ABSTRACT
Reasons for COVID-19 hesitancy are multi-faceted and tend to differ from those for general vaccine hesitancy. We developed the COVID-19 Vaccine Concerns Scale (CVCS), a self-report measure intended to better understand individuals’ concerns about COVID-19 vaccines. We validated the scale using data from a convenience sample of 2,281 emergency medical services providers, a group of professionals with high occupational COVID-19 risk. Measures included the CVCS items, an adapted Oxford COVID-19 vaccine hesitancy scale, a general vaccine hesitancy scale, demographics, and self-reported COVID-19 vaccination status. The CVCS had high internal consistency reliability (α = .89). A one-factor structure was determined by exploratory and confirmatory factor analyses (EFA and CFA), resulting in a seven-item scale. The model had good fit (X2[14] = 189.26, p < .001; CFI = .95, RMSEA = .11 [.09, .12], NNFI = .93, SRMR = .03). Moderate Pearson correlations with validated scales of general vaccine hesitancy (r = .71 , p < .001; n = 2144) and COVID-19 vaccine hesitancy (r = .82; p < .001; n = 2279) indicated construct validity. The CVCS predicted COVID-19 vaccination status (B = −2.21, Exp(B) = .11 [95% CI = .09, .13], Nagelkerke R2 = .55), indicating criterion-related validity. In sum, the 7-item CVCS is a reliable and valid self-report measure to examine fears and concerns about COVID-19 vaccines. The scale predicts COVID-19 vaccination status and can be used to inform efforts to reduce COVID-19 vaccine hesitancy.
Vaccination is a critical tool for combating the coronavirus disease 2019 (COVID-19) pandemic, as it can prevent infection, reduce transmission, and minimize severe illness.Citation1 As of September 2021, despite widespread availability and no costs associated with vaccination, only 76% of U.S. adults had received at least one dose of a COVID-19 vaccine.Citation2 Moderate-to-high levels of COVID-19 vaccine hesitancy have also been noted in many other countries, including but not limited to Russia,Citation3 Australia,Citation4 Poland,Citation5 and Japan.Citation6
The need to understand vaccine hesitancy is becoming increasingly important in light of the evolving situation related to the COVID-19 virus and vaccine administration. First, the evolving nature of the virus continues to underscore the importance of widespread vaccination. The COVID-19 vaccines available are highly effective at preventing severe diseaseCitation7,Citation8although breakthrough infections are possible. Even with breakthrough cases, vaccination is believed to be beneficial by reducing the time over which an infected individual is contagious, thus decreasing the risk of spreading infection to others.Citation8 Second, the introduction of booster shots, supported by evidence that protection from a COVID-19 vaccine declines over time,Citation9 will be critical to ensure protection is maintained. Booster shots for some of the COVID-19 vaccines are currently being suggested to be administered six months after the initial dose.Citation10 Third, the increasing use of mandates that require vaccination, recently bolstered in the U.S. by Food and Drug Administration (FDA) approval of the Comirnaty BNT162b2 (mRNA) vaccine,Citation11 means many vaccine-hesitant individuals may now be faced with decisions about getting vaccinated that will affect their employment or education.
Based on these important public health issues, understanding the drivers of vaccine hesitancy is important to enhance immunizations. Research studies have revealed concerns about safety, misinformation, lack of confidence, lack of trust, and politicization.Citation12,Citation13 While some of these themes are similar for the broad topic of vaccinations, evidence suggests that there are differences between general vaccine hesitancy and COVID-19 vaccine hesitancy. Specifically, drivers of COVID-19 vaccine hesitancy include unique factors such as endorsement of COVID-19 conspiracy theories, including COVID-19 being a biological weapon, a way to keep citizens in line, or part of a bigger plot,Citation14,Citation15 or that the vaccine contains microchips.Citation14 Thus, current measures of general vaccine hesitancy are not sufficient to study this issue. Prior work has led to the development of COVID-19 vaccine hesitancy measures,Citation16–19 which examine willingness to get, and perceived importance of, the COVID-19 vaccine. However, while these measures assess one’s hesitancy to get vaccinated, they do not provide an understanding of the root causes of COVID-19 vaccine hesitancy. Some recent scales have been developed that include items to examine contributors to COVID-19 vaccine hesitancy,Citation20, Citation21 but thus far, items in these scales tend to be those that are common to general vaccine hesitancy (e.g., adverse reactions, side effects) and not reflective of the nuanced hesitancy that surrounds COVID-19 vaccines.
For all of these pertinent and pressing reasons, addressing COVID-19 vaccine hesitancy is critical to increase vaccination rates. To do so, we must understand the sources and motivations of hesitancy to effectively address it with strategies such as targeted messaging and education. Being able to identify and measure COVID-19 vaccine concerns is the first step toward developing these strategies. Therefore, the goal of this study was to develop and validate a new measure for COVID-19 vaccine concerns.
Materials and methods
Participants
This study was an evaluation of vaccine hesitancy among U.S. emergency medical services (EMS) healthcare professionals. This specific population was chosen as EMS professionals are frontline providers in the health-care system who regularly enter homes and interface with the public. Thus, this population is at high risk for public exposure to COVID-19, and also need to be immunized to minimize public spread of disease. Participants ranged in age from 18 to 83 years old and were recruited from the National Registry of Emergency Medical Technicians’ (National Registry) database, which contains contact information for approximately 420,000 EMS professionals in the U.S. This evaluation is based on a larger study conducted to determine vaccine hesitancy in EMS professionals wherein we selected a simple random sample of 19,062 nationally certified EMS professionals from the database.Citation22 The voluntary, web-based survey invitation was sent via a unique link to EMS professionals’ provided e-mail addresses within the National Registry database. This unique link allowed for 1-to-1 matching with each respondent’s data in the National Registry database, preventing multiple attempts to be completed by the same individual or by multiple individuals with access to an invited participant’s e-mail. Our survey began with an informed consent form that explained the nature and risks of the study. The American Institutes for Research’s Institutional Review Board approved this study, which was deemed exempt from further review.
Study design
The objective of this cross-sectional study was to develop and validate a scale of COVID-19 vaccine concerns. We followed recommendations for writing items described by DeVellisCitation23 and the process outlined by HinkinCitation24 for scale validation. For assessment of internal consistency reliability, construct validity, and criterion-related validity, the developed scale was evaluated against other previously validated scales that test similar vaccine hesitancy constructs (the Oxford COVID-19 Vaccine Hesitancy measure,Citation16 and the Vaccine Hesitancy Scale,Citation25 described further below).
Procedure
Scale development
To develop a scale on COVID-19 vaccine concerns, we conducted a literature review using PubMed and Google Scholar. Specifically, we reviewed scales on general vaccine hesitancy,Citation25–28 and COVID-19 vaccine hesitancy and attitudes.Citation14,Citation16,Citation29 For content validity, we conducted interviews with 21 patrol officers, firefighters, and paramedics (the population who was of focus for the current survey validation) asking about their opinions and concerns around the COVID-19 vaccine to ensure our items adequately represented the full range of the construct. The most frequently mentioned concerns in these interviews included side effects, potential long-term effects of the vaccine, and potential for adverse safety issues due to the rapid development timeline. Due to the time sensitivity of the topic (i.e., needing to time the survey appropriately given the timing of vaccine releases), we were unable to release the survey in a pre-field test.
Data collection
Electronic questionnaires were sent to the study population in April 2021 following a tailored DillmanCitation30 method with reminders sent at one and two weeks after initial contact. Survey participation was voluntary and did not include an incentive to participate. Demographic data from the participants’ National EMS Certification database profile were linked to survey data and then deidentified for analysis.
Measures
COVID-19 vaccine concerns
The resulting scale was a 7-item measure on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree). The scale was then assessed against the following previously developed and validated scales for evidence of validity.
COVID-19 vaccine hesitancy
COVID-19 vaccine hesitancy was measured with two items adapted from the Oxford COVID-19 Vaccine Hesitancy measure.Citation16 Higher scores indicated more hesitancy: (a) “If my family or friends were thinking of getting a COVID-19 vaccination, I would: (1) Strongly encourage them, (2) Encourage them, (3) Not say anything to them about it, (4) Ask them to delay getting a vaccination, (5) Suggest they do not get a vaccination;” (b) “Taking a COVID-19 vaccination is: (1) Really important, (2) Important, (3) Neither important nor unimportant, (4) Unimportant, (5) Really unimportant.” Only two of the original seven items on the Oxford scale were relevant for inclusion due to the wording of the other five items being future-oriented (e.g., “If there is a COVID-19 vaccine available [I will want to get it as soon as possible/I will take it when offered/I’m not sure what I will do/I will put off (delay) getting it/I will refuse to get it/Don’t know]”), and the timing of the current study (which began months after widespread availability of the vaccines for our study population).
General vaccine hesitancy
General vaccine hesitancy was measured using the 9-item Vaccine Hesitancy Scale.Citation25 Items were on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree). Higher scores indicated more hesitancy.
COVID-19 vaccination status
Vaccination status was determined by asking participants “Have you received a COVID-19 vaccine?” Response options were yes or no.
Demographics
Demographics included sex, age, race/ethnicity, urbanicity (residing in urban/suburban vs. rural), high-risk condition status, and education level. The nominal variable of sex was categorically designated as male or female. Age was a continuous variable. Race and ethnicity were dichotomized to non-minority (white, non-Hispanic) or minority (including Black or African American, Asian, Hispanic or Latino, Asian or Native Hawaiian or Pacific Islander), due to a small proportion of minority EMS professionals. Educational level was a categorical variable including less than high school/completed high school/obtained a General Education Development (GED) degree, some college, Associate’s degree, and Bachelor’s degree, or graduate degree.
Data analysis
Missing data were dropped listwise rather than imputed to avoid making assumptions concerning predicted responses while developing the scale. Descriptive statistics were evaluated for demographics. For Likert scales, items were reverse coded when necessary, and mean composites were computed. Cronbach’s alpha was used to compute internal consistency reliability. Pearson correlations were used to assess construct validity. Logistic regression was used to examine criterion-related validity, with model fit being assessed by the Hosmer–Lemeshow test. Area under the curve (AUC) analyses of receiver operating characteristic (ROC) curves were examined for the logistic regression models to determine prediction accuracy. To examine incremental validity of the scale in predicting COVID-19 vaccination status above and beyond any predictive validity of general vaccine hesitancy, we also tested a multivariable logistic regression model using both variables as predictors. The sample was split randomly to allow for an exploratory factor analysis (EFA) on one subsample and a confirmatory factor analysis (CFA) on the other subsample. For the EFA, maximum likelihood extraction with direct oblimin rotation was selected. For the CFA, maximum likelihood estimation was used. SPSS v.27Citation31 was used for all analyses with the exception of CFA, for which LISREL v.10.3.3.26Citation32 was used. Statistical tests were two-tailed, with p < .05 indicating statistical significance.
Results
Descriptive statistics
A total of 2,281 participants completed the COVID-19 Vaccine Concerns Scale (CVCS) (response rate = 12%) with the majority being male, white and non-Hispanic; their average age was 40. Participants’ education levels ranged from high school/GED to doctoral degrees. Participants lived in rural, suburban and urban areas. Twenty-eight percent of participants had a condition such as heart disease or obesity that put them at high risk for COVID-19. See .
Table 1. Demographic characteristics of participants.
Measure reliability and validity
The CVCS was evaluated for reliability with a Cronbach’s alpha of .89, suggesting high internal consistency reliability.Citation33 All items exhibited adequate inter-item correlation.
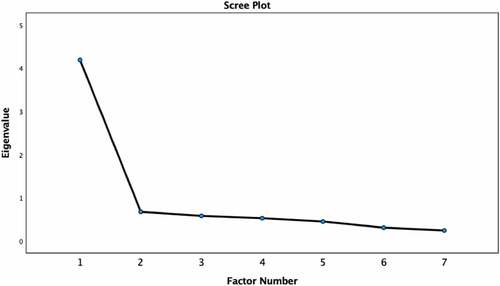
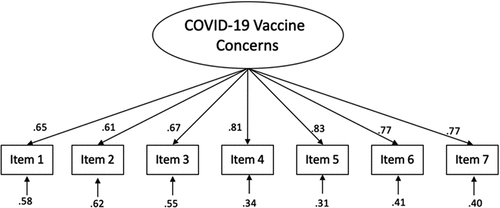
An EFA was conducted on a random subsample of 1,147 participants. The Kaiser–Meyer–Olkin value was .90 indicating sampling adequacy for factor analysis.Citation34 The determinant was .03, indicating lack of multicollinearity.Citation35 Only the first eigenvalue was above 1.0, with a value of 4.19 (explaining 59.86% of variance). The second eigenvalue was .68 (explaining 9.71% of variance). The scree plot () similarly suggested a one-factor solution. Factor loadings for all items were high () and all were above the recommended cutoff point for inclusion of .30.Citation35
Table 2. Factor loadings and inter-item correlations
A CFA was conducted on the remaining subsample of 1,134 participants. The overall model fit well, Χ2(14) = 189.26, p < .001; comparative fit index (CFI) = .95, root-mean-square error of approximation (RMSEA) = .11 (.09, .12), non-normed fit index (NNFI) = .93, standardized root mean square residual (SRMR) = .03. The CFI, NNFI, and SRMR values indicate good fit,Citation36 while the RMSEA value was out of bounds of the optimal criterion of .06.Citation36 All items loaded highly onto the latent factor (see ).
Construct validity
The adapted Oxford COVID-19 vaccine hesitancy scaleCitation16 and the general vaccine hesitancy scaleCitation25 were reliable (α = .91 and .90, respectively).Citation33 The 7-item CVCS scale was highly and positively correlated with the adapted Oxford COVID-19 vaccine hesitancy scaleCitation16 (r = .82 ; p < .001; n = 2279) and the general vaccine hesitancy scaleCitation25 (r = .71 , p < .001; n = 2144). These findings suggest high construct validity.
Criterion-Related validity
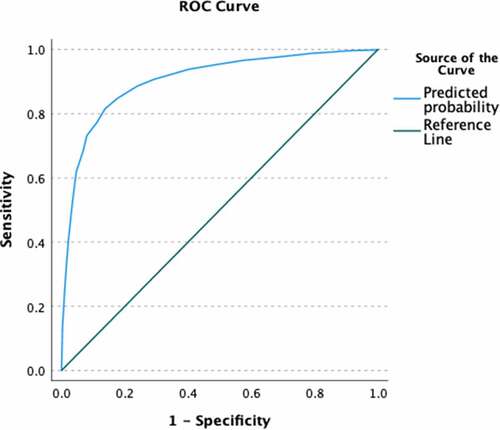
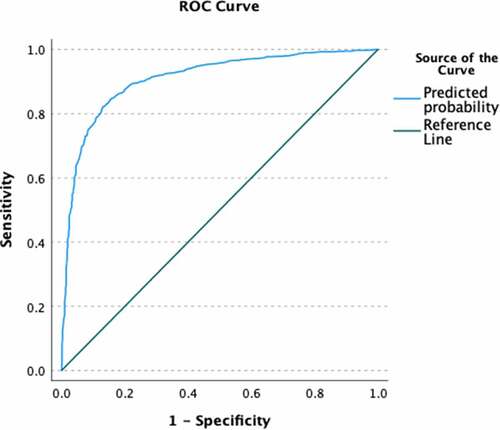
The mean score on the 7-item scale predicted COVID-19 vaccination status, B = −2.21, Exp(B) = .11 (95% CI = .09, .13) and was assessed for goodness of fit (non-significant Hosmer–Lemeshow test, 10 groups). The scale predicted a large amount of variance in vaccination status (Nagelkerke R2 = .55) and AUC was high (AUC = .90), see for ROC. This indicates high criterion-related validity. Further, in a multivariable model with general vaccine hesitancy, the CVCS predicted COVID-19 vaccination status (B = −2.07, Exp(B) = .13 [95% CI = .10, .16]) above and beyond general vaccine hesitancy (B = −.40, Exp(B) = .67 [95% CI = .52, .87]). This multivariable model predicted a large amount of variance (Nagelkerke R2 = .58), but only slightly more than the model with just the CVCS scale. The multivariable model had a high AUC (AUC = .91; see for ROC), but did not have good fit (significant Hosmer–Lemeshow test, 10 groups). Altogether, this indicates incremental validity of the CVCS scale above and beyond general vaccine hesitancy to predict COVID-19 vaccination status.
Discussion
This study sought to develop and validate a scale to assess concerns around COVID-19 vaccines. The resultant CVCS scale is unifactorial and is composed of seven self-report items on a 5-point Likert scale. The scale showed high internal consistency reliability, construct validity, and criterion-related validity. Scores on the CVCS were correlated with scales of general and COVID-19 vaccine hesitancy, and scores significantly predicted COVID-19 vaccination status in a high-risk group of frontline healthcare professionals.
Our scale development was conducted at a time during which understanding of vaccine hesitancy was increasingly important to public safety, but few validated scales existed that were specifically tailored to address COVID-19 vaccine hesitancy. Many of the available scales had adapted existing surveys related to perspectives on general vaccine hesitancy or childhood vaccination.Citation17,Citation37,Citation38 Those scales that were developed specifically for COVID-19 vaccines have limitations, including wording that is outdated in light of the current availability of a vaccine (e.g., “If a COVID-19 vaccine was available at my local pharmacy, I would … ”),Citation16 not considering specific COVID-19 vaccine concerns,Citation19 only focusing on one aspect of concerns (adverse effects of the vaccine),Citation20 or being lengthy. Our survey adds to these resources by providing a succinct tool to assess topics specifically relevant to our evolving discovery of individuals’ perspectives that impact COVID-19 vaccine hesitancy, such as the role of existing immunity, new vaccine technology, and conspiracy theories. Additionally, because each item of our scale represents a unique, specific concern, considering responses to each item individually can enable better understanding of individuals’ specific concerns around COVID-19 vaccines. This information can help to provide insight about the particular issues that may be driving COVID-19 vaccine hesitancy for an individual or community, which, in turn, can inform targeted education and messaging to address these issues. Moreover, our scale shows evidence of criterion-related validity by predicting actual COVID-19 vaccination status, in contrast to other published scales that have examined associations with vaccine intentions rather than actual vaccination status.Citation16,Citation17,Citation19–21
Furthermore, while several general vaccine hesitancy scales have been correlated to willingness to accept vaccination (i.e., prior to the availability of COVID-19 vaccines),Citation16,Citation39 few have yet been tested to predict self-reported vaccination status, now that vaccines have been available to all adults in the U.S. since April 2021.Citation40 A mean of the seven items on the CVCS can be used to predict both COVID-19 vaccine hesitancy and COVID-19 vaccination status. Our scale therefore offers a validated approach to understand COVID-19 vaccine hesitancy at a time in which the public is actively making the decision to receive the vaccine, or not. Addressing individuals’ concerns about COVID-19 vaccines, with messaging or education informed by efforts to understand vaccine hesitancy, could potentially increase COVID-19 vaccination rates. Increasing COVID-19 vaccinations will help efforts to reach herd immunity by reducing the prevalence of the virus and its transmission in the community, and can decrease the strain on overwhelmed healthcare systems by reducing the number of severely ill COVID-19 patients. It is critical that we continue to evaluate COVID-19 vaccine hesitancy as the pandemic evolves, especially as topics such as booster shots, FDA approval of vaccines, and vaccine mandates continue to shape perspectives about COVID-19 vaccination moving forward.
There are several limitations of this study. First, our sample was a random sample of EMS professionals, and there are possible limitations around generalizability to the general public. For example, racial/ethnic minorities, as well as females, are underrepresented in the EMS population, as compared the U.S. population.Citation41 In our study, it was important to test the scale in a group of professionals at high risk for contracting COVID-19 to improve generalizability to other high-risk occupational groups, especially as vaccine hesitancy may place undue burden on these types of professionals. Regardless, the scale should be further tested in a general population to examine generalizability and measurement equivalence. Similarly, it would have been ideal to test the CFA in a separate sample, instead of creating random halves of one sample; however, this practice has been commonly used and shown to be adequate.Citation24 In addition, the RMSEA fit index for the CFA was above the commonly accepted criterion of .06;Citation36 nonetheless, the other three fit indices tested were indicative of good model fit. Further, due to the time sensitivity of the topic, we did not have the opportunity to field test the survey before deployment. Although this was a risk, we felt confident that the work we did to develop the scale before it was released was robust. Additionally, our response rate was fairly low, at 12%. While this is a typical response rate for surveys conducted in an EMS population,Citation42,Citation43 it can lead to response and selection bias. Further, our measure of vaccine status was through self-report; future work should test its predictive validity with objective vaccine data. Finally, this scale was validated only in English; future work should seek to assess its validity in other languages.
Conclusions
The 7-item COVID-19 Vaccine Concerns scale showed high internal consistency reliability, construct validity, and criterion-related validity. We recommend a composite score of this scale be used to assess individuals’ level of concern, as a predictor of COVID-19 hesitancy and likeliness of COVID-19 vaccination. In addition, the individual items can be assessed to understand specific concerns to inform tailored messaging and education. As this scale was tested in the EMS population, future work should examine the validity of this scale in the general population.
Acknowledgments
The authors are grateful to the individuals who participated in this study, and to the National Registry of Emergency Medical Technicians that facilitated survey distribution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Centers for Disease Control and Prevention. Benefits of getting a COVID-19 vaccine. CDC; 2022 Jan 11 [accessed 2022 Jan 31]. http://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html
- Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. 2021 Sept 15 [ accessed 2021 Sept 15]. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- Solis Arce J, Warren S, Meriggi N, Scacco A, McMurry N, Voors M, Syunyaev G, Malik A, Aboutajdine S, Omer S. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–7. doi:10.1038/s41591-021-01454-y.
- Edwards B, Biddle N, Gray M, Sollis K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLOS One. 2021;16(3):e0248892. doi:10.1371/journal.pone.0248892.
- Raciborski F, Samel-Kowalik P, Gujski M, Pinkas J, Arcimowicz M, Jankowski M. Factors associated with a lack of willingness to vaccinate against COVID-19 in Poland: a 2021 nationwide cross-sectional survey. Vaccines. 2021;9(9):1–11. doi:10.3390/vaccines9091000.
- Okubo R, Yoshioka T, Ohfuji S, Matsuo T, Tabuchi T. COVID-19 vaccine hesitancy and its associated factors in Japan. Vaccines. 2021;9(6):662. doi:10.3390/vaccines9060662.
- Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. doi:10.1056/NEJMoa2108891.
- Centers for Disease Control and Prevention. Delta variant: what we know about the science. CDC; 2021 Aug 21 [accessed 2022 Jan 31]. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
- Centers for Disease Control and Prevention. Joint statement from HHS Public Health and medical experts on COVID-19 booster shots. CDC; 2021 Aug 18 [accessed 2022 Jan 31]. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html
- Centers for Disease Control and Prevention. Who is eligible for a COVID-19 vaccine booster shot? CDC; 2022 Jan 21 [accessed 2022 Jan 31]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- Shivaram D. Why Pfizer’s FDA approval matters and what it means for vaccine mandates. NPR; 2021 Aug 24 [accessed 2022 Aug 24]. https://www.npr.org/sections/coronavirus-live-updates/2021/08/24/1030267314/pfizer-vaccine-covid-fda-approval-kids-faq-mandate
- Cowan S, Mark N, Reich J. COVID-19 vaccine hesitancy is the new terrain for political division among Americans. Socius. 2021;7:1–3. doi:10.1177/23780231211023657.
- Razai M, Chaudhry U, Doerholt K, Bauld L, Majeed A. COVID-19 vaccination hesitancy. Bmj. 2021;373:n1138. doi:10.1136/bmj.n1138.
- Freeman D, Waite F, Rosebrock L, Petit A, Causier C, East A, Jenner L, Teale A, Carr L, Mulhall S, et al. Coronavirus conspiracy beliefs, mistrust, and compliance with government guidelines in England. Psychol Med. 2020;21:1–3. doi:10.1017/S0033291720001890.
- Bertin P, Nera K, Delouvée S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front Psychol. 2020;11:565128. doi:10.3389/fpsyg.2020.565128.
- Freeman D, Loe B, Chadwick A, Vaccari C, Waite F, Rosebrock L, Jenner L, Petit A, Lewandowsky S, Vanderslott S, et al. COVID-19 vaccine hesitancy in the UK: the Oxford Coronavirus Explanations, Attitudes, and Narratives Survey (OCEANS ii). Psychol Med. 2020;11:1–5. doi:10.1017/S0033291720005188.
- Rodriguez V, Alcaide M, Salazar A, Montgomerie E, Maddalon M, Jones D. Psychometric properties of a vaccine hesitancy scale adapted for COVID‑19 vaccination among people with HIV. AIDS Behav. 2022;26(1):96–101. doi:10.1007/s10461-021-03350-5.
- Kotta I, Kalcza-Janosi K, Szabo K, Eniko Marschalko E. Development and validation of the multidimensional COVID-19 vaccine hesitancy scale. Hum Vaccin Immunother. 2021; 1–10. Epub ahead of print. doi:10.1080/21645515.2021.2007708.
- Breslin G, Dempster M, Berry E, Cavanagh M, Armstrong N. COVID-19 vaccine uptake and hesitancy survey in Northern Ireland and Republic of Ireland: Applying the theory of planned behaviour. PLOS One. 2021;16(11):e0259381. doi:10.1371/journal.pone.0259381.
- Delgado-Gallegos J, Padilla-Rivas G, Zúñiga-Violante E, Avilés-Rodríguez G, Arellanos-Soto D, Gastelum-Arias L, Villareal H, de Los Ángeles Cosío-León M, Romo-Cardenas G, Moreno-Treviño M, et al. Determinants of COVID-19 vaccine hesitancy: a cross-sectional study on a Mexican population using an online questionnaire (COV-AHQ). Front Public Health. 2021;9:728690. doi:10.3389/fpubh.2021.728690.
- Kumari A, Ranjan P, Chopra S, Kaur D, Upadhyay A, Kaur T, Bhattacharyya A, Arora M, Gupta H, Thrinath A, et al. Development and validation of a questionnaire to assess knowledge, attitude, practices, and concerns regarding COVID-19 vaccination among the general population. Diabetes Metab Syndr. 2021;15(3):919–25. doi:10.1016/j.dsx.2021.04.004.
- Gregory ME, Powell JR, MacEwan SR, Kurth JD, Kenah E, Panchal AR, McAlearney AS. COVID-19 vaccinations in EMS professionals: prevalence and predictors. Prehosp Emerg Care. 2021; 1–9. Epub ahead of print. doi:10.1080/10903127.2021.1993391.
- DeVellis R, Thorpe C. Scale development: theory and applications. 4th. Thousand Oaks (CA): Sage; 2016.
- Hinkin T. A brief tutorial on the development of measures for use in survey questionnaires. Organ Res Methods. 1998;1(1):104–21. doi:10.1177/109442819800100106.
- Shapiro GK, Tatar O, Dube E, Amsel R, Knauper B, Naz A, Perez S, Rosberger Z. The vaccine hesitancy scale: psychometric properties and validation. Vaccine. 2018;36(5):660–67. doi:10.1016/j.vaccine.2017.12.043.
- Shapiro GK, Holding A, Perez S, Amsel R, Rosberger Z. Validation of the vaccine conspiracy beliefs scale. Papillomavirus Res. 2016;2:167–72. doi:10.1016/j.pvr.2016.09.001.
- Zingg A, Siergrist M. Measuring people’s knowledge about vaccination: developing a one-dimensional scale. Vaccine. 2012;30(25):3771–77. doi:10.1016/j.vaccine.2012.03.014.
- Sarathchandra D, Navin MC, Largent MA, McCright AM. A survey instrument for measuring vaccine acceptance. Preventative Med. 2018;109:1–7. doi:10.1016/j.ypmed.2018.01.006.
- Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;21(45):7002–06. doi:10.1016/j.vaccine.2020.09.041.
- Dillman D, Smyth J, Christian L. Internet, phone, mail, and mixed-mode surveys: the tailored design method. 4th. Hoboken (NJ): John Wiley & Sons; 2014.
- IBM. SPSS Statistics for Macintosh. Ver. 27.0 [software]. Armonk (NY): IBM; 2020.
- Jöreskog K, Sörbom D. LISREL 10 for Windows. Ver. 10.3.3.26 [software]. Skokie (IL): Scientific Software International, Inc.; 2018.
- Taber C. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48(6):1273–96. doi:10.1007/s11165-016-9602-2.
- Dziuban C, Shirkey E. When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychol Bull. 1974;81(6):358–61. doi:10.1037/h0036316.
- Yong A, Pearce S. A beginner’s guide to factor analysis: focusing on exploratory factor analysis. Tutor Quant Methods Psychol. 2013;9(2):79–94. doi:10.20982/tqmp.09.2.p079.
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. doi:10.1080/10705519909540118.
- Akel K, Masters N, Shih S, Lu Y, Wagner A. Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Hum Vaccin Immunother. 2021;17(8):2639–46. doi:10.1080/21645515.2021.1884476.
- Larson H, Jarrett C, Schulz W, Chaudhuri M, Zhou Y, Dube E, Schuster M, MacDonald N, Wilson R. Measuring vaccine hesitancy: the development of a survey tool. Vaccine. 2015;33(34):4165–75. doi:10.1016/j.vaccine.2015.04.037.
- Ebrahimi O, Johnson M, Ebling S, Amundsen O, Halsøy Ø, Hoffart A, Skjerdingstad N, Johnson S. Risk, trust, and flawed assumptions: vaccine hesitancy during the COVID-19 pandemic. Front Public Health. 2021;9. doi:10.3389/fpubh.2021.700213.
- Treisman R. Biden says all adults will be vaccine eligible by April 19. NPR; 2021 Apr 6 [accessed 2022 Apr 6]. https://www.npr.org/sections/coronavirus-live-updates/2021/04/06/984745020/biden-will-direct-states-to-make-all-adults-vaccine-eligible-by-april-19
- Crowe R, Krebs W, Cash R, Rivard M, Lincoln E, Panchal A. Females and minority racial/ethnic groups remain underrepresented in emergency medical services: a ten-year assessment, 2008-2017. Prehosp Emerg Care. 2019;24(2):180–87. doi:10.1080/10903127.2019.1634167.
- Schmuhl P, Van Duker H, Gurley K, Webster A, Olson L. Reaching emergency medical services providers: is one survey mode better than another? Prehosp Emerg Care. 2010;14(3):361–69. doi:10.3109/10903121003760184.
- Cash R, Rivard M, Camargo C, Powell J, Panchal A. Emergency medical services personnel awareness and training about personal protective equipment during the COVID-19 pandemic. Prehosp Emerg Care. 2021;25(6):777–84. doi:10.1080/10903127.2020.1853858.