE, five pregnancies which occurred during the study were reported, and the outcome of each pregnancy was tracked and reported. One participant in the TV003 group reported two pregnancies; the first resulted in a spontaneous abortion and the second resulted in a live birth. One participant in TV005 had a live birth, and two participants in the placebo group had pregnancies that ended in spontaneous abortion.
Vaccine viremia
The percent of participants with vaccine viremia was assessed for each dengue serotype after each vaccination (, Supplemental Tables 3-6). Apart from 1 participant (2.6%) in the TV003 BFE group, viremia was only detected after the first vaccination. Vaccine viremia from all four dengue serotypes was detected after TV003 and TV005 vaccination. After the first vaccination, numerically higher percentage of participants with vaccine viremia were observed overall in the TV003 and TV005 BFN subgroups compared to the respective BFE subgroups. Across all dengue types, a numerically higher percent of participants with detectable vaccine viremia were observed after the first dose of TV003 as compared TV005. This observed vaccine viremia was transient. Only one instance of vaccine viremia was detected at Day 28 after any vaccination, all other viremia was only detected on Days 7 or 12. (). As expected, there was no detectable vaccine viremia at any time point in the placebo group.
Figure 3. Vaccine viremia after each vaccination, stratified by baseline flavivirus serostatus.
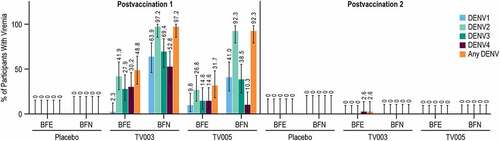
The vaccine viremia was analyzed over days 7, 12, and 28 PD1 to determine the valency (i.e. the maximum number of serotypes detected in each participant after vaccination). This vaccine viremia was categorized as tetravalent (i.e. viremia detected all four DENV serotypes), trivalent, bivalent, monovalent, or none. A higher percentage of participants in the BFN groups had tri-or tetravalent viremia in the 28 days following the first vaccination as compared to the BFE group. In the BFN subgroup, 63.9% and 25.6% of TV003 and TV005 participants, respectively, demonstrated tri- or tetravalent vaccine viremia following the first vaccination (Supplemental Table 7). In contrast, only 14.0% and 12.2% of the TV003 and TV005 BFE subgroups, respectively, demonstrated tri-or tetravalent vaccine viremia PD1 (Supplemental Table 8).
Immunogenicity
Dengue seropositivity frequency
The percentage of participants seropositive for each dengue type was measured using VRNT60 on Day 1 (prevaccination) and time points up to 1 year after the second vaccination (, Supplemental Tables 9,10). After a single dose of TV003 or TV005, an increase in the percent of participants who were seropositive for each dengue serotype was observed in both respective BFE and BFN subgroups as compared to Day 1 percentages. The second dose of TV003 or TV005 minimally impacted seropositivity.
Figure 4. Longitudinal viral reduction neutralization test (Vrnt60) seropositivity frequency, stratified by baseline flavivirus serostatus.
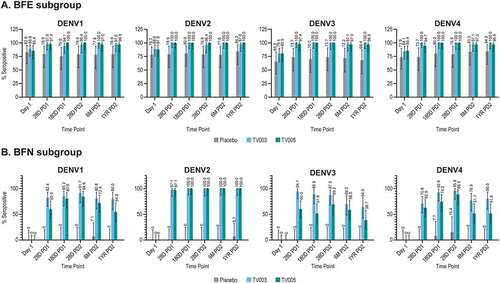
At 28 days PD1 in the BFE subgroups, 97.6%, 100%, 100%, and 100% of TV003 participants and 97.4%, 100%, 97.4%, and 94.7% of TV005 participants were seropositive for DENV1, 2, 3 or 4, respectively (). A high level of seropositivity ranging from 94.1 to 100% was maintained across all four serotypes out to 1-year PD2. In the BFE placebo group, the seropositivity frequency remained relatively constant over time points tested and ranged from 65.2% to 84.2% across the 4 dengue serotypes at all time points.
In the BFN subgroups, the observed seropositivity rates were numerically higher after vaccination with TV003 as compared to TV005, for DENV1, DENV3, and DENV4, and comparable for DENV2 (). At 28 days PD1, 82.4%, 97.1%, 94.1%, and 70.6% of TV003 participants and 60.0%, 97.1%, 60.0%, and 62.9% of TV005 participants were seropositive for DENV1, 2, 3, or 4, respectively. Seropositivity to DENV2 was maintained in 100% of BFN participants vaccinated with TV003 or TV005 through 1-year PD2, and decreased slightly over time for DENV 1, 3, and 4 (ranging from 64.0%-80.0% in TV003 and 38.7%-54.8% in TV005 at 1-year PD2) (). Seropositivity frequencies in the BFN placebo group remained low throughout the course of the study across all four dengue types.
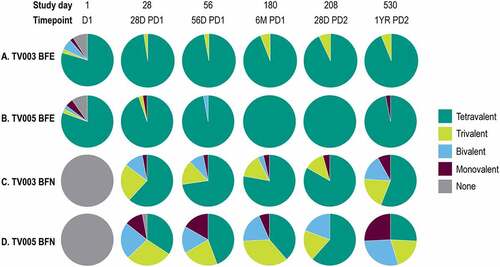
The valency of the VRNT60 seropositivity was also explored. The proportion of participants from the BFN and BFE subgroups with each valency (tetravalent, trivalent, bivalent, monovalent, or none) are shown after vaccination with TV003 and TV005 at time points across the study in . In the BFE subgroups, 100%, and 97.3% of TV003 or TV005 participants, respectively, demonstrated tri- or tetravalent seropositivity at Day 28 PD 1 (, Supplemental Table 11). In the BFN subgroups, 85.3% of TV003 participants and 62.9% of TV005 participants demonstrated tri-or tetravalent seropositivity at Day 28 PD 1 (, Supplemental Table 12). There was an observed trend of increased valency level over time in TV003 and TV005 BFN subgroups from Day 28 to Day 180. At study Day 180, tri-or tetravalency was demonstrated in 92.6% of BFN TV003 participants and 74.2% of BFN TV005 participants. Overall, the levels of tri-or tetravalent seropositivity were numerically higher in BFN TV003 subgroup as compared to BFN TV005 subgroup.
Figure 5. Valency of viral reduction neutralization test (Vrnt60) seropositivity, stratified by baseline flavivirus serostatus.
Dengue VRNT60 titers
As compared to Day 1 (prevaccination), increases in VRNT60 titers were observed after vaccination with TV003 and TV005 in both the BFE and BFN subgroups, for all dengue serotypes (, Supplemental Tables 13, 14). In the BFE subgroup, the highest observed GMT responses across all time points tested were 1349, 1449, 705, or 864 after TV003 vaccination and 890, 1165, 396, and 636 after TV005 vaccination for DENV 1 to 4, respectively ().
Figure 6. Longitudinal geometric mean titers stratified by baseline flavivirus status.
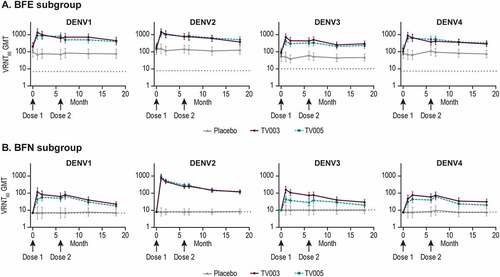
In the BFN subgroups, the highest observed GMT responses after TV003 were 116, 753, 156, and 76 for DENV1 to 4 respectively, and 66, 888, 43, and 61 in the TV005 group for DENV 1 to 4, respectively across all time points tested (). Overall, the BFN TV003 group GMTs were numerically higher as compared to BFN TV005 at all time points for DENV1, 3, and 4, and comparable for DENV2. For both the BFE and BFN subgroups, minimal increases in GMTs were observed after the second TV003 or TV005 immunizations. Across DENV serotypes and flavivirus subgroups, GMTs remained at least 2–3 times higher than pre-vaccination levels at 1-year PD2.
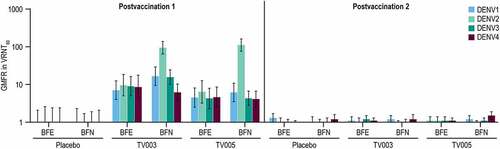
The geometric mean fold rise (GMFR) in VRNT60 titers were analyzed over the respective pre-vaccination levels (Day 28/Day 1 for postvaccination 1 and Day 208/Day 180 for postvaccination 2) (, Supplemental Tables 13, 14). In the BFE subgroups, the GMFRs PD1 were generally comparable between TV003 and TV005 for all serotypes, ranging from 7.0 to 9.6 and 4.3 to 6.4-fold, respectively. In the TV003 and TV005 BFN subgroups, the highest GMFRs PD1 were observed to DENV2 (94.2 and 111.0-fold, respectively) amongst the four serotypes. For the remaining serotypes, the GMFR was numerically higher in the BFN TV003 subgroup as compared to BFN TV005 subgroup (16.6 vs 6.2 for DENV1, 15.6 vs 4.3 for DENV3, and 6.2 vs 4.1-fold for DENV4, respectively). After the second vaccination with TV003 or TV005, all GMFR were ≤1.5 demonstrating a minimal effect of a second dose on DENV neutralization titers. All GMFRs in the placebo group at both PD1 and PD2 were at or near to 1.0 as expected.
Figure 7. Geometric mean fold rise in VRNT60 stratified by baseline flavivirus serostatus.
Immunogenicity analyses within sex, race, ethnicity, and age ranges were generally comparable to the overall population (data not shown).
Discussion
This phase I study of V181 demonstrated that both the TV003 and TV005 formulations were generally well tolerated in healthy adults. No SAEs related to the investigational vaccine formulations were reported, and the majority of the solicited AEs including headache, rash, fatigue, myalgia, and injection-site reactions were mild. Rashes occurred in a subset of participants after TV003 or TV005 vaccination, almost exclusively after the first dose. The rashes were mostly mild in severity, and self-limiting in nature. The safety profile shown in the current study is consistent with previous reports of the analogous live-attenuated dengue vaccine TV003 and TV005 manufactured and evaluated by the NIAIDCitation16,Citation17,Citation19–21 and Instituto Butantan.Citation22
V181 induced robust neutralizing antibody titers and increased dengue seropositivity to all four DENV serotypes in both baseline flavivirus-experienced and flavivirus-naive participants. At 6 months after a single dose of TV003 or TV005, 100% tri- or tetravalent dengue seropositivity was demonstrated in the BFE subgroups. A very high level of tri-or tetravalent dengue seropositivity was also induced after a single dose in participants who were flavivirus-naïve at the start of the study (92.6% after TV003 and 74.2% after TV005 by 6 months PD1). This is an important finding because although a precise correlate of protection has not yet been defined for this vaccine, it is generally accepted that the ideal dengue vaccine should induce a robust neutralization response to all four dengue serotypes.Citation26
A recent study of TV003 produced by the NIAID used epitope mapping and antibody depletion assays to show that the vaccine can induce type-specific responses to each of the four DENV.Citation27 In the current study, we evaluated vaccine viremia and the associated valency. With a quadrivalent live virus vaccine it is likely necessary to have replication of all four viral components to ensure robust and type-specific immunity to all four serotypes. In this study, viremia was demonstrated with at least one DENV, in 97.2% of BFN participants after the first vaccination with TV003. Importantly, the proportion of these participants with viremia was 63.9%, 97.2%, 69.4%, and 52.8% for DENV1, 2, 3, and 4, respectively, demonstrating that the 4 V181 vaccine viral components replicate in the majority of baseline seronegative participants. The lower percentage of BFE participants with demonstrated vaccine viremia compared to BFN participants was expected given that prior dengue infection(s) would result in protective immunity against the infecting serotype(s) and was anticipated to result in reduced vaccine virus replication. In contrast, a study of the live-attenuated vaccine candidate TAK-003 reported vaccine viremia from only the DENV2 serotype in a phase 1 study.Citation28 Similarly, a phase 2 study in 101 dengue-naïve adults who received CYD-TDVTM showed 86.7% of the vaccine viremia at day 7 was from the CYD-4 component, with no CYD-1 viremia detected, and only 1 subject with CYD-2 viremia.Citation29 This could be related to some extent to the finding that TAK-003 shows higher efficacy against DENV-2 compared to other serotypesCitation30 and CYD-TDV efficacy is greatest against DENV4 serotype.Citation31
In early studies published by the NIAID, the analogous TV005 vaccine showed higher percentages of DENV2 seropositivity as compared to TV003 after vaccination of BFN participants (86.4% vs 64% for TV005 and TV003, respectively), cumulative across two studies.Citation32 This result differs from the current study in which 100% DENV2 seropositivity was achieved after the first vaccination of BFN participants with TV003 or TV005. The reasons for the difference in these findings are unknown. There are a number of differences between the respective studies including vaccine manufacturers, cohorts, and assays, all or some of which could potentially contribute. Results from the current study show that the V181 TV003 formulation levels of vaccine viremia and immunogenicity was comparable to or higher across all four dengue types compared to TV005. The difference between the two formulations is the 10-fold higher DENV2 potency in TV005. As such the lower immunogenicity observed after TV005 as compared to TV003 was an unexpected result. The reasons for this are not fully known at this time but dengue viral interactions can be complexCitation28,Citation33,Citation34 and interference of replication of the other viral components by the higher TV005 DENV2 component is a hypothesis. Based upon the totality of data currently available from studies conducted by the NIAID, Instituto Butantan, and our own study, the TV003 formulation has been selected for study in future clinical evaluations of V181.
The viral neutralization titers after V181 TV003 immunization were durable as measured at 6 months after the first vaccination, in both BFN and BFE subgroups, with minimal decreases observed in VRNT60 GMTs from study Day 28 to Day 180. The second dose of TV003 did not result in substantial boosting, as demonstrated by GMFR of the VRNT60 near 1.0 in both BFE and BFN subgroups. Further, there were limited increases in the seropositivity frequency or valency of seropositivity after the second immunization. These findings align with previous reports by the NIAID showing a lack of substantial boosting with a second dose of dengue LATV formulations.Citation16, Citation17 The lack of boosting is consistent with the inhibition of replication of the vaccine viruses administered in the second dose linked to the immune response induced by the first dose. This concept is further supported by the lack of viremia and rash observed after the second dose, consistent with data from studies conducted by the NIAID.Citation16,Citation17,Citation20 In addition, a single dose of the TV003 vaccine produced by the NIAID demonstrated 100% protection from DENV2 challenge virus viremia and rash in a controlled human infection model study.Citation20 In total, these data support a single-dose vaccine regimen for the V181 TV003 formulation.
There are a few important limitations to the current study. The safety assessments are primarily limited by study size and larger studies will be needed to better understand the complete safety profile of this dengue vaccine. In terms of immunogenicity analysis, a precise correlate of protection for dengue has not yet been established and may vary depending upon the vaccine candidate attributes. Here, we have measured DENV neutralization titers as a measure of immunogenicity, but T cell-mediated immunity and levels of memory B cells may also contribute. These immune responses have been explored by others after vaccination with the analogous vaccine produced by NIAID.Citation35,Citation36 Lastly, the study enrolled a majority of white and Hispanic or Latino participants due to the demographics of the population where the study took place, as well as more females than males overall. Larger and more diverse studies including additional age groups will be required to fully evaluate the vaccine.
In summary, V181 TV003 and TV005 formulations induced vaccine viremia and robust humoral immune responses to all four dengue serotypes in baseline flavivirus-naïve and flavivirus-experienced adults after a single dose. Both formulations were generally well tolerated. Overall, dengue neutralization titers were generally higher in TV003 as compared to TV005. These results support additional studies of V181 TV003 formulation as a single-dose vaccine for the prevention of dengue disease.
Supplemental Material
Download PDF (1.5 MB)Supplemental Material
Download MS Word (153 KB)Acknowledgements
The authors would like to thank the participants and study staff for the V181-001 study. We also thank Karyn Davis of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA for editorial assistance.
Disclosure statement
K.L.R., A.W.L., T.F., K.C., A.R., M.S., J.M., D.H., S.G-K., A.B. and B.C. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. R.R reports grants or contracts from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. C.D-P. reports grants or contracts from Takeda Pharmaceutical Company, Leidos, Pfizer Inc., Sanofi and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. C.A. reports employment at Diagnostic Research Group, which was a site for this research and received payment for performing the study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2046960
Additional information
Funding
References
- Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, Pigott DM, Shearer FM, Johnson K, Earl L, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4(9):1508–12. doi:10.1038/s41564-019-0476-8.
- Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi:10.1371/journal.pntd.0001760.
- Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet. 2019;393(10169):350–63. doi:10.1016/s0140-6736(18)32560-1.
- Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med. 2020;12(528). doi:10.1126/scitranslmed.aax4144.
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, et al. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis. 2016;16(6):712–23. doi:10.1016/s1473-3099(16)00026-8.
- Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999:5335–70. doi:10.1016/s0065-3527(08)60342-5.
- Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP Jr., Srikiatkhachorn A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77(5):910–13. doi:10.4269/ajtmh.2007.77.910. PMID: 17984352.
- Reich NG, Shrestha S, King AA, Rohani P, Lessler J, Kalayanarooj S, Yoon IK, Gibbons RV, Burke DS, Cummings DA. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J R Soc Interface. 2013;10(86):20130414. doi:10.1098/rsif.2013.0414.
- Montoya M, Gresh L, Mercado JC, Williams KL, Vargas MJ, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013;7(8):e2357. doi:10.1371/journal.pntd.0002357.
- Anderson KB, Gibbons RV, Cummings DA, Nisalak A, Green S, Libraty DH, Jarman RG, Srikiatkhachorn A, Mammen MP, Darunee B, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis. 2014;209(3):360–68. doi:10.1093/infdis/jit436.
- Halstead SB. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol Spectr. 2014;2(6). doi:10.1128/microbiolspec.AID-0022-2014.
- Halstead SB. Safety issues from a phase 3 clinical trial of a live-attenuated chimeric yellow fever tetravalent dengue vaccine. Hum Vaccin Immunother. 2018;14(9):2158–62. doi:10.1080/21645515.2018.1445448.
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–206. doi:10.1056/NEJMoa1506223.
- Gailhardou S, Skipetrova A, Dayan GH, Jezorwski J, Saville M, Van der Vliet D, Wartel TA. Safety overview of a recombinant live-attenuated tetravalent dengue vaccine: pooled analysis of data from 18 clinical trials. PLoS Negl Trop Dis. 2016;10(7):e0004821. doi:10.1371/journal.pntd.0004821.
- Dengue vaccine: WHO position paper-September 2018. World Health Organization Weekly Epidemiological Record. Dengue vaccines: WHO position paper – September 2018; 2018 [accessed 2022 Feb 16]. p. 457–76.
- Kirkpatrick BD, Durbin AP, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Diehl SA, Elwood D, Jarvis AP, et al. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent dengue vaccine to healthy, flavivirus-naive adults. J Infect Dis. 2015;212(5):702–10. doi:10.1093/infdis/jiv082.
- Durbin AP, Kirkpatrick BD, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Opert K, Jarvis AP, Sabundayo BP, et al. A 12-month-interval dosing study in adults indicates that a single dose of the national institute of allergy and infectious diseases tetravalent dengue vaccine induces a robust neutralizing antibody response. J Infect Dis. 2016;214(6):832–35. doi:10.1093/infdis/jiw067.
- Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am J Trop Med Hyg. 2001;65(5):405–13. doi:10.4269/ajtmh.2001.65.405.
- Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: A randomized, double-blind clinical trial. J Infect Dis. 2013;207(6):957–65. doi:10.1093/infdis/jis936.
- Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, et al. The live attenuated dengue vaccine tv003 elicits complete protection against dengue in a human challenge model. Sci Transl Med. 2016;8(330):330ra336. doi:10.1126/scitranslmed.aaf1517.
- Whitehead SS, Durbin AP, Pierce KK, Elwood D, McElvany BD, Fraser EA, Carmolli MP, Tibery CM, Hynes NA, Jo M, et al. In a randomized trial, the live attenuated tetravalent dengue vaccine tv003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl Trop Dis. 2017;11(5):e0005584. doi:10.1371/journal.pntd.0005584.
- Kallas EG, Precioso AR, Palacios R, Thomé B, Braga PE, Vanni T, Campos LMA, Ferrari L, Mondini G, da graça Salomão M, et al. Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine butantan-dv in adults in brazil: A two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect Dis. 2020;20(7):839–50. doi:10.1016/s1473-3099(20)30023-2.
- Blaney JE Jr., Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006;19(1):10–32. doi:10.1089/vim.2006.19.10.
- Whiteman MC, Bogardus L, Giacone DG, Rubinstein LJ, Antonello JM, Sun D, Daijogo S, Gurney KB. Virus reduction neutralization test: A single-cell imaging high-throughput virus neutralization assay for dengue. Am J Trop Med Hyg. 2018;99(6):1430–39. doi:10.4269/ajtmh.17-0948.
- Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventative vaccine clinical trials. 2007 [accessed 2022 Feb 16]. http://www.fda.gov/cber/guidelines.htm.
- Katzelnick LC, Harris E. Immune correlates of protection for dengue: State of the art and research agenda. Vaccine. 2017;35(36):4659–69. doi:10.1016/j.vaccine.2017.07.045.
- Nivarthi UK, Swanstrom J, Delacruz MJ, Patel B, Durbin AP, Whitehead SS, Kirkpatrick BD, Pierce KK, Diehl SA, Katzelnick L, et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat Commun. 2021;12(1):1102. doi:10.1038/s41467-021-21384-0.
- Rupp R, Luckasen GJ, Kirstein JL, Osorio JE, Santangelo JD, Raanan M, Smith MK, Wallace D, Gordon GS, Stinchcomb DT. Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (tdv) in healthy adults: A phase 1b randomized study. Vaccine. 2015;33(46):6351–59. doi:10.1016/j.vaccine.2015.09.008.
- Dayan GH, Thakur M, Boaz M, Johnson C. Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine. 2013;31(44):5047–54. doi:10.1016/j.vaccine.2013.08.088.
- Biswal S, Borja-Tabora C, Martinez Vargas L, Velásquez H, Theresa Alera M, Sierra V, Johana Rodriguez-Arenales E, Yu D, Wickramasinghe VP, Duarte Moreira E, et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: A randomised, placebo-controlled, phase 3 trial. Lancet. 2020;395(10234):1423–33. doi:10.1016/s0140-6736(20)30414-1.
- Dayan GH, Langevin E, Gilbert PB, Wu Y, Moodie Z, Forrat R, Price B, Frago C, Bouckenooghe A, Cortes M, et al. Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine. 2020;38(19):3531–36. doi:10.1016/j.vaccine.2020.03.029.
- Durbin AP. Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate tv003/tv005. Curr Opin Virol. 2020:4379–87. doi:10.1016/j.coviro.2020.09.005.
- Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, Kanesa-Thasan N, Vaughn DW, Innis BL, Sun W. Phase i trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69(6 Suppl):48–60. doi:10.4269/ajtmh.2003.69.48.
- Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, Sirivichayakul C, Pengsaa K, Pojjaroen-Anant C, Chambonneau L, et al. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J. 2004;23(2):99–109. doi:10.1097/01.inf.0000109289.55856.27.
- Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, et al. The human cd8+ t cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol. 2015;89(1):120–28. doi:10.1128/JVI.02129-14.
- Tu HA, Nivarthi UK, Graham NR, Eisenhauer P, Delacruz MJ, Pierce KK, Whitehead SS, Boyson JE, Botten JW, Kirkpatrick BD, et al. Stimulation of b cell immunity in flavivirus-naive individuals by the tetravalent live attenuated dengue vaccine tv003. Cell Rep Med. 2020;1(9):100155. doi:10.1016/j.xcrm.2020.100155.