ABSTRACT
Rotavirus (RV) is the most common cause of severe gastroenteritis (GE) in infants and young children worldwide and is associated with a significant clinical and economic burden. The objective of this study was to analyze the characteristics, healthcare resource utilization and the direct medical costs related to RVGE hospitalizations in Spain. An observational, multicenter, cross-sectional study was conducted from June 2013 to May 2018 at the pediatric departments of 12 hospitals from different Spanish regions. Children under 5 years of age admitted to the hospital with a confirmed diagnosis of RVGE were selected. Data on clinical characteristics, healthcare resource use and costs were collected from patient records and hospital databases. Most children hospitalized for RVGE did not have any previous medical condition or chronic disease. Forty-seven percent had previously visited the Emergency Room (ER), 27% had visited a primary care pediatrician, and 15% had received pharmacological treatment prior to hospital admission due to an RVGE episode. The average length of a hospital stay for RVGE was 5.6 days, and the mean medical costs of RVGE hospitalizations per episode ranged from 3,940€ to 4,100€. The highest direct medical cost was due to the hospital stay. This study showed a high burden of health resource utilization and costs related to the management of cases of RVGE requiring hospitalization. RV vaccination with high coverage rates should be considered to minimize the clinical and economic impacts of this disease on the health-care system.
Introduction
Rotavirus (RV) remains one of the leading causes of severe diarrhea requiring hospitalization in children worldwide.Citation1 A recent systematic review and meta-analysis, performed in highly developed countries, estimated an average proportion of cases of acute gastroenteritis (AGE) caused by RV of 41% (IC95%, 36–47%) at the hospital level and of 29% (IC95%, 25–34%) for nosocomial infections, leading to a considerable number of hospitalizations every year.Citation2 This use of direct health-care resources places relevant economic burden to the health-care system and society.Citation3
RV vaccines have demonstrated to be highly effective, achieving 60% to 90% reductions in outpatient visits, emergency room (ER) visits, and hospitalizations due to severe AGE in children in many Western European countries.Citation3–7 In Spain, Rotavirus vaccines, RotaTeq®, (MSD)Citation8 and Rotarix®, (GSK),Citation9 have been available since 2006, but are not included in the National Immunization Program. They are used under pediatricians’ recommendations and fully paid by parents, reaching an intermediate vaccination coverage rate with significant differences among regions. It has been shown that, in this context of suboptimal vaccination, the burden of hospitalization due to RVGE in Spain is still relevantCitation10 and, therefore, may lead to a substantial economic impact to the healthcare system.
Previous studies have estimated the use of health-care resources and costs related to the hospital management of RVGE disease in Spain. Most of them by using hospital administrative databases that have been found to underestimate the actual hospital admission incidence, likely due to the selected hospital discharge codification.Citation11–13 Furthermore, estimation of the use of medical resources and related costs might be hampered by the limited data available in those databases.Citation11,Citation13,Citation14 The aim of this study was to describe the clinical characteristics and healthcare resource utilization of children under 5 years of age hospitalized due to RVGE in Spain and perform a medical costs analysis using different sources of information that may provide wide and complete data related to RVGE hospitalizations.
Materials and methods
Study design
This was an observational, multicenter, cross-sectional study conducted at the pediatric departments of Spanish hospitals from different regions.
Hospitals that systematically performed microbiological tests to confirm the etiology to all children <5 years admitted due to AGE and which have electronic records in the Microbiology and Pediatric Departments were considered for selection. The analyzed data corresponded to a 5-year period (from June 2013 to May 2018).
This study was designed, conducted and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology.Citation15 The study was reviewed and approved by the Euskadi Independent Ethics Committee for Research with Medicines (CEIC-E).
Study population
All children under 5 years of age hospitalized with a diagnosis of RVGE during the 5-year period were considered for the study. Selection criteria included: age <5 years; admitted to the hospital (at least 24 h of stay); RVGE confirmation through a microbiological laboratory test; and with available patients’ medical charts including the necessary data for study objectives analyses (clinical, diagnostic and treatment-related data).
Data collection
The pediatric population (≤14 years of age) of the catchment area of each hospital was collected from the hospital administrative database to estimate the population covered by the participating hospitals. Data of population assigned to a specific hospital is divided into pediatric and adult population, but not disaggregated by age groups.
The Microbiology Department databases of each participating hospital were reviewed to identify all hospitalizations with a RVGE confirmed diagnosis in children <5 years during the study period. The type of test used for microbiological determination by each hospital was also collected.
Hospital medical records of children included in the analysis were reviewed to collect information on the type of RVGE (community acquired or nosocomial), their epidemiological and clinical characteristics and use of health-care resources. Nosocomial RVGE was defined as children <5 years of age hospitalized with symptoms of RV infection and laboratory confirmation appearing from 48 h after admission in hospital to 72 h after hospital discharge.Citation16
Variables collected from the patients’ medical records included sociodemographic data (age, gender, nationality); previous medical history and epidemiological data (prematurity, previous medical conditions, type of lactation, attendance to daycare, family members with similar symptoms); hospitalization data (admission and discharge dates), clinical characteristics of the episode (presence and duration of symptoms, complications during hospitalization, hospitalizations outcome); diagnostic test, pharmacological treatment and hygienic-dietary measures for the management of the RVGE. Also, the utilized medical resources prior to hospital admission (primary care and ER visits and treatments) noted in the medical records of patients and readmissions due to the same RVGE episode were collected. All study data were reviewed and recorded in an electronic case report form by the investigators.
In the case of nosocomial RV infections, classification was verified by investigators according to case definitions and only resource consumption related to the management of the RVGE, according to the investigator criteria, were collected. In those patients presenting dehydration, categorization of the severity was performed by the investigators based on the information in the medical record.
To assess direct medical costs related to health care resource utilization, the unit costs were obtained from the cost accounting of each hospital. In case the unit cost information for a given resource was not available from the hospital databases, Oblikue e.Salud database was used as source. Oblikue database collects Spanish unit costs obtained from more than 300 data sources retrieved from published articles, official health services tariffs of the Autonomous Communities, and discharge records from the National Health System hospitals.Citation17
Statistical analysis
For the description of the clinical characteristics and use of health-care recourses of the RVGE hospitalizations, a sample size of 969 patients was estimated to allow the detection of a patient profile with the characteristics present in 8% of the studied population with a precision of ±1.8% percentage units and a two-sided alpha risk with p = .05 (type I error). Based on the Orrico-Sánchez et al. study,Citation18 the proportion of RVGE cases among all hospitalizations in children <5 years of age during the postvaccine introduction period was 1.9%. The study was performed in 20 hospitals from the Autonomous Community of Valencia over a 7-year period, representing approximately 20 RVGE hospitalizations per hospital and year. Therefore, by selecting a convenience sample of 12 hospitals would provide a total of 1,200 hospitalizations due to RVGE during the 5-year period with a precision of .79% and a level of confidence of 95%. Subsequently, according to our sample size calculation, a study sample of approximately 1,000 cases was selected by a simple random sampling per hospital among the total sample of children hospitalized due to RVGE in the participating hospitals during the study period.
For the clinical characteristics and resource consumption descriptive analysis, absolute and relative frequency distributions for the qualitative variables and the mean and standard deviation (SD) for quantitative variables are presented. For each variable, percentages were calculated using as denominator the number of subjects for which data on that variable was reported by investigators in the study electronic case report.
The direct medical costs of hospitalization for RVGE were calculated as the sum of the costs of the hospital stay, diagnostic tests, pharmacological treatment and hygienic-dietary measures. Additionally, the total medical costs per RVGE episode, defined as the direct medical cost of hospitalization for RVGE and the cost derived from ER visits, out-of-hospital visits, treatment prior to hospitalizations and the length of readmissions were calculated. Hospital length of stay for community-acquired RVGE episodes was calculated as follows: hospital discharge date minus the date hospital admission plus 1; and, in cases of nosocomial RVGE episodes as: date of hospital discharge minus the date of microbiological diagnosis of RVGE plus 1. The unit costs related to the use of resources were calculated as the average unit costs obtained from the cost accounting of each hospital. If not available (hygienic-dietary measures and special diets), it was obtained from the Oblikue-e.Salud database. Unit costs of pharmacologic treatments were obtained from the database of the General Council of the official Colleges of Pharmacists.Citation19 Tests/treatment for which no available unit cost was identified or for which the duration was not recorded were not included in the cost analysis. The costs were quantified by multiplying the registered natural units of the use of health-care resources by the unit costs obtained for each resource. All costs were expressed in euros and corrected according to the consumer index price for 2019. The costs were estimated for each case, per hospital and year during the study period.
All statistical analyses were performed using the statistical program Statistical Package SAS version 9.4. A level of statistical significance of .05 was applied to all statistical tests.
Results
Twelve hospitals from 11 provinces throughout the country were included in the study. Average aggregated pediatric population (≤14 years of age) of the catchment area of hospitals during the study period, from June 2013 to May 2018, was 792,739 children. This figure represents an 11% of the total Spanish population ≤14 years according to the census data for the same period.Citation20
During the study period, 1,731 RVGE hospitalizations in children <5 years were collected from the microbiology department databases of the study hospitals. Of those, a random sample of 1,002 admissions were selected and included in the clinical characteristics descriptive analysis. For the cost estimations, the episodes for which there were data available in the study sources on the health-care resources considered for the analysis and their corresponding costs were selected (N = 994).
Epidemiological and clinical characteristics of hospitalizations
The mean age of cases was 13.70 (SD ± 11.71) months, and 59.1% (592/1,002) were boys. Ninety percent (740/821) of children and 75.9% (1,091/1,438) of parents had Spanish nationality and 70.6% (707/1,002) of RVGE hospitalizations occurred between January and April.
Among all RVGE hospitalizations, prematurity and low birth weight were reported in 13.4% and 10.1% of cases, respectively, and in 19.3% of patients, a chronic disease was informed (). Sixty-one percent of patients were nursing children, 26.1% of whom were breastfed (). Among other epidemiological characteristics of interest, 27.0% of patients were attending a daycare center or school at the time of admission, and 21.3% were confirmed to have household contacts with similar symptomatology (), with an average of 1.34 (SD ± .74) contacts affected.
Table 1. Epidemiological characteristics of RVGE episodes hospitalized in children <5 years of age
The most commonly reported symptoms of RVGE were diarrhea (94.4%; 940/996), vomiting (64.6%; 636/985), and fever (55.8%; 547/981), with mean durations of 2.97 (SD ± 1.63), 2.16 (SD ± 1.12), and 2.11 (SD ± 2.25) days, respectively. The mean number of episodes per day was 7.1 (SD ± 4.26) for diarrhea and 5.4 (SD ± 4.48) for vomiting. Abdominal pain was reported in 11.5% (75/649), and other symptoms in 21.1% (201/952) of cases.
Dehydration was the most frequent complication registered (48.5%;484/997), followed by electrolyte disorders (38.3%;380/991). The severity of dehydration is described in . Almost all children with dehydration (98.8%;478/484) received, at the time of admission, an intravenous rehydration bolus. Seizures were reported in 5.0% of subjects (50/999) (), with an average of 2.48 (SD ± 2.59) episodes per patient. ICU admission was needed in 2.1% (21/1,000) of children for a mean time of 2.5 h (SD ± 1.29).
Figure 1. Frequency of complications reported in RVGE hospitalization episodes in children <5 years of age.
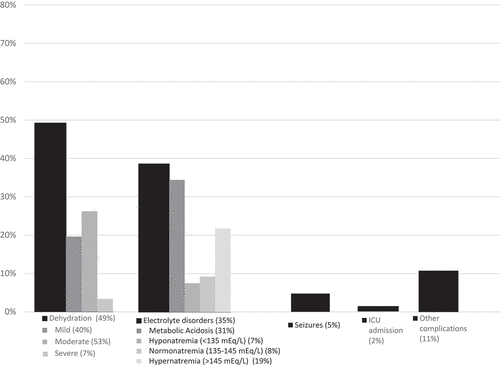
Among the total RVGE episodes, 16.5% (165/1,002) were identified as nosocomial infections. Regarding hospitalization outcome, of the total sample, 999 patients (99.7%) were discharged, and 3 died. Two of the deaths were reported as unrelated to the RVGE episode, and 1 was possibly related. Finally, .9% of cases (9/995) had to be readmitted following discharge due to a cause related to their previous RVGE episode.
Use of resources prior to hospital admission
Forty-six point seven percent of the children (462/990) attended the ER before the hospitalization with a mean of 1.48 (SD ± .75) visits, and 27.4% (211/770) attended primary care pediatric office with a mean of 1.24 (SD ± .61) visits due to the RVGE episode. In addition, 15.4% (150/973) of patients were prescribed a pharmacological treatment before their hospital admission. The frequencies of the medications and their respective treatment durations are displayed in .
Table 2. Pharmacological and therapeutic interventions for the RVGE episodes before and during hospitalization
Use of resources during the hospitalization
The mean length of hospital stay due to RVGE was 5.59 (SD ± 7.61) days, with a split of 5.54 (SD ± 7.56) days for hospital inpatient stay and .05 (SD ± .37) days for children admitted to the ICU. Mean length of stay was 4.66 (SD ± 5.00) days in community-acquired RVGE and 10.57 (SD ± 14.32) days in nosocomial infections.
Immunochromatography was the most widely used test for the microbiological determination of RVGE during hospital admission (). In 94.0% (935/995) of the RVGE episodes for which this data was collected, other tests were performed to determine the presence of other pathogens (). Globally, 66 RVGE hospital cases tested positive for coinfection, the most frequent pathogen being adenovirus and bacteria (stool culture).
Table 3. Diagnostic test performed for RVGE confirmation at admission and other tests during hospitalization
In 90.2% (901/999) of subjects, other diagnostic tests were performed during hospital admission (accounting for a total of 2,138 diagnostic tests). The most frequently performed tests are summarized in .
Of the 991 children hospitalized due to RVGE for which information on pharmacological treatment was available, 306 (30.9%) received at least one treatment during hospitalization, mainly, antibiotics, antipyretics, and oral rehydration solutions, with a mean treatment duration of 5.31 (SD ± 3.29), 1.90 (SD ± 1.28), and 1.98 (SD ± 1.34) days, respectively (). Among the 20 subjects with a positive stool culture for bacteria (), only 6 received antibiotics. Additionally, among the dietary and hygiene interventions during hospital admission, and although dehydration was recorded in 484 of the children hospitalized as mentioned above, intravenous fluid replacement was administered in 79.5% (785/988) of the RVGE episodes during hospitalization, with an average duration of 33.31 (SD ± 34.67) hours ().
Direct medical costs of hospitalization for RVGE
The average direct medical cost related to the RVGE hospitalization during the study period was €3,940.38 (SD ±€5,248.85). The total average associated cost for RVGE episodes, including the costs of medical resources reported before, during hospitalization and related to readmissions, was of €4,100.11 (SD ±€5,283.63) (). Among the direct medical costs during hospitalization, the highest was due to hospital stay with an average cost of €3,676.63 (SD ±€5,013.00), followed by hygienic dietary measures (€97.24; SD ±€645.81). Prior to hospitalization, ER visits represented the highest cost (). No differences were observed in the direct medical costs across the study period. Direct medical costs in each of the participating sites ranged from €3,163.47 (SD ±€3,083.06) to €5,843.24 (SD ±€8,716.44) (Table S1).
Table 4. Direct Medical Costs (€) related to the RVGE episodes
Diagnostic tests and treatment for which a unit cost could not be identified were excluded for the cost analysis. Those represent a 2.2% and a 5.2% of the total diagnostic test and treatments collected, respectively.
Discussion
The aim of this study was to describe the clinical characteristics, use of health-care resources and costs of RVGE hospitalizations in Spain by analyzing a wide sample of patients from different centers and regions, and using different sources of information, that may expand and complete the information previously available for Spain. Our findings showed a significant health resource utilization and costs related to the management of severe cases of RVGE requiring hospital treatment in a country with intermediate vaccination coverage rate. Results from a previous analysis in the hospitals participating in this study, published elsewhere,Citation10 showed that the RVGE-related hospitalization ratios were highly dependent on the vaccination coverage rates, suggesting that the increase in the vaccination coverage rate would significantly impact the related healthcare resource utilizations and costs.
The description of the characteristics of children hospitalized with RVGE showed that most of them were previously healthy, not reporting previous medical conditions or chronic diseases, and that attendance to daycare or school centers is not predominant, similarly to what has been observed for RVGE cases managed at primary care.Citation21–24 As expected, clinical characteristics were similar to those reported in other studiesCitation1,Citation23,Citation24 with a high presence and intensity of symptoms, if we take into consideration the duration and number of episodes per day of diarrhea and vomiting, which are the potential cause of the high frequency of dehydration among RVGE cases.Citation23 In our sample almost half of children hospitalized reported to present dehydration with a not negligible proportion of cases suffering a severe presentation. However, our study was not designed to evaluate the rate of dehydration in RVGE, and the high proportion of this complication could be biased, as dehydration may have been the cause of admission in many of the children. Also, it should be noted that, independently of the proportion of dehydration observed in this study, almost 80% of children hospitalized received IV rehydration treatment during admission, in many cases, probably as a preventive measure for reducing the risk of severe rehydration or prolonged hospital stay. Seizures have been previously described as a complication related to RVGE. The frequency reported in our study (5%) is within the range of what has been observed in other studies.Citation24–28 Although RV-related seizures are usually benign and self-limited, they may lead to ER visits, additional diagnostic and treatment measures and considerable stress for parents.Citation29 Overall, these clinical characteristics of the RVGE hospitalizations have implications in the need of health care resources needed to manage these cases.
We found that almost half of the patients who were hospitalized due to an RVGE episode had attended the ER, and approximately 30% had visited a primary care pediatrician on more than one occasion before hospital admission. Altogether, this suggests the significant impact of RVGE episodes on the disease burden at different health-care levels. Anyway, only approximately 15% of all RVGE cases received treatment before admission, being these oral rehydration solutions in 47% of those cases. This represent a very low proportion of oral rehydration prescriptions in primary care, although there is consensus in the guidelines on the recommendation of oral rehydration for the management of AGE in children, especially in cases of mild-to-moderate dehydration.Citation30–32 Other authors have previously stated that guidelines are not frequently followed pointing out that, despite it is generally known among pediatricians that oral rehydration should be given to prevent invasive procedures or hospital admissions, it remains unclear ‘how’ and to ‘whom’, leading to a suboptimal adherence to treatment.Citation33
Among the health-care resources used during the hospitalization, it is important to highlight the long average length of the hospital stay (5.6 days) observed. There is a high variability in the length of stay reported in previous studies, ranging from 2.5 to 6.5 days, approximately.Citation18,Citation25,Citation34 Results from the study performed in the Autonomous Community of Valencia including a long period of time (2002–2015), showed the relationship of the average hospital stay with age, with children <2 years having an approximately 50% longer average length of stay than the older groups,Citation18 therefore, the age distributions in the different studies may partly explain these differences. Mean age of the subjects in our study was 13.7 months which is consistent with a longer hospital stay. Our study also took into consideration nosocomial infections, for whom length of hospital stay is difficult to estimate and may overestimate the days of admission related to the RV infection. We, anyhow, used the date of the microbiological diagnosis confirmation for the nosocomial infection as a reference for its calculation, to minimize that potential overestimation. Apart from the consequent high use of medical resources and costs, we assume that this long hospital stay must have had an important impact on the number of workdays lost by parents and caregivers, as well as other indirect costs. In fact, it has been estimated that RVGE hospitalizations of children cause work absenteeism in nearly 70% of parents, with a mean number of days off from work of 4, negatively impacting their quality of life.Citation3
Only 31% of children received at least one pharmacological treatment during hospitalization, and, surprisingly, of those, more than 40% (13,4% among the total number of hospitalizations) were prescribed with antibiotics, despite the low frequency of detected bacterial coinfection. Anyway, even in the case of bacterial coinfection, antibiotics are not routinely used, being only indicated for very specific cases, according to guidelines.Citation31–33,Citation35 This result should be interpreted with caution due to retrospective collection of data in our study and other studies to confirm the misuse of antibiotics in RVGE cases are warranted. In this regard, a study performed in 8 European countries including Spain, assessing the appropriateness of antibiotic prescription in febrile children, showed that one-third of all antibiotic prescriptions in ED were of inappropriate or inconclusive indication.Citation36 This may suggest that prevention of infectious diseases may contribute to an improvement in rational use of antimicrobial. The use of vaccines, as has been stated in the Action Framework recently released by the World Health Organization, are already contributing to the battle against antimicrobial resistance, mainly by reducing cases of infections for which antibiotics were prescribed. In the survey performed by GAVI to measure the vaccine impact on antimicrobial resistance, vaccination strategies targeting bacterial pathogens generally received higher scores, but experts also attributed significant value to some vaccines against viral pathogens, including rotavirus vaccines.Citation37,Citation38
The mean medical cost of RVGE hospitalizations per episode estimated was 3,940€, increasing to 4,100€ when out-of-hospital costs related to the episode were considered. It is important to note that these last costs may be underestimated due to the retrospective collection of data based in the hospital medical records of patients in which not all medical visits and treatments occurring before hospitalization may be recorded. As expected, more than 95% of that cost was attributable to the hospital stay, as there is no specific pharmacological treatment for RV infection.Citation1
Important variability in the average costs per RVGE hospitalization from one participating hospital to another was observed, ranging from €3,000 to €5,800, probably due to differences in patient management protocols. Other studies performed previously in Spain have also reported variable results.Citation12–14,Citation39 Different factors may explain these differences. First, the time periods analyzed in the different studies and the increase in costs over time. Second, in previous studies the costs were mainly estimated by using the Diagnosis Related Groups for Disease (DRG). According to the DRG reimbursement system, patients belong to a group of diagnostically homogeneous cases; therefore, patients within the same category are similar clinically and are expected to use the same level of hospital resources. There is no specific DRG for RV, and a DRG including AGE and other miscellaneous digestive disorders in age <18 years was mainly used.Citation13,Citation14 As RV is known to cause more severe AGE than other pathogens that lack of a specific GRD for RVGE may be responsible for an underestimation of RVGE hospitalization costs when using the DRG cost assignation. Similar variability in direct cost related to RVGE hospitalization was observed in other European countries that may be explained by the same factors described above. Studies were mainly performed in periods prior to vaccine introduction and used primarily the DRG system for hospital costs estimation. Moreover, differences in health care systems and its utilization can limit comparisons between countries.Citation16,Citation40
Our study only considered costs related to the most severe presentation of disease. Although cases requiring hospitalization represent a significant proportion of the economic burden of disease, the number of cases is much higher in primary care, and the indirect costs assumed by families are not negligible. The study by Bouzón-Alejandro et al.Citation41 prospectively assessed indirect costs related to RVGE in Spain, showing a mean cost per case of 192.7€ (SD ± 219.8€). Therefore, additional research contributing to the global economic impact of disease estimation is warranted.
This study has several strengths. The participating hospitals systematically performed microbiological testing to all children <5 years admitted due to RV to confirm the etiology. Therefore, by using the Microbiology Department records as source of information is less subject to underestimation than the use of hospital administrative databases. Additionally, we included a large sample size from different hospitals and different regions and used hospital medical records as source of information, allowing us to obtain more detailed information on health care resource use. Also, unit costs were obtained directly from the hospital’s accountability. This use of different sources to complete data, may have led to a more precises estimation of use of medical resources and costs related to the hospital management of RVGE cases.
Study limitations are mainly related to the retrospective design. Some healthcare resources may have not been recorded in the medical records of patient leading to an underestimation of estimated costs. Also, for some tests and treatment no unit costs were identified and were excluded from the analysis, although these represented a small proportion of the total tests and treatments collected that may have contributed minimally to the overall costs. A proportion of episodes in our sample were nosocomial and/or reported having previous medical conditions. It is known that RV infection may complicate the course of patients with previous conditions especially immunocompromising diseases.Citation42 This may have an impact on the severity and resource consumption analysis (including antibiotic use). While we cannot exclude the possibility of nosocomial transmission complicating the course of disease and resulting in cost overestimation due to RVGE, we have tried to minimize this bias by only including costs related to the treatment of RVGE in the estimations for nosocomial cases.
Conclusion
Health-care resource utilization associated with RVGE hospitalization and the derived direct costs in Spanish hospitals are high, representing a substantial economic burden of RV severe cases requiring hospital management. This fact reinforces the importance of RV vaccination with high vaccination coverage rates for the minimization of the clinical and economic impact of disease in the health-care system. Other studies that may help to estimate the global economic burden of disease related to RV infections in Spain and the potential pharmacoeconomic impact of preventive measures are granted.
Ethics approval and consent to participate
The study was designed, conducted and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology, with the ethical principles of the Declaration of Helsinki, and with the current Spanish legislation related to observational studies (Ministerial Order SAS/3470/2009).
This study was reviewed and approved by the Euskadi Independent Ethics Committee for Research with Medicines (CEIC-E).
According to the Guidelines for Good Pharmacoepidemiology Practices and the Spanish regulation for non-interventional observational studies, when using secondary data sources in which a procedure for de-identification of data is implemented, there is no need for subject informed consent collection. In the present study, only already existing data from databases and clinical records was collected, there was no interview with subjects, and all data was securely and adequately dissociated. The Euskadi Independent Ethics Committee for Research with Medicines (CEIC-E) approved this informed consent waiver.
Supplemental Material
Download MS Word (21.9 KB)Acknowledgements
We gratefully acknowledge all site investigators of the participating hospital for their contribution to the study: Leyre Román (Hospital Universitario 12 de Octubre), Andrea Seoane (Hospital Universitario 12 de Octubre), Isabel Gimeno (Hospital Universitario 12 de Octubre), Pilar Cedena (Hospital Universitario 12 de Octubre), María Ángeles Orellana (Hospital Universitario 12 de Octubre), Bárbara Calero (Hospital Clínico Universitario Virgen de la Arrixaca), Raúl Morcillo (Hospital Clínico Universitario Virgen de la Arrixaca), Paula Vidal Lana (Hospital Regional Universitario de Málaga), Jaime Gutierrez del Álamo López (Hospital Regional Universitario de Málaga), Cristina Antúnez Fernández (Hospital Regional Universitario de Málaga), Elisa Garrote (Hospital Universitario de Basurto), Elia Doménech (Hospital Germans Trias i Pujol), Emilio Monteagudo (Hospital La Fe), Laura Pérez (Hospital La Fe), Carlos Ortí (Hospital Universitario y Politécnico La Fe), Gustavo Cilla (Hospital Universitario de Donostia), Miriam Alkorta (Hospital Universitario de Donostia), Marta Illan (Hospital Universitario Clínico San Carlos), Claudia García (Hospital Juan Ramón Jiménez), Pedro Márquez (Hospital Juan Ramón Jiménez), Mónica Frutos (Hospital Universitario Río Hortega), Carlos Alcalde (Hospital Universitario Río Hortega), Marina G. Sánchez (Hospital Universitario Río Hortega), Lucía Palacios (Hospital Universitario Río Hortega), Rosalía Cebrián (Hospital Universitario Río Hortega), Ana Isabel Elola (Hospital Universitario Central de Asturias), Alicia Pérez (Hospital Universitario Central de Asturias), María Angustias Álvarez (Hospital Universitario Central de Asturias).
Participating hospitals: Hospital Clínico Universitario Virgen de la Arrixaca (Murcia), Hospital Universitario 12 de Octubre (Madrid), Hospital Regional Universitario de Málaga (Málaga), Hospital Universitario de Basurto (Bilbao), Complejo Hospitalario de Navarra (Pamplona), Hospital Germans Trias i Pujol (Barcelona), Hospital La Fe (Valencia), Hospital Universitario de Donostia (Guipúzcoa), Hospital Universitario Clínico San Carlos (Madrid), Hospital Juan Ramón Jiménez (Huelva), Hospital Universitario Río Hortega (Valladolid), Hospital Universitario Central de Asturias (Asturias).
Disclosure statement
MC and MSM are employees of MSD Spain. All other authors do not have any competing interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2046961.
Additional information
Funding
References
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3(1):17083. doi:10.1038/nrdp.2017.83.
- Ardura-García C, Kreis C, Rakic M, Jaboyedoff M, Mallet MC, Low N, Kuehni CE. Rotavirus disease and health care utilisation among children under 5 years of age in highly developed countries: A systematic review and meta-analysis. Vaccine. 2021;39(22):2917–10. doi:10.1016/j.vaccine.2021.04.039.
- Álvarez Aldeán J, Aristegui J, López-Belmonte JL, Pedrós M, Sicilia JG. Economic and psychosocial impact of rotavirus infection in Spain: a literature review. Vaccine. 2014;32:3740–51. doi:10.1016/j.vaccine.2014.04.058.
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of Rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017;65(5):840–50. doi:10.1093/cid/cix369.
- Diez-Domingo J, Garces-Sanchez M, Gimenez-Sanchez F, Colomina-Rodriguez J, Martinon-Torres F. What have we learnt about rotavirus in Spain in the last 10 years? An Pediatr (Barc). 2019;91:166–79.
- Pereira P, Vetter V, Standaert B, Benninghoff B. Fifteen years of experience with the oral live-attenuated human rotavirus vaccine: reflections on lessons learned. Expert Rev Vaccines. 2020;19(8):755–69. doi:10.1080/14760584.2020.1800459.
- de Hoog MLA, Vesikari T, Giaquinto C, Huppertz HI, Martinon-Torres F, Bruijning-Verhagen P. Report of the 5th European expert meeting on rotavirus vaccination (EEROVAC). Hum Vaccin Immunother. 2018;14(4):1027–34. doi:10.1080/21645515.2017.1412019.
- Rotateq SMPC. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/rotateq-epar-product-information_en.pdf
- Rotarix SRMPC. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/rotarix-epar-product-information_en.pdf
- Ruiz-Contreras J, Alfayate-Miguelez S, Carazo–gallego B, Onísís E, Díaz-Munilla L, Mendizabal M, Méndez Hernández M, Ferrer-Lorente B, Unsaín-Mancisidor M, Ramos-Amador JT, et al. Rotavirus gastroenteritis hospitalizations in provinces with different vaccination coverage rates in Spain, 2013–2018. BMC Infect Dis. 2021;21(1):1138. doi:10.1186/s12879-021-06841-x.
- Garcia-Basteiro AL, Bosch A, Sicuri E, Bayas JM, Trilla A, Hayes EB. Hospitalizations due to rotavirus gastroenteritis in Catalonia, Spain, 2003-2008. BMC Res Notes. 2011;4(1):429. doi:10.1186/1756-0500-4-429.
- Gil A, Carrasco P, Jimenez R, San-Martin M, Oyaguez I, Gonzalez A. Burden of hospitalizations attributable to rotavirus infection in children in Spain, period 1999–2000. Vaccine. 2004;22(17–18):2221–25. doi:10.1016/j.vaccine.2003.11.037.
- Lopez-de-Andres A, Jimenez-Garcia R, Carrasco-Garrido P, Alvaro-Meca A, Galarza PG, de Miguel AG. Hospitalizations associated with rotavirus gastroenteritis in Spain, 2001–2005. BMC Public Health. 2008;8(1):109. doi:10.1186/1471-2458-8-109.
- Luquero FJ, Hernan Garcia C, Eiros Bouza JM, Castrodeza Sanz J, Sanchez-Padilla E, Simon Soria F, Ortiz de Lejarazu Leonardo R. Perfil de ingresos y urgencias pediátricas en período epidémico de rotavirus en Valladolid. Utilidad de un modelo predictivo. Gac Sanit. 2009;23(1):58–61. doi:10.1016/j.gaceta.2008.03.004
- Resources. Guidelines for good pharmacoepidemiology practices (GPP). 2015. https://www.pharmacoepi.org/resources/policies/guidelines-08027/ .
- Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis. 2012;12(1):62. doi:10.1186/1471-2334-12-62.
- Oblikue. eSalud platform. https://www.oblikue.com/en/esalud.html
- Orrico-Sanchez A, Lopez-Lacort M, Perez-Vilar S, Diez-Domingo J. Long-Term impact of self-financed rotavirus vaccines on rotavirus-associated hospitalizations and costs in the Valencia Region, Spain. BMC Infect Dis. 2017;17(1):267. doi:10.1186/s12879-017-2380-2.
- Base de datos de medicamentos y parafarmacia (Botplus). Consejo General de colegios Farmacéuticos. Botplus. https://www.farmaceuticos.com/botplus/
- Instituto Nacional de Estadística. Demografía y población. https://www.ine.es/index.htm
- Dennehy PH, Cortese MM, Bégué RE, Jaeger JL, Roberts NE, Zhang R, Rhodes P, Gentsch J, Ward R, Bernstein DI, et al. A case-control study to determine risk factors for hospitalization for Rotavirus Gastroenteritis in U.S. children. Pediatr Infect Dis J. 2006;25(12):1123–31. doi:10.1097/01.inf.0000243777.01375.5b.
- Vesikari T, Van Damme P, Giaquinto C, Dagan R, Guarino A, Szajewska H, Usonis V. European society for paediatric infectious diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015;34(6):635–43. doi:10.1097/INF.0000000000000683.
- Aristegui J, Ferrer J, Salamanca I, Garrote E, Partidas A, San-Martin M, San-Jose B. Multicenter prospective study on the burden of rotavirus gastroenteritis in children less than 3 years of age in Spain. BMC Infect Dis. 2016;16(1):549. doi:10.1186/s12879-016-1890-7.
- Gimenez-Sanchez F, Delgado-Rubio A, Martinon-Torres F, Bernaola-Iturbe E; Rotascore Research Group. Multicenter prospective study analysing the role of rotavirus on acute gastroenteritis in Spain. Acta Paediatr. 2010;99(5):738–42. doi:10.1111/j.1651-2227.2010.01684.x.
- GarcíGarcíA-Magán C, de Castro-López MJ, Llovo-Taboada J, Curros-Novo C, Puente-Puig M, Sánchez-Fauquier A, Martinón-Torres F. Caracterización microbiológica de las gastroenteritis agudas virales atendidas en un servicio de pediatría en un área de alta cobertura vacunal frente a rotavirus. Enfermedades Infecciosas Y microbiología Clínica. 2014;32(4):246–49. doi:10.1016/j.eimc.2013.09.008.
- Kang B, Kim DH, Hong YJ, Son BK, Kim DW, Kwon YS. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure. 2013;22:560–64. doi:10.1016/j.seizure.2013.04.007.
- Le Saux N, Bettinger JA, Halperin SA, Vaudry W, Scheifele DW. Canadian Immunization Monitoring Program, Active (IMPACT). Substantial morbidity for hospitalized children with community-acquired rotavirus infections: 2005-2007 IMPACT surveillance in Canadian hospitals. Pediatr Infect Dis J. 2010;29(9):879–82. doi:10.1097/INF.0b013e3181e20c94.
- Lloyd MB, Lloyd JC, Gesteland PH, Bale JJ. Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol. 2010;42(6):404–08. doi:10.1016/j.pediatrneurol.2010.03.002.
- Payne DC, Baggs J, Zerr DM, Klein NP, Yih K, Glanz J, Curns AT, Weintraub E, Parashar UD. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clin Infect Dis. 2014;58(2):173–77. doi:10.1093/cid/cit671.
- Gavilán Martín C, García Avilés B, González Montro R. Gastroenteritis aguda. Protocolos diagnóstico-terapéuticos de infectología. Madrid: Ergon; 2011pp. 113–24.
- Costa I Pagés J, Polanco Allué I, Rodrigo Gonzalo de Liria C. Guía Práctica Clínica. Gastroenteritis guda en el el niño. SEGHNP-SEIP 2010. https://portal.guiasalud.es/wp-content/uploads/2018/12/GPC_464_Gastroenteritis.pdf
- De la Torre A. Gastroenteritis Aguda (V.4.0/2019). Guia ABE-. Infecciones en pediatría. guía rápida para la selección del tratamiento antomicrobiano empírico. Available at: https://www.guia-abe.es/temas-clinicos-gastroenteritis-aguda
- van den Berg J, Berger MY. Guidelines on acute gastroenteritis in children: a critical appraisal of their quality and applicability in primary care. BMC Fam Pract. 2011;12(1):134. doi:10.1186/1471-2296-12-134.
- de la Flor J. Gastroenteritis aguda. Pediatr Integral. 2019;23:348–55.
- Giaquinto C, van Damme P, Huet F, Gothefors L, van der Wielen M; . REVEAL Study Group; Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL study. J Infect Dis. 2007;195 Suppl 1(s1):S36–44. doi:10.1086/516716.
- Hagedoorn NN, Borensztajn DM, Nijman R, Balode A, von Both U, Carrol EN, Eleftheriou I, Emonts M, van der Flier M, de Groot R, et al. Variation in antibiotic prescription rates in febrile children presenting to emergency departments across Europe (MOFICHE): A multicentre observational study. PLoS Med. 2020;17(8):e1003205. doi:10.1371/journal.pmed.1003208.
- Vekemans J, Hasso-Agopsowicz M, Kang G, Hausdorff WP, Fiore A, Tayler E, Klemm EJ, Laxminarayan R, Srikantiah P, Friede M, et al. Leveraging vaccines to reduce antibiotic use and prevent antomicrobial resistance: a World Health Organization action framework. Clin Infect Dis. 2021;73(4):e1011–7. doi:10.1093/cid/ciab062.
- Malarski M, Hasso-Agopsowicz M, Soble A, Mok W, Mathewson S, Vekemans J. Vaccine impact on antimicrobial resistance to inform GAVI, the Vaccine Alliance’s 2018 vaccine investment strategy: report from an expert survey. F1000 Research. 2019;8:1685. doi:10.12688/f1000research.20100.1.
- Gil-Prieto R, San Martín M, de Andrés AL, Alvaro-Meca A, González A, de Miguel AG. Hospital-Acquired rotavirus infections in Spain over a ten-year period (1998-2007). Hum Vaccin. 2009;5(11):748–53. doi:10.4161/hv.5.11.9792.
- Tran AN, Husberg M, Bennet R, Brytting M, Carlsson P, Eriksson M, Storsaeter J, Österlin B, Johansen K. Impact on affected families and society of severe rotavirus infections in Swedish children assessed in a prospective cohort study. Infect Dis (Lond). 2018;50(5):361–71. doi:10.1080/23744235.2017.1416162.
- Bouzón-Alejandro M, Redondo-Collazo L, Sánchez-Lastres JM, Martinón-Torres N, Martinón-Sánchez JM, Martinón-Torres F, et al. Prospective evaluation of indirect costs due to acute rotavirus gastroenteritis in Spain: the ROTACOST study. BMC Pediatr. 2011;11:81. doi:10.1186/1471-2431-11-81.
- Bruijning-Verhagen P, Nipshagen MD, de Graaf H, Bonten MJM. Rotavirus disease course among immunocompromised patients: 5-year observations from a tertiary care medical centre. J Infect. 2017;75(5):448–54. doi:10.1016/j.jinf.2017.08.006.
