ABSTRACT
As of 05/28/2021, SARS-CoV-2 (COVID-19) had caused 3.9 million infections in the United States (US) pediatric population since its discovery in December of 2019. The development and expansion of vaccination has markedly changed the shape of the epidemic. In this qualitative study, we report on pediatric hematology/oncology provider views on the COVID-19 vaccine prior to approval in the adolescent population <16 years of age. Results from interviews with 20 providers across the state of Indiana showed that most were supportive of the COVID-19 vaccine for healthy adults. However, the majority also expressed a need to see more data on the safety and effectiveness of COVID-19 vaccinations in pediatric hematology/oncology populations. While they recognized the public health importance of vaccination, their duty to protect their patients led to a need for more specific safety and efficacy data.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), commonly referred to as COVID-19, was first identified in December 2019. This novel virus has gone on to cause a devastating global pandemic, which at the time of this writing has infected more than 168 million persons and caused >3.5 million deaths worldwide.Citation1 In the US, the overall rate of COVID-19 infections in the pediatric population is 8,803/100,000, with 6.63 million confirmed cases as of November 2021.2 Children account for a total of 16.8% of all cases reported in the US. Rates of hospitalization and mortality with COVID-19 in the pediatric population range from .1–1.9% and .0–.03%, respectively.Citation2 Pediatric hematology/oncology patients represent a particularly vulnerable subset of the population; their immunocompromised states place them at much higher risk than the general pediatric population for suffering from adverse events if they were to contract COVID-19. In addition, vaccination of children and adolescents is likely to enhance protection of adults with whom they come into contact (e.g., parents, grandparents, and teachers).
Despite the development of multiple vaccines against COVID-19, misinformation and the politicization of vaccines has caused hesitancy within the general population about receiving the vaccine. Children and adolescents who are cancer survivors or who have sickle cell disease may be at increased risk for morbidity and mortality from COVID-19 infection. As a result, vaccination of these children and their families is particularly important. Based on previous literature, a strong recommendation for vaccination from a trusted provider can make a difference in those who are vaccine hesitant.Citation3–6 Studies have found that there is some hesitancy among health-care workers in general with regard to the COVID-19 vaccine.Citation7,Citation8 We evaluated pediatric subspecialty provider perspectives regarding the COVID-19 vaccine for pediatric patients with sickle cell disease and childhood cancer. At the time of the data collection for this article, no COVID-19 vaccine had yet been approved for adolescents under 16 years of age.
Methods
Procedures
As part of larger study of subspecialty provider vaccination practices, 18 physicians and 2 nurse practitioners specializing in pediatric hematology/oncology care in the state of Indiana completed one-on-one qualitative interviews. Participants work in a wide variety of clinical settings, including dedicated clinics focusing on leukemia/lymphoma, bone marrow transplant (BMT), sickle cell disease, solid tumor/neuro-oncology, as well as more generalized hematology/oncology practices. A total of 30 individuals, representing the known cohort of pediatric hematology/oncology clinicians in the entire state of Indiana, were invited via in-person communication or e-mail to participate in a 30-minute interview study assessing provider attitudes toward influenza, human papilloma virus (HPV), and COVID-19 vaccines. Twenty agreed to participate (response = 66.7%). Due to the COVID-19 pandemic, interviews were conducted remotely by phone or via Zoom. No compensation was offered for participation. This study was approved by the Indiana University Institutional Review Board (IRB).
Data collection
Individuals were provided a study information sheet and electronic consent was obtained prior to the interview. Demographic information on participants’ gender, race/ethnicity, age, workplace organization, as well as years in practice was collected via a brief electronic survey. While attitudes regarding multiple vaccines were assessed during the interviews, this paper focuses on the unique issues related to COVID-19 vaccination, given it is a new vaccine while the other vaccines have long been established as standard of care in the pediatric population (and all established vaccines were unanimously supported by our respondents). Additionally, we did not directly ask the clinicians to compare and contrast their attitudes about COVID-19 vaccination versus other vaccines. Thus, this paper focuses solely on clinicians’ COVID-19 vaccine attitudes. The interviews took place between January 2021-March 2021 and participants were asked their thoughts on COVID-19 vaccine development, data regarding the vaccine, provider vaccine concerns, vaccine hesitancy, misconceptions, concerns among families, and barriers to COVID-19 vaccination.
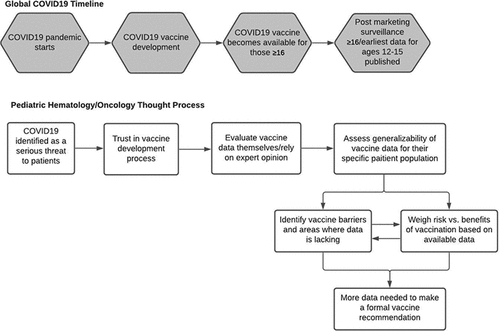
Interviews were audio-recorded and transcribed. During each individual interview, the interviewer took notes and completed a field note after the interview. The authors were all in agreement that the 20 completed interviews reached theoretical saturation,Citation9 providing adequate representation of attitudes given the emergence of common themes with little new variation regarding COVID-19 vaccination. Codes were organized into an overarching model ().
Analysis
We used a thematic approach to analysis.Citation10 A codebook was created based on a literature review and re-occurring themes encountered during review of transcripts. Each transcript was coded by two authors, with differences resolved by discussion. Example codes included published data, scientific concerns, vaccine misconceptions, and potential barriers of COVID-19 vaccination.
Results
Participants
Of the 20 interviewees, 65% identified as female and 35% identified as male. The majority of those interviewed self-identified as white (85%) and 60% were between the ages 31–40 years. Thirteen (65%) had practiced in their subspecialty for ≤10 years. For further participant information, see . At the time the interviews took place, the COVID-19 vaccine was available for all health-care workers in the state of Indiana. Out of the 20 interviewees, 19 had either completed COVID-19 vaccination, received the first dose, or had imminent plans to receive the vaccine. One individual expressed reservation toward receiving the vaccine, citing concerns about potential side effects due to personal health concerns. Personal motivations for getting the vaccine included: reducing chances of getting COVID-19 (n = 4), personal health (n = 3), help protect their families (n = 3), reducing COVID-19 severity if they acquired it (n = 2), help protect their patients (n = 2), and reduce the chances of spread of COVID-19 to others (n = 1).
Table 1. Participant Demographics
Overview
provides an overview of findings and how those findings relate to the COVID-19 vaccine development process. Providers uniformly identified COVID-19 as a global threat to their subspecialty patients and trust in the scientific process. Once efficacy data is published, providers like to review it themselves and/or rely on expert opinion. This is then followed by assessing how the vaccine may be applicable to their specific patient population, weighing the risk and benefits, and concluding whether or not to recommend the vaccines to their patients. These track to the global COVID-19 timeline, from the recognition of COVID-19 as global pandemic to vaccine development, vaccine availability for individuals ≥16, to post marketing surveillance and the start of pediatric trials. For specific quotes regarding the above please see .
Table 2. Participant quotes
Desire for pediatric data
The majority of those interviewed (n = 15) expressed a general need to see more pediatric subspecialty data prior to recommending the vaccine to patients. Participants wanted more data in: pediatric populations (n = 8), pediatric immunocompromised/cancer patients (n = 7), breast feeding infants (n = 3), pregnant women (n = 2), long-term effects (n = 1), long-term efficacy (n = 1), transmission (n = 1), and vaccine efficacy in immunocompromised patients (n = 1). One provider expressed concerns about lack of adequate distribution of the data from the clinical trials and indicated that they would like to be able to personally review the information themselves, although most would be satisfied by published data or data-based recommendations from organizations like the CDC.
Trust in the scientific process
Most interviewees (n = 16) would either recommend the COVID-19 vaccine to their patients or would plan on recommending the COVID-19 vaccine if it was found to be safe and efficacious for their specific patient population, pediatric hematology and oncology patients. At the time of the interviews, only two said that they would not recommend the COVID-19 vaccine, citing the lack of data in the pediatric immunocompromised population. One indicated willingness to recommend the vaccine for those ≥18 years but were more tempered about those under age 18 years due to lack of pediatric data at the time of the interview. Rationale for wanting to be able to give the COVID-19 vaccination to their patients included: reducing the patient’s chances of acquiring COVID-19 (n = 4), protecting family members of the patient (n = 3), reducing the severity of COVID-19 infection if their patients were to contract it (n = 2), viewing their patient population as more at risk for COVID-19 (n = 1), public health benefit (n = 1), and due to the increased risk of thrombosis associated with COVID-19 (n = 1). For the topic of trust in the scientific process and for all other issues covered in these interviews we did not observe differences in responses based on provider group (physician vs nurse practitioner), provider gender, or provider age.
Discussion
When considering whether to recommend COVID-19 vaccine to their pediatric hematology/oncology patients, the majority of the providers interviewed preferred to have data specific to their patient population. Participants also described assessing gaps in the existing data available. Participants uniformly exhibited trust in the scientific process (and would recommend the vaccine to healthy adults), but they hesitated to recommend the vaccine to their patients. Participants also prefer to independently review the data available prior to accepting reported outcomes.
We hypothesize that this finding is due to the relationship between pediatric subspecialty providers and their patients. Pediatric hematology/oncology providers develop a strong rapport with their patients and their patients’ families. This relationship is the foundation for developing the safest and best recommendation for their vulnerable patients. While data is currently lacking on how provider recommendations may influence either adult or pediatric uptake of the COVID-19 vaccine, findings from a study done relatively early in the pandemic (May 2020) indicated that a physician recommendation modestly, but significantly increased intention to get the COVID-19 vaccine among adults.Citation11
Although there was no mistrust in the scientific process, providers listed many of the same concerns about COVID-19 vaccination as patients list. A survey of adults found that full Food and Drug Administration (FDA) approval, Centers for Disease Control (CDC) and World Health Organization (WHO) recommendations for the vaccine, a higher margin of vaccine efficacy, and decreased chances of serious adverse reactions would increase their likelihood of receiving the vaccine.Citation6,Citation12 Surveys of 1,541 caregivers of children in Emergency Departments internationally found that 65% intended to vaccinate their child with the most common reason being to protect their child (62%). The most common reason for vaccine refusal was the novelty of the vaccine (52%). Factors associated with a greater willingness to vaccinate included the child being an older age, children who were up to date on vaccines or received a flu vaccine in the past year, those who had no chronic illnesses, and if the caregiver was more concerned that they or the child had COVID-19 on arrival to the ED.Citation13 A second survey of 1,321 mothers of children aged 9–12 years assessed intention to vaccinate against COVID-19 and found that 60.4% planned to vaccinate, 8.6% were not planning to vaccinate, and the remaining 31% unsure. Factors associated with uncertainty or no intent to vaccinate included low education levels, lower income, and a history of being unvaccinated or partially vaccinated. These factors were also confirmed in a systemic review, with increased hesitancy to COVID-19 vaccination among those of African American descent, pregnant and breastfeeding women, and lower income status.Citation14 Lower vaccine hesitancy was found amid those who had either a college degree or higher, being age >45, or being male.Citation14 Regardless of intention to vaccinate, themes common to both providers and patients in the decision-making process including vaccine safety and efficacy, recommendation from a health authority, and individual risk.Citation15
Since the time that these interviews have taken place, knowledge surrounding the COVID-19 vaccination has continued to evolve. In May 2021, there were publications regarding the safety, immunogenicity, and efficacy of the Pfizer (BNT162b2) two dose COVID-19 vaccine in adolescents aged ≥12–15 years, followed by a recommendation to vaccinate down to 12 years of age.Citation16 While the safety profile was acceptable in their study population, children with chronic diseases or who were immunosuppressed were excluded, limiting extrapolation of this data to children with a history of cancer. Finally, in a most recent update on October 29th, 2021, the FDA authorized emergency use of the Pfizer-BioNTech COVID-19 vaccine in children ages 5–11 for the prevention of COVID-19.Citation17
Limitations
While we were able to obtain in-depth information regarding attitudes, knowledge, barriers, and concerns of pediatric hematology/oncology providers, there are limitations to this study. The respondents were predominantly female and White, with samples taken largely from a metro area in one state, so our results may not be applicable to other groups from different geographic locales. At the time of these interviews, the COVID-19 vaccine was approved for adolescents/adults ≥16 years of age, but every state had individual approaches to COVID-19 vaccine prioritization. In the state of Indiana, this was done initially on an age-related basis and the vaccine did not become available to adolescents until March 2021. As a result, during the timeframe interviews took place, there was a lack of real-world experience with the vaccine in this subspecialty pediatric population. To date, there is still limited information available regarding pediatric patients less than 12 years of age. Data is limited not only in immunocompromised adults, but even more so in the pediatric population. It is unknown how effective the vaccines may be compared to healthy individuals given the potential for a blunted immune response. Additionally, given the rapidly expanding knowledge of COVID-19 and the vaccines, the interviewees stances on COVID-19 vaccination as above may have shifted since the time during which these interviews were completed. Finally, only a specific subspecialty of pediatrics was included in this study, limiting the ability to generalize these findings. Further understanding and longer-term data of COVID-19 vaccination will help clarify these current unknowns.
Conclusion
Although there are still many unknowns regarding the long-term data with COVID-19 vaccination, most subspecialty pediatric hematology/oncology providers are likely supportive of the COVID-19 vaccine once sufficient data are available in immunocompromised children and children with chronic disease. This continues to be a rapidly evolving area as new data emerges. This article serves to offer insight into the thought processes of subspecialty pediatric hematology/oncology providers for the novel COVID-19 vaccines and can inform provider-focused interventions to strengthen vaccine recommendations among pediatric subspecialty providers.
Disclosure statement
Gregory D. Zimet has served as a consultant and advisory committee member for Merck regarding HPV vaccination and as an advisory committee member for Moderna regarding COVID-19 vaccination. He has also received investigator-initiated research funding from Merck, administered through Indiana University.
Additional information
Funding
References
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. [ updated 2021 May 28; accessed 2021 May 28]. https://covid19.who.int/ .
- American Academy of Pediatrics. Clinical updates on COVID-19. [ updated 2021 Nov; accessed 2021 Nov 15]. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ .
- Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, Devere V, Zhou C, Robinson JD. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037–6. doi:10.1542/peds.2013-2037.
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139(1):e20161764. doi:10.1542/peds.2016-1764.
- Sturm L, Donahue K, Kasting M, Kulkarni A, Brewer NT, Zimet GD. Pediatrician-parent conversations about human Papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61(2):246–51. doi:10.1016/j.jadohealth.2017.02.006.
- Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021;c118(10):e2021726118. doi:10.1073/pnas.2021726118.
- Ahmed G, Almoosa Z, Mohamed D, Rapal J, Minguez O, Abu Khurma I, Alnems A, Al Mutair A. Healthcare provider attitudes toward the newly developed COVID-19 vaccine cross-sectional study. Nurs Rep. 2021;11:187–94. doi:10.3390/nursrep11010018.
- Ledda C, Costantino C, Cuccia M, Maltezou HC, Rapisarda V. Attitudes of healthcare personnel towards vaccinations before and during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(5):2703. doi:10.3390/ijerph18052703.
- Grady MP. Qualitative and action research: a practitioner handbook. Bloomington: Phi Delta Kappa Educational Foundation; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa.
- Head KJ, Kasting ML, Sturm LA, Hartsock JA, Zimet GD. A national survey assessing SARS-CoV-2 vaccination intentions: implications for future public health communication efforts. Sci Commun. 2020;42(5):698–723. doi:10.1177/1075547020960463.
- Kreps S, Prasad S, Brownstein JS, Hswen Y, Garibaldi BT, Zhang B, Kriner DL. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi:10.1001/jamanetworkopen.2020.25594.
- Goldman RD, Yan TD, Seiler M, Parra Cotanda C, Brown JC, Klein EJ, Hoeffe J, Gelernter R, Hall JE, Davis AL; International COVID-19 Parental Attitude Study (COVIPAS) Group. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020;38(48):7668–73. doi:10.1016/j.vaccine.2020.09.084.
- Yasmin F, Najeeb H, Moeed A, Naeem U, Asghar MS, Chughtai NU, Yousaf Z, Seboka BT, Ullah I, Lin CY. COVID-19 vaccine hesitancy in the United States: a systematic review. Front Public Health. 2021;9:770985. doi:10.3389/fpubh.2021.770985.
- Hetherington E, Edwards SA, MacDonald SE, Racine N, Madigan S, McDonald S, Tough S. SARS-CoV-2 vaccination intentions among mothers of children aged 9 to 12 years: a survey of the All Our Families cohort. CMAJ Open. 2021;9(2):E548–E555. doi:10.9778/cmajo.20200302.
- Frenck RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S Bailey, R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. NEJM. 2021. doi:10.1056/NEJMoa2107456.
- U.S Food and Drug Administration. FDA authorizes Pfizer-BioNtech COVID-19 vaccine for emergency use in children 5 through 11 years of age. [ updated 2021 Oct; accessed 2021, Nov 07]. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age.