ABSTRACT
We evaluated the cost-utility of replacing trivalent influenza vaccine (TIV) with quadrivalent influenza vaccine (QIV) in the current target populations in Uruguay. An existing decision-analytic static cost-effectiveness model was adapted for Uruguay. The population was stratified into age groups. Costs and outcomes were estimated for an average influenza season, based on observed rates from 2013 to 2019 inclusive. Introducing QIV instead of TIV in Uruguay would avoid around 740 additional influenza cases, 500 GP consultations, 15 hospitalizations, and three deaths, and save around 300 workdays, for the same vaccination coverage during an average influenza season. Most of the influenza-related consultations and hospitalizations would be avoided among children ≤4 and adults ≥65 years of age. Using QIV rather than TIV would cost an additional ~US$729,000, but this would be partially offset by savings in consultations and hospitalization costs. The incremental cost per quality-adjusted life-year (QALY) gained with QIV would be in the order of US$18,000 for both the payor and societal perspectives, for all age groups, and around US$12,000 for adults ≥65 years of age. The main drivers influencing the incremental cost-effectiveness ratio were the vaccine efficacy against the B strains and the percentage of match each season with the B strain included in TIV. Probabilistic sensitivity analysis showed that switching to QIV would provide a favorable cost-utility ratio for 50% of simulations at a willingness-to-pay per QALY of US$20,000. A switch to QIV is expected to be cost-effective for the current target populations in Uruguay, particularly for older adults.
Introduction
Most developed countries have an influenza vaccination program, which may target groups at increased risk of severe effects from influenza or who are heavily exposed. Trivalent influenza vaccines (TIVs) contain antigens derived from two influenza type-A virus subtypes and one influenza type-B virus subtype (either B/Victoria or B/Yamagata lineage).Citation1 Each year, the World Health Organization (WHO) informs vaccine manufacturers which two influenza type-A subtypes should be included in their vaccines (an AH1N1-like strain and an AH3N2-like strain). In addition, WHO predicts which type-B virus is expected to be the predominant circulating B strain for the forthcoming influenza season. However, mismatches between the vaccine and the circulating viruses, or co-circulation of strains from both B lineages, occur. For example, data from the Global Influenza B Study based on over 1.8 million influenza cases from 31 countries during 2000–2018 showed a lineage-level mismatch for the trivalent vaccine in 30% to >40% of seasons.Citation2 In these cases, TIV effectiveness was shown to be reduced.Citation1
Quadrivalent influenza vaccines (QIVs), containing strains of both influenza B lineages, have been developed and are already included alongside TIVs in the national immunization programs of some countries.Citation1 The safety and efficacy of QIVs have been previously demonstrated in multiple trialsCitation3, Citation4and are recognized by WHO as potentially offering wider protection against influenza type-B vaccines than TIVs.Citation5 When the implementation of a vaccination program is being evaluated in a context of limited resources, health economic analysis is a useful tool to help ensure that resources are allocated optimally.Citation6–9 Costeffectiveness analyses evaluate the additional costs of a particular vaccine strategy against the expected benefits compared with the current standard of care, using mathematical models that provide a simplified representation of the disease.
The benefits of QIV versus TIV will be reduced in seasons with a low circulation of B subtypes or a good match with the B strain included in the TIV vaccine, and greater in seasons with a high circulation of B subtypes and a poor match with the B strain included in the TIV vaccine. Even allowing for this variability, numerous health economic evaluations have suggested that replacing TIV with QIV is cost-effective in eligible populations (generally children ≤4 years of age, adults ≥65 years of age, other adults at high risk of influenza complications, or subgroups of these categories). The switch to QIV has been demonstrated to be cost-effective in a number of local settings around the world, as reported in a systematic reviewCitation1and for several countries, for example Canada,Citation10 Italy,Citation11 Germany,Citation12 Spain,Citation13 Brazil,Citation14 China,Citation15 South Korea,Citation16 and Japan.Citation17 Benefits from switching from TIV to QIV have also been demonstrated in a Latin American context, for example in Brazil, Colombia, Panama, and Mexico.Citation14, Citation18–20
The national immunization program in Uruguay recommends influenza vaccination for children ≤4 years of age, adults ≥65 years of age, healthcare professionals, residents and staff in nursing homes, pregnant women, and individuals with >1 chronic medical condition that place them at high risk.Citation21 The current standard of care for influenza vaccination is TIV. Here we report the results of a model-based study we conducted to assess the cost-effectiveness of replacing TIV with QIV in eligible populations in Uruguay.
Materials and methods
Model structure
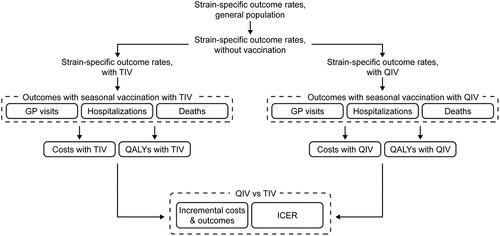
In order to predict influenza-related costs and outcomes for seasonal vaccination of eligible populations in Uruguay with either TIV or QIV, an existing decision-analytic static costeffectiveness modelCitation10was adapted for the local setting. The validity of the model had been previously assessed by an internal panel of cross-functional experts. The model takes into account the pathway of an individual with influenza as presented by WHO (physician visit, hospitalization, workday loss, and death).Citation22 A static model was chosen in line with WHO guidelines for evaluations in which no positive externalities such as herd effect are taken into account (case 7 in Figure 5 of the WHO guideline),Citation9 although it was acknowledged that the results would underestimate the cost-effectiveness of using QIV in the Uruguayan context.
The model structure is shown in The same coverage rate and target population were considered for both TIV and QIV. The difference in outcomes was, therefore, driven only by the difference in vaccine effectiveness and cost.
The following outcomes were considered: influenza cases avoided; influenza-related physician consultations and hospitalizations avoided; deaths avoided; productivity losses due to illness or death; life-years gained; quality-adjusted life-years (QALYs) gained; and incremental costeffectiveness ratios (ICERs, i.e., the cost per QALY gained with QIV versus TIV).
Analyses
Base case and sensitivity analyses
We estimated cost-effectiveness in terms of cost-utility—i.e., we report results as ICERs. Total costs were obtained by multiplying each outcome by its unit cost estimate. To estimate QALYs, outcome data were combined with the utility values associated with each outcome. Costs were reported as US dollars. The analysis for the base case was carried out from the payor and societal perspectives.
ICERs were calculated by considering the incremental costs and the number of QALYs gained with a switch from TIV to QIV in an average influenza season in Uruguay, based on the seasons from 2013 to 2019 inclusive. The incremental cost covered both the difference in the cost of vaccination and potential savings in resource use for the healthcare system due to the prevention of cases. Because QIV is considered to be more effective but is also more expensive than TIV, the cost-effectiveness depends on the balance between the clinical effectiveness, additional vaccination costs, and disease-management savings.
For the payor perspective, the model included only estimated health costs directly associated with treating, managing, and caring for patients with influenza. For the societal perspective, indirect costs—specifically, loss of productivity due to influenza among the employed population—were also considered. Premature deaths were not considered as a factor in the loss of productivity.
The counts for each outcome of interest were estimated for one average season. However, the model also considered the long-term consequences of these outcomes during that season. Each influenza-related death was associated with number of life-years and QALYs lost. These effects and the associated costs were discounted by a 3.0% annual rate, as recommended by WHO guidelines.Citation9, Citation23
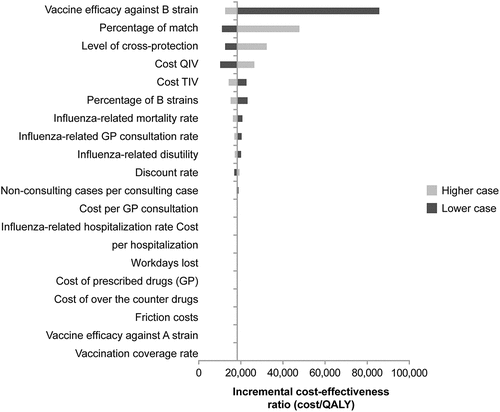
We performed deterministic sensitivity analyses by varying key model input parameters individually within a plausible interval (considering one lower bound and one upper bound), to measure their influence on the model. The variations used to assess uncertainty in parameters are shown in . In addition, a probabilistic sensitivity analysis was conducted varying all the parameters together, each according to a defined probabilistic distribution (Suppl material). One thousand Monte Carlo simulations were performed to generate the costeffectiveness acceptability curve.Citation24
Table 1. Input values used in the model and, where applicable, ranges used in the sensitivity analyses
Model inputs and assumptions
Where possible, data from Uruguay were used. Where data for Uruguay were not available, data from other countries were used, as reported below.
Population and life expectancy
The number of high-risk individuals in the population in 2018 was then calculated based on the prevalence of the conditions and the population size (Suppl. Table S1). The age-specific population size was derived from national statistics with projections for 2018 and served as a basis to calculate the percentages of individuals ≤4 years of age, individuals ≥65 years of age, and those at high risk among 5 to 64 years of age. The percentage of individuals categorized as high risk is the percentage of patients having at least one of the following conditions: human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), tuberculosis, cancers with direct immunosuppression, cancers with possible immunosuppression, cardiovascular diseases, chronic respiratory diseases, chronic liver diseases, obesity, diabetes mellitus, chronic kidney diseases, chronic neurological disorders, and sickle cell disorders. This percentage was estimated for Uruguay in 2018 using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 and United Nations (UN) population estimates for 2018. Double counting between asthma and chronic obstructive pulmonary disease, and diabetes and chronic kidney disease was avoided using data in the source references on the overlap between these diseases.Citation33, Citation34
The life expectancies (LEs) for each age group were derived from the UN website.Citation26 The lower LEs of the high-risk population were calculated using values from the population with diabetes in Canada,Citation35 as local data were not available (Suppl. Table S2). The calculations were adjusted for the weighted average between males and females. The loss in LE due to diabetes was assumed to be representative of all the medical conditions as diabetes, like other chronic diseases, contributes to years of life lost and this allows for a conservative simplification.
Vaccine efficacy, strain distribution, and coverage rate
Vaccine efficacy in the different age groups was derived from a cost-effectiveness study in the USA by Clements et al.Citation36 in which clinical efficacy is assessed using other sources.Citation25, Citation37–40 These authors obtained estimates of age-specific efficacy for TIV primarily from metaanalyses. The authors assumed that QIV efficacy for influenza B was equivalent to the efficacy for a matched TIV, and calculated overall estimated QIV efficacies by applying this efficacy to the proportion of circulating influenza B not covered by TIV/trivalent liveattenuated influenza vaccine. We recalculated their vaccine efficacy values to match the 5–19- and 20–49-year age groups used in our study (). Vaccine efficacy was specific to A strain, B vaccine-matched strain and B vaccine-mismatched strain. The same vaccine efficacy was used for strains from both the A subtypes (i.e., A/H1N1 and A/H2N3). We conservatively assumed a 67% level of cross-protection by TIV against the mismatched B strain, also based on Clements et al.Citation36 Proportions of A (H1N1 and H3N2) and B influenza cases were obtained from the FluNet database.Citation27 For the B strains, the vaccine efficacy of TIV needed to be adjusted according to its efficacy against both the matched strain and the mismatched strain. The average percentage of cases caused by the B lineage not included in the vaccine was assumed to be a fixed value of 50%, derived from the study by Reed et al.Citation29
Vaccination coverage rates were derived from a report on influenza immunization in Uruguay (),Citation41 and were assumed constant over the different seasons.
Rates of influenza-related health parameters
A set of steps were required in order to estimate the overall effects of TIV and QIV. The incidence rates were estimated based on those obtained in the placebo (unvaccinated) arm of clinical trials. These rates were distributed over the seasons using a season severity coefficient specific to Uruguay (Suppl. Material). The influenza-related consultation, hospitalization, and mortality rates were obtained by applying the probability of consultation, hospitalization, and death per influenza cases. Outcome rates were distributed depending on the strain. The rates in an unvaccinated population were corrected by the vaccine coverage (tested) and the strain-specific vaccine efficacy (A, B match, and B mismatch, for TIV only) to obtain rates in two scenarios: the population vaccinated with TIV and the population vaccinated with QIV. Finally, for these two scenarios, the total incidence of infection was calculated.
Once the outcome rates were calculated for each strain, they were aggregated to obtain the overall rates for each outcome. Outcome rates for TIV and QIV were then multiplied by the population size to obtain the number of influenza-related physician visits, hospitalizations, deaths, and workdays lost (Suppl. Tables S3 and S4).
Costs and outcomes were estimated for an average season, based on the average outcome rates observed in Uruguay in the seasons from 2013 − 2019 inclusive. Seven years were considered to cover fluctuations in influenza attack rates between influenza seasons, dominant strains, and TIV mismatch with the predominant circulating B lineage.
Resource use and costs
The costs of vaccines, medical visits, hospitalizations and over-the-counter or GP-prescribed medications are shown in .Citation30, Citation31, Citation42 The costs of a GP consultation and of hospitalization were derived from the CINVE Consultora de Salud report.Citation30 The costs were converted into US dollars from Uruguayan pesos using an exchange rate of 32.406 Uruguayan pesos to 1 US dollar and were adjusted to 2019 values using inflation rates for each year as recommended by the Central Bank of Uruguay. We assumed the same cost for all age groups. To obtain the cost of vaccination with each strategy, the unit cost of TIV or QIV was multiplied by the size of the vaccinated population.
Indirect costs resulting from productivity losses due to illness and to early death were estimated based on the human capital approach. This takes into consideration the value of time lost to an individual, household, or society due to morbidity, premature mortality, providing care, or accessing vaccination. Value is determined according to an individual’s gross earnings.Citation43 As no local data were available, the average number of workdays lost due to influenza-related sick leave was estimated based on a study undertaken in 14 countries in Europe and North America, which assumed a value of 3.28.Citation44 The number of days lost per age group in our study () was calculated based on employment patterns in Uruguay. Employment rates by age group were derived from the National Institute of Statistics of Uruguay.Citation45 It was assumed that the minimum age to start a professional activity was 14 years. The weighted average national minimum wage in the private sector was used to provide a conservative estimate of daily wages by age group.Citation46
Health-related quality of life
In order to calculate QALYs lost, utility norms (i.e., utility values for the different age groups without influenza) were needed, as it could not be assumed that the populations were in perfect health. For the age groups ≤4 years and ≥65 years, the age-specific utility norms for the Uruguayan population as reported in Augustovski et al.Citation47 were used (). For the high-risk groups, utility norms were obtained from Arrospide 2019, Van Wilder 2019, Yang 2014, and Tran 2012Citation28, Citation32, Citation48, Citation49for the following chronic medical conditions; metabolic diseases, respiratory diseases, cardiovascular disease, HIV/AIDS, renal disease, cancer. The utilities were weighted by the prevalence of chronic conditions in Uruguay. The values shown reflect the presence of at least one chronic condition.
The loss in QALYs attributable to an influenza infection could then be applied to these norms. These were derived from Sander et al.,Citation50 and recalculated to match the age distribution in the current study (). As Sander et al. estimated the quality-of-life outcomes for overall influenza-like illness, the estimates are assumed to capture influenza complications.
Results
Health outcomes
The central trend numbers of each health outcome with TIV and QIV are shown in . The uncertainty of them is addressed through deterministic and probabilistic sensitivity analysis (DSA & PSA) and described later in the text.
Table 2. Health outcomes during an average influenza season (base case), number avoided with QIV vs TIV, and life-years and QALYs gained
Switching from QIV to TIV in Uruguay would avoid around 700 additional influenza cases, 500 GP consultations, 15 hospitalizations, and three deaths, for the same vaccination coverage during an average influenza season (). These would translate into ~300 additional workdays saved, 24 life-years gained, and 37 QALYs gained in an average season with QIV compared with TIV.
Most of the influenza-related consultations and hospitalizations would be avoided among children ≤4 years of age and adults ≥65 years of age: 41% and 31% of avoided influenza cases, 41% and 33% of avoided GP consultations, and 28% and 64% of avoided hospitalizations, respectively, were in these two age groups. All the avoided deaths were in the age group ≥65 years.
Cost-Utility
The central trend figures of the additional vaccination costs and total incremental costs of replacing TIV with QIV are shown by age in and their uncertainty is addressed through DSA & PSA and described later in the text.
Table 3. Additional vaccination cost and total incremental cost for switching from TIV to QIV (base case)
Using QIV rather than TIV would lead to an additional vaccination cost of ~US$729,000 due to the higher cost of QIV. However, this would be partially offset by savings in consultation and hospitalization costs. Taking these savings into account, the total incremental cost would be ~US$679,000 from a payor perspective and ~US$673,600 from a societal perspective.
The costs saved from the improved healthcare and productivity outcomes due to switching to QIV, and the resultant ICERs, are shown in . The incremental cost per QALY gained when using QIV over TIV would be US$18,368 (payor perspective) and US$18,224 (societal perspective) (). The ICERs were lower for the ≥65 years age group (US$12,291 and US$12,259, for a payor and societal perspective, respectively) due to the greater burden of influenza, and thus the potential for greater benefits, in this age group relative to the others.
Table 4. Costs saved by switching from TIV to QIV for the targeted population in Uruguay during an average influenza season and ICERs (base case).
Sensitivity analyses
In the deterministic sensitivity analysis, the main drivers influencing the ICER appeared to be the vaccine efficacy against the B strains and the percentage of match (). The degree of cross-protection and costs of the vaccines also impacted the results.
Probabilistic sensitivity analysis confirmed that switching from TIV to QIV has a 50% probability to be cost-effective at a willingness-to-pay per QALY gained of US$20,000 (i.e., around one gross domestic product [GDP] per capita) (Suppl. Figure S1). Considering a willingness to pay per QALY gained of $40,000 (~2 GDP per capita), switching to QIV has a probability of 87.7% to be a cost-effective strategy and at $60,000 willingness to pay per QALY (~3 GDP per capita), a probability of 94.9% to be cost-effective.
Discussion
Our cost-utility model shows that switching from TIV to QIV in Uruguay is cost-effective from both a payor and a societal perspective. Although no national threshold level for cost-effectiveness has been explicitly defined for Uruguay, the ICER estimates (US$18,368 from the payor perspective and US$18,244 from the societal perspective) were around one GDP per capita, which is considered acceptable in many countries around the world. Switching to QIV would be cost-effective because the additional cost of vaccination would be partly offset by a reduction in the number of influenza-related GP consultations, hospitalizations, and deaths compared with TIV. Decision-makers will of course need to consider other competing alternatives before implementing a switch. The results from our study are in line with those reported from other countries.Citation10–17
Switching to QIV showed the greatest impact in the ≥65-year-old age group. People in the ≥65-year-old age group are known to be the population most likely to experience poor outcomes from influenza; thus, there is greater scope for benefits from a more effective vaccine to be apparent. The switch would also have a great impact among young children and those 5–19 years of age. The benefit in these groups is likely to be even greater than suggested by our estimates, as we did not account for herd immunity in our static model, although children are traditionally an important vector of transmission. The use of a static model was deemed acceptable because no negative externalities were expected from the strategy, but not accounting for positive externalities would underestimate the benefit of the vaccine (providing the vaccine coverage was high enough to result in a herd effect). Therefore, the modeled population focused on the populations at risk of developing severe forms of influenza rather than on the vector.
High ICER values were observed in those considered at high risk in the 20–49 and 50–64 years of age categories. As such, the health outcomes avoided in these groups by vaccinating them with QIV do not compensate for the additional costs to the same extent as in other age groups.
The cost-effectiveness of QIV was shown to depend heavily on vaccine efficacy against the B strain, the degree of matching between the circulating B lineage and the B lineage strain included in TIV, the degree of cross-protection of TIV against the strain not included in the vaccine, and the cost of the vaccines (). The model results can vary widely depending on these input values. For example, the sensitivity analyses used a very broad range of values for vaccine efficacy against the B strain. A lower efficacy of QIV against the B strain included in TIV would reduce the overall benefit of QIV. Alternatively, if fewer cases in a particular season were due to the strain included in TIV—i.e., there was a lower level of B strain match—this would increase the relative benefit of QIV and, therefore, increase the costs saved with QIV. A higher degree of cross-protection and a higher cost of QIV would both reduce the benefits seen with QIV and increase the ICER values.
As mentioned above, our base case ICER estimates of US$18,368 (payor perspective) and US$18,244 (societal perspective) were approximately one GDP per capita. It is worth noting that an informal suggestion by WHO that ICERs of up to three times the GDP per capita of a country could be considered cost-effective has not found general acceptance.Citation23, Citation51, Citation52 WHO itself has since dismissed this suggestion, and recommends that each country should use cost-effectiveness information alongside other country-specific considerations, such as budget impact and feasibility, in deciding on the use of medical interventions.Citation23 Also of note, the difference between the payor and societal perspective is small compared with that typically reported for high-income countries, e.g., Italy, Germany, and Spain.Citation11–13 This is explained by the fact that the average wage considered for the productivity losses in Uruguay is quite low in comparison with international standards, yielding savings in this regard of approximately US$5,000 for the average year, while the incremental investment is more significant. Furthermore, focusing only on 1 year (instead of longer horizons as in standard cost-effectiveness analyses) narrows the potential for savings with QIV. Additionally, influenza cases are concentrated in the ≤4 and ≥65 age groups which, due to the methodology, have reduced the impact of productivity loss due to absenteeism to the societal perspective. The impact of premature deaths in the ≥65 age group on productivity loss is also reduced compared with younger age groups and may have contributed to a reduced societal perspective. Other potential contributors to the small difference between the two figures were the increased impact of vaccination in the elderly, where productivity losses are not considered, and low USD salaries in Uruguay. Uruguay operates a social security scheme in healthcare funded by payroll contributions, with premiums potentially being required in some cases. Citizens not covered by this scheme are eligible for a publicly funded scheme which is primarily used by the poorer subset of the population. Private health insurance is also available and used by richer subsets of the population.Citation53
Our study has a number of strengths. The assumptions used for inputs to the model were based on the strongest evidence available. The efficacy of the vaccines has been widely studied, and the values we used were obtained from a meta-analysis of published values.Citation11–13The average level of B lineage match over the last seven seasons in Uruguay was 65.6%, varying from 5.7% to 100%. Given the variability and non-predictability of influenza strain circulation, we assumed that a 50% match was a fair assumption, and varied it from 20% to 80% in sensitivity analyses. The degree of cross-protection of TIV against the mismatched B strain is not very well understood;Citation1 as there is no consensus, we conservatively assumed a level of cross-protection of 67% based on the same study as the one used to derive the vaccine efficacy.Citation36 Influenza-related health parameters such as the attack rate and hospitalizations were estimated separately for each of the years 2013–2019 inclusive, thus accounting for seasonal variability. Inputs to the model were adapted to the Uruguayan context using national data. Finally, our costs are reported in US dollars, which facilitates comparison with other studies.
The model also has some limitations. As a static model, it did not account for herd immunity. According to WHO guidelines for the economic evaluation of immunization programs, the use of static models is appropriate for evaluations of influenza vaccination of groups that do not contribute heavily to transmission, such as the elderly. However, as the results from static models are generally more conservative than those from dynamic models, our study would underestimate rather than overestimate effectiveness. A further limitation was the need to use inputs from other countries where Uruguay-specific data from official sources were not available, and the uncertainties associated with calculated estimates due to this limitation; the sensitivity of all analyses would be improved with more local data.Citation1
Conclusion
The findings from this health economic model indicate that in Uruguay, switching from TIV to QIV in the national influenza immunization program is likely to be cost-effective in the eligible populations overall due to predicted reductions in influenza-related consultations, hospitalizations, and deaths. Probabilistic sensitivity analysis confirmed that switching from TIV to QIV would be cost-effective for 50% of simulations at a willingness-to-pay per QALY gained of US$20,000. These findings suggest that using a vaccine that includes a strain from both B lineages is worthwhile for the eligible population in Uruguay and is particularly cost-effective for older adults. Further studies using country-specific data are required to confirm the sensitivity of these results.
Supplemental Material
Download MS Word (93.4 KB)Disclosure statement
PMB, AP, HD, and JGL are employees Sanofi Pasteur, a company that manufactures and commercializes influenza vaccines.
LB is an employee of CreativCeutical, which received funding from Sanofi Pasteur to conduct the quality control of the data and run the analyses.
IO, CG, GM, and LL are employees of CINVE, which received funding from Sanofi to perform economic evaluations in Uruguay, Argentina, Chile, and Paraguay.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2050653
Additional information
Funding
References
- de Boer PT, van Maanen BM, Damm O, Ultsch B, Dolk FCK, Crépey P, Pitman R, Wilschut JC, Postma MJ. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):249–10. PMID: 28613092. doi:10.1080/14737167.2017.1343145.
- Caini S, Kusznierz G, Garate VV, Wangchuk S, Thapa B, de Paula Júnior FJ, Ferreira de Almeida WA, Njouom R, Fasce RA, Bustos P, et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS One. 2019;14(9):e0222381. doi:10.1371/journal.pone.0222381.
- World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76.
- Pepin S, Dupuy M, Borja-Tabora CFC, Montellano M, Bravo L, Santos J, de Castro J-A, Rivera-Medina DM, Cutland C, Ariza M, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6–35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern Hemispheres. Vaccine. 2019;37(13):1876–84. doi:10.1016/j.vaccine.2018.11.074.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- Hendriks J, Hutubessy RCW, Grohmann G, Torelli G, Friede M, Kieny MP. Quadrivalent influenza vaccines in low and middle income countries: cost-effectiveness, affordability and availability. Vaccine. 2018;36(28):3993–97. doi:10.1016/j.vaccine.2018.05.099.
- Mauskopf J, Standaert B, Connolly MP, Culyer AJ, Garrison LP, Hutubessy R, Jit M, Pitman R, Revill P, Severens JL, et al. Economic analysis of vaccination programs: an ISPOR good practices for outcomes research task force report. Value Health. 2018;21(10):1133–49. doi:10.1016/j.jval.2018.08.005.
- Ultsch B, Damm O, Beutels P, Bilcke J, Brüggenjürgen B, Gerber-Grote A, Greiner W, Hanquet G, Hutubessy R, Jit M, et al. Methods for Health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European vaccine economics community. Pharmacoeconomics. 2016;34(3):227–44. doi:10.1007/s40273-015-0335-2.
- World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes. 2nd. Published2019. [Accessed 2020 Dec 8].
- Chit A, Roiz J, Aballea S, Chuang J-H. An assessment of the expected cost-effectiveness of quadrivalent influenza vaccines in Ontario, Canada using a static model. PLoS One. 2015;10(7):e0133606. doi:10.1371/journal.pone.0133606.
- Mennini FS, Bini C, Marcellusi A, Rinaldi A, Franco E. Cost-Effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum Vaccines Immunother. 2018;14(8):1867–73. doi:10.1080/21645515.2018.1469368.
- Damm O, Garbe J, Thomas OBH, Alvarez FP, Bellier L, Greiner W, Greiner W. Cost-Effectiveness of quadrivalent influenza vaccination in Germany. Value Health. 2018;21(3):S232. doi:10.1016/j.jval.2018.09.1386.
- Crépey P, Redondo E, Díez-Domingo J, Ortiz de Lejarazu R, Martinón-Torres F, Gil de Miguel Á, López-Belmonte JL, Alvarez FP, Bricout H, Solozabal M, et al. From trivalent to quadrivalent influenza vaccines: public health and economic burden for different immunization strategies in Spain. PLoS One. 2020;15(5):e0233526. doi:10.1371/journal.pone.0233526.
- Crépey P, Boiron L, Araujo RR, Lopez JG, Petitjean A, de Albuquerque Luna EJ. Impact of quadrivalent influenza vaccines in Brazil: a cost-effectiveness analysis using an influenza transmission model. BMC Public Health. 2020;20(1):1374. doi:10.1186/s12889-020-09409-7.
- Jiang M, Li P, Wang W, Zhao M, Atif N, Zhu S, Fang Y. Cost-Effectiveness of quadrivalent versus trivalent influenza vaccine for elderly population in China. Vaccine. 2020;38(5):1057–64. doi:10.1016/j.vaccine.2019.11.045.
- Kim Y-K, Song JY, Jang H, Kim TH, Koo H, Varghese L, Han E. Cost effectiveness of quadrivalent influenza vaccines compared with trivalent influenza vaccines in young children and older adults in Korea. PharmacoEconomics. 2018;36(12):1475–90. doi:10.1007/s40273-018-0715-5.
- Tsuzuki S, Schwehm M, Eichner M. Simulation studies to assess the long-term effects of Japan’s change from trivalent to quadrivalent influenza vaccination. Vaccine. 2018;36(5):624–30. doi:10.1016/j.vaccine.2017.12.058.
- Van Bellinghen LA, Marijam A, Tannus Branco de Araujo G, Gomez J, Van Vlaenderen I. Cost-Utility of quadrivalent versus trivalent influenza vaccine in Brazil - comparison of outcomes from different static model types. Braz J Infect Dis. 2018;22(1):1–10. doi:10.1016/j.bjid.2017.11.004.
- Jamotte A, Clay E, Macabeo B, Caicedo A, Lopez JG, Bricks L, Romero Prada M, Marrugo R, Alfonso P, Moreno Arévalo B, et al. Public health impact and economic benefits of quadrivalent influenza vaccine in Latin America. Hum Vaccin Immunother. 2017;13(4):877–88. doi:10.1080/21645515.2016.1256928.
- Ruiz-Palacios GM, Beigel JH, Guerrero ML, Bellier L, Tamayo R, Cervantes P, Alvarez FP, Galindo-Fraga A, Aguilar-Ituarte F, Lopez JG, et al. Public health and economic impact of switching from a trivalent to a quadrivalent inactivated influenza vaccine in Mexico. Hum Vaccin Immunother. 2020;16(4):827–35. doi:10.1080/21645515.2019.1678997.
- Ministry of Health Uruguay (Ministerio de Salud Publica). Vacunación Antigripal y antineumocócica. Published 2018. [Accessed 2020 Sep 28]. https://www.gub.uy/ministerio-salud-publica/comunicacion/comunicados/vacunacion-antigripal-y-antineumococica
- World Health Organization. Guidance on the economic evaluation of influenza vaccination. [Accessed 2020 Nov 18]. https://apps.who.int/iris/bitstream/handle/10665/250086/WHO-IVB-16.05-eng.pdf
- Bertram M, Lauer J, De Joncheere K, Edejer T, Hutubessy R, Kieny M-P, Hill SR. Cost–effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;64(12):925–30. doi:10.2471/BLT.15.164418.
- Cost-Effectiveness Acceptability Curve (CEAC). York Health Economics Consortium. [ Accessed 2016]. https://yhec.co.uk/glossary/cost-effectiveness-acceptability-curve-ceac/.
- DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31(1):49–57. doi:10.1016/j.vaccine.2012.10.084.
- United Nations. Record view, life expectancy at birth for both sexes combined (years). Published 2019. [Accessed 2020 Sep 28]. https://population.un.org/wpp/Download/Standard/Mortality/
- World Health Organization. FluNet. https://www.who.int/influenza/gisrs_laboratory/flunet/en/. [Accessed 2020 Sep 30].
- Tran BX, Ohinmaa A, Nguyen LT. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual Life Outcomes. 2012;10(1):132. doi:10.1186/1477-7525-10-132.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30(11):1993–98. doi:10.1016/j.vaccine.2011.12.098.
- CINVE Consultora Salud (CINVE Health Consultancy). ‘Gasto Medio por Individuo 2010-2018' (Average spending per individual 2010-2018). [Unpublished data presented at Sector Inform presentation]. 2019.
- Pan American Health Organization. PAHO revolving fund vaccine prices for 2019. Published 2019. [Accessed 2020 Sep 30]. https://www.paho.org/en/documents/paho-revolving-fund-vaccine-prices-2019
- Van Wilder L, Rammant E, Clays E, Devleesschauwer B, Pauwels N, De Smedt D. A comprehensive catalogue of EQ-5D scores in chronic disease: results of a systematic review. Qual Life Res. 2019;28(12):3153–61. doi:10.1007/s11136-019-02300-y.
- Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M; GBD Chronic Kidney Disease Collaboration; Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. doi:10.1016/S0140-6736(20)30045-3.
- Hosseini M, Almasi-Hashiani A, Sepidarkish M, Maroufizadeh S. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res. 2019;20(1):229. doi:10.1186/s12931-019-1198-4.
- Loukine L, Waters C, Choi BC, Ellison J. Impact of diabetes mellitus on life expectancy and health-adjusted life expectancy in Canada. Popul Health Metr. 2012;10(1):7. doi:10.1186/1478-7954-10-7.
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-Effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother. 2014;10(5):1171–80. doi:10.4161/hv.28221.
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;2012:Cd004879.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi:10.1016/S1473-3099(11)70295-X.
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11(1):153. doi:10.1186/1741-7015-11-153.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010;3:Cd004876. doi:10.1002/14651858.CD001269.
- Ministry of Health Uruguay (Ministerio de Salud Publica). 2da encuesta nacional de factores de riesgo de enfermedades no transmisibles (National survey of risk factors for noncommunicable diseases). Published 2019. [ Accessed 2020 Sep 30].
- Uruguay Pharmacy Center (Centro de Farmacias del Uruguay). [Accessed 2020 Feb 21]. http://cfu.org.uy/medicamentos.html
- Drummond MSM, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th. Oxford: Oxford University Press; 2015.
- Aoki FY, Fleming DM, Griffin AD, Lacey LA, Edmundson S. Impact of zanamivir treatment on productivity, health status and healthcare resource use in patients with influenza. Zanamivir Study Group. Pharmacoeconomics. 2000;17(2):187–95. doi:10.2165/00019053-200017020-00007.
- National Institute of Statistics of Uruguay. Actividad, Empleo y Desempleo. http://www.ine.gub.uy/actividad-empleo-y-desempleo. Published 2018. [Accessed 2020 Sep 30].
- Castiglia A, Echagüe T. ‘Ministro de Trabajo y Seguridad Social Mtro. Ernesto Murro’, Unidad Estadística del Trabajo y la Seguridad Social. Published 2019. [Accessed 2020 Sep 30]. https://www.gub.uy/ministerio-trabajo-seguridad-social/sites/ministerio-trabajo-seguridad-social/files/documentos/publicaciones/SMN%20y%20salarios%20bajos%20en%20Uruguay%20-%20sueldo%20base%20%28g126%29_actualizado_2017_6_febrero-1.pdf
- Augustovski F, Rey-Ares L, Irazola V, Garay OU, Gianneo O, Fernández G, Morales M, Gibbons L, Ramos-Goñi JM. An EQ-5D-5L value set based on Uruguayan population preferences. Qual Life Res. 2016;25(2):323–33. doi:10.1007/s11136-015-1086-4.
- Arrospide A, Machón M, Ramos-Goñi JM, Ibarrondo O, Mar J. Inequalities in health-related quality of life according to age, gender, educational level, social class, body mass index and chronic diseases using the Spanish value set for Euroquol 5D-5L questionnaire. Health Qual Life Outcomes. 2019;17(1):69. doi:10.1186/s12955-019-1134-9.
- Yang F, Lau T, Lee E, Vathsala A, Chia KS, Luo N. Comparison of the preference-based EQ-5D-5L and SF-6D in patients with end-stage renal disease (ESRD). Eur J Health Econ. 2015;16(9):1019–26. doi:10.1007/s10198-014-0664-7.
- Sander B, Kwong JC, Bauch CT, Maetzel A, McGeer A, Raboud JM, Krahn M. Economic appraisal of Ontario’s Universal Influenza Immunization Program: a cost-utility analysis. PLoS Med. 2010;7(4):e1000256. doi:10.1371/journal.pmed.1000256.
- Chi YL, Blecher M, Chalkidou K, Culyer A, Claxton K, Edoka I, Glassman A, Kreif N, Jones I, Mirelman AJ, et al. What next after GDP-based cost-effectiveness thresholds? Gates Open Res. 2020;4:176. doi:10.12688/gatesopenres.13201.1.
- Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–24. doi:10.2471/BLT.14.138206.
- Bernales-Baksai P. Tackling segmentation to advance universal health coverage: analysis of policy architectures of health care in Chile and Uruguay. Int J Equity Health. 2020;19(1):106. doi:10.1186/s12939-020-01176-6.