ABSTRACT
Mass vaccination with a safe and effective vaccine may be the best way to control the COVID-19 pandemic. Heterologous prime-boost vaccination with the CoronaVac and AZD1222 vaccines may increase the immunogenicity elicited by either vaccine alone. This study sought to compare the immunogenicity of a heterologous CoronaVac and AZD1222 prime-boost with a homologous CoronaVac prime-boost. From July 13 to September 2, 2021, 88 participants were enrolled in the study. Half (n = 44) of the participants were assigned to the AZD1222/CoronaVac cohort and half were assigned to the CoronaVac/AZD1222 cohort. Both cohorts had a prime-boost interval of 4 weeks. A control group of 136 health care personnel who received the homologous CoronaVac/CoronaVac prime-boost was matched by age and sex to the experimental cohorts. The primary endpoint was the geometric mean ratio (GMR) of the anti-receptor binding domain (RBD) antibody concentration 4 weeks after the booster dose was administered. The CoronaVac/CoronaVac cohort served as the reference group. Baseline age and sex were similar, and the median age was 42.5 years. The GMR was 2.58 (95% confidence interval [CI] 1.80–3.71) and 8.69 (95% CI 6.05–12.47) in the AZD1222/CoronaVac and CoronaVac/AZD1222 cohorts, respectively. Reactogenicity was similar following prime and booster doses with the same vaccine. Findings indicated that the heterologous CoronaVac and AZD1222 prime-boost combination elicited a more robust immune response than the homologous CoronaVac prime-boost. While both heterologous prime-boost combinations showed similar reactogenicity, the immunogenicity of the CoronaVac/AZD1222 cohort was higher, indicating that the order of prime-boost vaccine administration was important.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a major global health issue, responsible for more than 233 million cases and 4.7 million deaths worldwide as of 2 October 2021.Citation1 To reduce the disease burden, vaccines have been developed and distributed globally. By October 2021, seven vaccines were approved by the World Health Organization (WHO): mRNA-1273 (mRNA vaccine), BNT162b2 (mRNA vaccine), Ad26.COV2.S (adenoviral vector vaccine), ChAdOx1 nCoV-19 (AZD1222) (adenoviral vector vaccine), BBIBP-CorV (inactivated vaccine), and CoronaVac (inactivated vaccine).Citation2 To date, only four of these vaccines, CoronaVac, BBIBP-CorV, AZD1222, and BNT162b2, are available in Thailand, of which CoronaVac and AZD1222 are the most accessible and widely used.Citation3
While studies have assessed the efficacy or effectiveness of the four vaccines against COVID-19 in homologous prime-boost vaccination,Citation4-7 research on heterologous prime-boost vaccination is limited. Following the B.1.617.2 (Delta) variant outbreak, however, the Thailand Ministry of public health approved the use of a heterologous CoronaVac/AZD1222 prime-boost vaccination regimen to increase the flexibility of vaccination, shorten the interval between primary and booster doses, and accelerate population immunity.Citation8 There is evidence support that heterologous vaccination improve immunogenicity than homologous vaccination.Citation9 Heterologous vaccination involves the use of vaccines with different platforms, allowing patients to benefit from the advantages of each vaccine regimen and increasing the flexibility of vaccine management. The heterologous AZD1222/BNT162b2 and BNT162b2/AZD1222 prime-boost vaccination regimens, for example, induce stronger humoral and cell-mediated immune responses than the homologous AZD1222 prime-boost vaccine regimen.Citation10,Citation11 These findings suggest that AZD1222 and BNT162b2 together elicit powerful immunogenicity. However, there are no established data on the use of CoronaVac or BBIBP-CorV in combination with AZD1222 or BNT162b2 for prime-boost vaccination.
The current study assessed the efficacy of a CoronaVac and AZD1222 heterologous prime-boost approach. Both vaccines were used as a prime or a boost vaccine, and vice versa, with a 4-week interval in between each dose.
Materials & methods
Study design and participants
A single-center, prospective cohort study was designed to demonstrate the immunogenicity of a heterologous prime-boost COVID-19 vaccine approach at Chulabhorn Hospital, Bangkok, Thailand. The study aims to compare the immunogenicity of the combination between the CoronaVac and AZD1222 vaccine to the homologous CoronaVac. Between July 13 and 2 September 2021, 88 participants were enrolled. The study compared the immunogenicity of the CoronaVac and AZD1222 vaccine combination to the homologous CoronaVac vaccine. Of the participants, 44 were primed with the CoronaVac vaccine and boosted with the AZD1222 vaccine (CoronaVac/AZD1222), and 44 were primed with the AZD1222 vaccine and boosted with the CoronaVac vaccine (AZD1222/CoronaVac), with a 4-week interval between each dose. Data on homologous prime-boost with the CoronaVac vaccine were obtained from 136 health care workers in our institute who were sex- and age-matched to the study participants. The health care workers in our institute were invited to participate in the study of immunogenicity and the safety of the COVID-19 vaccines available in Thailand (TCTR20210517006).
Healthy participants who were ≥18 years of age and had no history of SARS-CoV-2 infection or receipt of the SARS-CoV-2 vaccine were included in the study. Patients were excluded if they were lactating or pregnant, had an underlying disease, had received any vaccine within 14 days before enrollment, or had respiratory tract infection symptoms or fever within 14 days before enrollment. Written informed consent was obtained from all participants before enrollment. The study protocol, consent form, and case records form were reviewed and approved by the Chulabhorn Ethics Committee (reference number: 057/2564). This trial was registered with thaiclinicaltrials.org (TCTR20210714003).
Procedures
After enrollment, baseline spike protein anti-receptor binding domain (RBD) antibody levels were measured for all participants. The standard dose (.5 mL) of the CoronaVac or AZD1222 vaccine was administered intramuscularly into the deltoid muscle. Four weeks after receipt of the booster dose, the total antibody concentration of the anti-receptor binding domain (RBD) of SARS-CoV-2 (Elecsys S, Roche Diagnostics, Mannheim, Germany) was assessed for each participant. Both vaccines were manufactured and vialed using Good Manufacturing Practices and were approved by Thailand’s regulatory agency.
Sample size calculation
The sample size was determined using the anti-RBD total antibody concentration, 103.2 BAU/mL with a standard deviation (SD) of 96.8 BAU/mL, after homologous prime-boost CoronaVac vaccination at our institute. Assuming that the heterologous prime-boost approach would increase immunogenicity by at least 60%, the effect size dz was estimated at .64. Using a significance level of 5% (α = .05), a power of 80%, and a 10% drop-out estimation, G power software calculated the sample size at 44 participants per cohort.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9 and IBM SPSS statistic version 26. A p-value <.05 was considered statistically significant. The geometric mean concentration (GMC) of the anti-RBD post-vaccine antibody levels were compared for each cohort using a multiple linear regression model. The CoronaVac/CoronaVac cohort served as a reference. Summary statistics were presented as medians and interquartile ranges (IQRs) or geometric mean concentration and 95% confidence intervals (CIs).
Efficacy endpoints
The geometric mean ratio (GMR) at 4 weeks post-vaccination served as the primary endpoint and was compared between the experimental and control cohorts. Reactogenicity served as the secondary endpoint for each cohort.
Safety
All participants were observed for at least 15 minutes after each vaccination to monitor immediate adverse events. Short Message Service (SMS) was used to assess the wellbeing of each participant after 1, 7, and 30 days post-vaccination. Severity was defined as (i) mild: not interfering with daily activity or a local reaction <5 cm, (ii) moderate: some interference with daily activity or a local reaction of ≥5.1 cm to <10 cm, (iii) severe: significant interference with daily activity or a local reaction ≥10 cm, or (iv) potentially life-threatening: emergency care/hospitalization or a local reaction including necrosis or exfoliative dermatitis.
Anti-RBD antibody measurements
There was evidence supporting that binding antibodies assays correlated with the neutralizing antibody test.Citation12,Citation13 In addition, binding antibodies also correlated with vaccine efficacy.Citation14 An automated electrochemiluminescence immunoassay (ECLIA), the Elecsys Anti-SARS-CoV-2 S kit (Elecsys-S, Roche Diagnostics, Mannheim, Germany), was used to detect the total anti-RBD antibody concentration in each patient cohort. This kit is designed to detect antibodies against wild-type SARS-CoV-2. The manufacturer cutoff value for a positive response was >.8 U/mL. Based on the WHO international standard for anti-SARS-CoV-2 immunoglobulin concentration,Citation15 the Elecsys-S unit was converted to binding antibody units (BAU) using the equation: Elecsys-S U = .972 × BAU.Citation16
Results
From July 13 to 2 September 2021, 88 participants were enrolled in the study. Of these, 10 AZD1222/CoronaVac cohort participants and 10 CoronaVac/AZD1222 cohort participants were lost to follow-up during the 4 weeks between the booster dose and anti-RBD antibody measurements. Summarization of the participants’ flow was demonstrated in As a result, results were obtained for 34 participants in both the AZD1222/CoronaVac and CoronaVac/AZD1222 cohorts. The median age of all participants was 42.5 years, and 58.8%, 55.8%, and 57.4% of the individuals in the AZD1222/CoronaVac, CoronaVac/AZD1222, and CoronaVac/CoronaVac cohorts, respectively, were women (). Baseline anti-RBD antibody levels were negative for all participants except one in the AZD1222/CoronaVac cohort who had a positive result of 1.14 BAU/mL (normal range: <.8 BAU/mL) prior to enrollment. This level was too low to represent a recent SARS-CoV-2 infection and may instead have been the result of antibody cross-reactivity or sample or reagent contamination.Citation17 Of the participants, 70.6%, 76.5%, and 91.2% in the AZD1222/CoronaVac and CoronaVac/AZD1222, and CoronaVac/CoronaVac cohorts, respectively, had no underlying disease.
Figure 1. Participants’ flow. Eighty-eight participants in the study group were enrolled. Forty-four participants were assigned to CoronaVac/azd1222 group and 44 to AZD1222/CoronaVac group. Twenty participants lost to follow-up, 10 in CoronaVac/azd1222 group and 10 in AZD1222/CoronaVac group. a comparison group was collected from health care workers who completed 2 doses of CoronaVac and available immunogenicity data. 136 health care workers with age and sex matching were chosen for analysis.
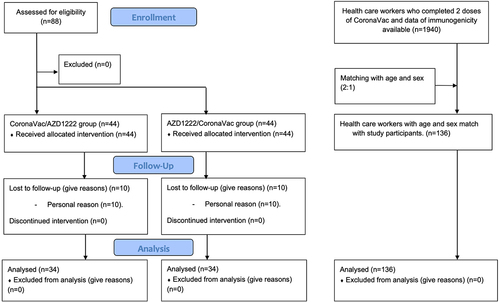
Table 1. Demographic and clinical characteristic at baseline of all participant in each cohort
Participants in the AZD1222/CoronaVac cohort (GMR 2.58, 95% CI 1.80–3.71) and the CoronaVac/AZD1222 cohort (GMR 8.69, 95% CI 6.05–12.47) had a higher GMR at 4 weeks after the booster dose than those in the CoronaVac/CoronaVac cohort ( and ).
Figure 2. Anti-Receptor binding domain (RBD) total antibodies 1 month after the second dose of COVID-19 vaccine. AZ: AZD1222 vaccine. SV: CoronaVac vaccine. SV/SV: prime with CoronaVac, boost with CoronaVac. AZ/SV: prime with AZD1222, boost with CoronaVac. SV/AZ: prime with CoronaVac, boost with AZD1222.
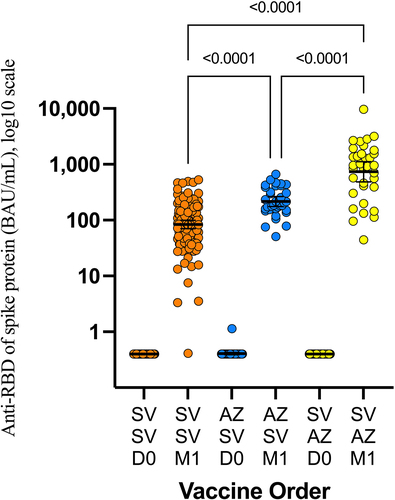
Table 2. Geometric mean ratio of anti-RBD of the spike protein of SARS-CoV-2 at 4-week after the 2nd dose of heterologous prime-boost approach
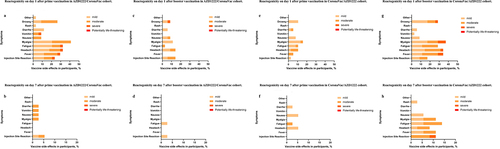
The most common systemic reactogenicity after the AZD1222/CoronaVac prime dose included myalgia, fatigue, and headache (41%, 32%, and 32%, respectively) and 24% experienced local reactogenicity. In contrast, the reactogenicity of individuals in the CoronaVac/AZD1222 cohort was significantly lower. The three most common symptoms were myalgia, fatigue, and headache (16%, 11%, and 11%, respectively), and the local reactogenicity was only 2.6%.
The most common reactogenicity after the CoronaVac/AZD1222 booster dose included myalgia, headache, and fever (37%, 34%, and 34%, respectively), and 17% of individuals experienced local reactogenicity. The reactogenicity was significantly lower for individuals in the AZD1222/CoronaVac cohort after the CoronaVac booster. The most common symptoms observed in this cohort were headache, myalgia, and fatigue (13%, 11%, and 9%, respectively), and the local reaction was 14%. More detail on reactogenicity within 7 days after vaccination is shown in .
Figure 3. Reactogenicity within 7 days after vaccination. (a) Reactogenicity on day 1 after prime vaccination in AZD1222/CoronaVac cohort. (b) Reactogenicity on day 7 after prime vaccination in AZD1222/CoronaVac cohort. (c) Reactogenicity on day 1 after booster vaccination in AZD1222/CoronaVac cohort. (d) Reactogenicity on day 7 after booster vaccination in AZD1222/CoronaVac cohort. (e) Reactogenicity on day 1 after prime vaccination in CoronaVac/azd1222 cohort. (f) Reactogenicity on day 7 after prime vaccination in CoronaVac/azd1222 cohort. (g) Reactogenicity on day 1 after booster vaccination in CoronaVac/azd1222 cohort. (h) Reactogenicity on day 7 after booster vaccination in CoronaVac/azd1222 Cohort.
Discussion
This study showed that a heterologous combination of the CoronaVac and AZD1222 vaccines elicited a more robust immune response than homologous CoronaVac vaccination with similar time intervals between doses. Moreover, the order of the prime-boost approach was important. The CoronaVac/AZD1222 cohort elicited higher immunogenicity than the AZD1222/CoronaVac cohort. In addition, booster dose reactogenicity did not differ from the reactogenicity of the prime dose with the same vaccine. These findings support the hypothesis that the heterologous prime-boost approach improves the immune response to SARS-CoV-2.
Recent studies of the COVID-19 vaccine combination have focused on adenoviral vector and messenger RNA platforms;Citation10,Citation11,Citation18,Citation19 this is the first study to evaluate the immunogenicity elicited by combining the inactivated COVID-19 vaccine and adenoviral vector platform. Although they used different vaccine platforms, the findings from this study were similar to those from the COM-COV study. The COM-COV study showed that the neutralizing antibody levels induced by AZD1222/BNT162b2 were higher than those induced by AZD1222/AZD1222, with a GMR of 8.5. The cell-mediated immune response was also higher in the AZD1222/BNT162b2 cohort, with a GMR of 3.8. The prime-boost vaccine order was also important in the COM-COV study; indeed, the humoral and cell-mediated responses were higher in response to the AZD1222/BNT162b2 combination than the BNT162b2/AZD1222 combination.Citation10
There are a few hypotheses for why immunogenicity is improved in response to heterologous prime-boost vaccination.Citation20 First, there is variability in the strength of the immune response mounted by different vaccine platforms. CoronaVac is an inactivated vaccine platform and the adjusted vaccine effectiveness is only 15.5% (95% CI 14.2%–16.8%) after a single dose but increases to 65.9% (95% CI 65.2%–66.6%) after the second dose.Citation4 This suggests that a single CoronaVac vaccine dose elicits a low-level immune response with memory lymphocytes. In contrast, a single dose of AZD1222 had an adjusted vaccine effectiveness of 48.7% (95% CI 45.2%–51.9%) that increased to 74.5% (95% CI 68.4%–79.4%) after the second dose.Citation21 These results showed that a single dose of AZD1222 elicited a more robust immune response than a single dose of CoronaVac. Thus, priming with the CoronaVac vaccine and boosting with the AZD1222 could be expected to have strong immunogenicity.
Immunogenicity may also be improved in response to heterologous prime-boost vaccination because different vaccine platforms elicit distinct types of immune responses. Basically, after inoculum with the inactivated vaccine, the antigen-presenting cells (APCs), mainly dendritic cells (DCs), bound the vaccine antigen using pattern recognition receptors (PRRs) and then expressed co-stimulatory molecules, secreted chemokine, and cytokines.Citation22 After processing by the endocytic pathway, the antigen will be presented to CD4+ T cells.Citation23 Some vaccine antigens attach to the B-cell; it activated B cell and was internalized, processed, and presented to the helper T cell. The humoral immune response was the main result of this process.Citation24,Citation25 On the other hand, vaccination with an adenoviral vector vaccine can elicit both humoral and cell-mediated immune responses.Citation25, Citation26 Following adenoviral vector vaccination, the transgene can be expressed in both nonimmune and immune cells. Transgene expression in nonimmune cells results in a release of transgene product from the cells and then mainly elicits a humoral immune response. In addition, transgene expression in immune cells mediates antigen cross-presentation, resulting in cytotoxic T lymphocyte (CTL).Citation26,Citation27 Moreover, different vaccine vectors elicit different robustness of immune response.Citation28 This complementary effect occurred after heterologous prime-boost with different vaccine vectors.Citation29
The dose of prime and boost vaccine is also pivotal. The dose of the prime vaccine did not influence the immunogenicity after completing heterologous prime-boost vaccination in some studies.Citation30,Citation31 However, a higher vaccine booster dose showed a significant final immune response than a low booster dose.Citation32 Although the inactivated vaccine elicited a low antibody level compared to another vaccine platform,Citation28 specific memory B cells also induced and promptly responded after the booster dose.Citation33 The immune response after vaccination with the AZD1222 vaccine was more robust than CoronaVac, which reflected a higher dose of vaccine in AZD1222 to CoronaVac.Citation28 This may explain why the CoronaVac/AZD1222 group had a higher immune response than the AZD1222/CoronaVac group.Citation34
A vaccine containing protein antigen can elicit the T cell-dependent B cell responses. This response includes proliferation, isotype switching, and maturation affinity of antigen-specific B cells. Not only antibodies production, but it also generates the memory B cells and long live plasma cells that can immediately respond to antigen-specific stimulation.Citation35 Moreover, innate immunity also enhances response upon restimulation. Primary specific antibodies and memory T cells after prime vaccination provide a distinct environment at the time of boost vaccination. In addition to detecting pathogen-associated molecular patterns (PAMPs), the innate cell also detects immune complexes of vaccine immunogens and primary antibodies. They are cleared and trigger inflammation by Fc receptors expressing phagocytic cells.
In addition, the innate cells can be trained. They mediated enhanced effector response after restimulation. The memory-like feature of the innate cells is so-called trained immunity. The mechanisms of trained immunity include metabolic rewriting, epigenetic reprogramming, and change in gene expression.Citation36 The trained immunity may enhance the boost vaccine in the heterologous prime-boost approach.
The heterologous prime-boost approach may also induce a robust immune response because it is not impacted by vector immunity. Voysey et al. showed that the greater the interval between the prime and boost vaccine doses, the more effective the response.Citation37 A heterologous approach using the Gam-COVID-Vac vaccine showed excellent vaccine efficacy, an effect also found in response to the CoronaVac vaccine.Citation38 The neutralizing antibody titer was significantly higher when a third CoronaVac vaccine dose was administered 6 months rather than only 1 month after the second dose.Citation39
High anti-RBD antibody levels correlate with vaccine efficacy.Citation28 Thus, this study supports Thailand Department of Disease Control policies to vaccinate the public using a CoronaVac vaccine prime followed by a AZD1222 vaccine boost rather than homologous CoronaVac prime-boost vaccination.Citation8 The current study will influence further considerations of the most optimal way to immunize the general population and achieve the highest level of herd immunity in resource-limited settings.
This study had a few limitations. First, it used a non-randomized design that may make it difficult to compare the experimental and control groups. Second, because a novel heterologous prime-boost approach was used along with new vaccines, many participants expressed hesitancy about the vaccination protocol. To address this, the participants were permitted to choose which vaccine regimen they would receive. Repeating this study as a randomized controlled trial would help to strengthen its validity. Second, the rate of loss to follow-up in both groups was quite high (22.7%, 10/44). Most of these participants were not available at the time scheduled for the anti-RBD antibodies test. This may contribute to the interpretation of the anti-RBD antibodies concentration. However, the rate of loss to follow-up was equal in both groups. Third, the duration of the immunogenicity after heterologous prime-boost was unknown. Further study is needed to establish this duration. Fourth, the population was considerable healthy, the results cannot be generalized to other populations. Fifth, although this finding support that heterologous prime-boost with CoronaVac and AZD1222 vaccine was superior to homologous CoronaVac vaccine, its lack of the homologous AZD1222 vaccine as the control. Further study is needed to demonstrate that heterologous prime-boost with CoronaVac/AZD1222 was not inferior to homologous AZD1222 vaccine. Finally, the antibody response is only one element of immune protection,Citation28 the T-cell function, and innate immunity are not mentioned in this study.Citation25 In addition, further research is needed to compare the immunogenicity of the heterologous CoronaVac/AZD1222 prime-boost vaccine regimen with the homologous AZD1222/AZD1222 prime-boost vaccine regimen.
Ethics
The study was conducted in accordance with the principles laid out in the Declaration of Helsinki guidelines for research involving human subjects. The study protocol was reviewed and approved by the Ethics Committee for Human Research, Chulabhorn Research Institute (Certificate No. 057/2564).
Acknowledgments
We thank the clinical research management unit for managing this project. We also thank the central laboratory of Chulabhorn Hospital for the laboratory testing, and gratefully acknowledge funding from Chulabhorn Royal Academy and National Vaccine Institute, Thailand. Finally, we thank Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time [ published correction appears in Lancet Infect Dis2020 Sep;20(9):e215]. Lancet Infect Dis. 2020;20(5):533–7. doi:10.1016/S1473-3099(20)30120-1.
- WHO. Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. 2021 [accessed 2021 Oct 16].
- COVID-19 WHO Thailand Situation Reports. [accessed 2021 Oct 16]. https://cdn.who.int/media/docs/default-source/searo/thailand/2021_10_14_tha-sitrep-205-covid-19.pdf?sfvrsn=8ba57507_3
- Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–84. doi:10.1056/NEJMoa2107715.
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. Jama. 2021;326(1):35–45. doi:10.1001/jama.2021.8565.
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al.; . AstraZeneca AZD1222 Clinical Study Group. Phase 3 safety and efficacy of AZD1222 (ChAdox1 nCov-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–60. doi:10.1056/NEJMoa2105290.
- Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384(16):1576–77. doi:10.1056/NEJMc2036242.
- Department of Disease Control MoPH. Thailand. a guide to COVID-19 vaccines in Thailand. 2021 [accessed 2021 Oct 16]. https://www.who.int/thailand/news/detail/08-07-2021-a-guide-to-covid-19-vaccines-in-thailand
- Fournillier A, Frelin L, Jacquier E, Ahlén G, Brass A, Gerossier E, Holmström F, Broderick KE, Sardesai NY, Bonnefoy J-Y, et al. A heterologous prime/boost vaccination strategy enhances the immunogenicity of therapeutic vaccines for hepatitis C virus. J Infect Dis. 2013;208(6):1008–19. doi:10.1093/infdis/jit267.
- Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–69. doi:10.1016/S0140-6736(21)01694-9.
- Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, Dopfer-Jablonka A, Heidemann A, Ritter C, Friedrichsen M, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdox1 nCov-19/bnt162b2 vaccination. Nat Med. 2021;27(9):1525–29. doi:10.1038/s41591-021-01449-9.
- Poon RW, Lu L, Fong CH, Ip TC, Chen LL, Zhang RR, Yip CC, Cheng VC, Chan KH, Yuen KY, et al. Correlation between commercial anti-RBD IgG titer and neutralization titer against SARS-CoV-2 beta variant. Diagnostics (Basel). 2021;11(12):2216. [accessed 2021 Nov 27]. doi:10.3390/diagnostics11122216.
- Morinaga Y, Tani H, Terasaki Y, Nomura S, Kawasuji H, Shimada T, Igarashi E, Saga Y, Yoshida Y, Yasukochi R, et al. Correlation of the commercial anti-SARS-CoV-2 receptor binding domain antibody test with the chemiluminescent reduction neutralizing test and possible detection of antibodies to emerging variants. Microbiol Spectr. 2021;9(3):e0056021. doi:10.1128/Spectrum.00560-21.
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–40. doi:10.1038/s41591-021-01540-1.
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, Plotkin S, Knezevic I. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–48. doi:10.1016/S0140-6736(21)00527-4.
- Resman Rus K, Korva M, Knap N, Avšič Županc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi:10.1016/j.jcv.2021.104820.
- Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sens. 2021;6(3):593–612. doi:10.1021/acssensors.0c02621.
- Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdox1 nCov-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11:100249. doi:10.1016/j.lanepe.2021.100249.
- Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, Peyrot L, Allatif O, Fassier JB, Massardier-Pilonchéry A, et al. Immunogenicity and efficacy of heterologous ChAdox1-BNT162b2 vaccination. Nature. 2021;600(7890):701–06. doi:10.1038/s41586-021-04120-y.
- Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21(3):346–51. doi:10.1016/j.coi.2009.05.016.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. doi:10.1056/NEJMoa2108891.
- Stoel M, Pool J, de Vries-Idema J, Zaaraoui-Boutahar F, Bijl M, Andeweg AC, Wilschut J, Huckriede A. Innate responses induced by whole inactivated virus or subunit influenza vaccines in cultured dendritic cells correlate with immune responses in vivo. PLoS One. 2015;10(5):e0125228. accessed 2015 May 1. doi:10.1371/journal.pone.0125228.
- Couture A, Garnier A, Docagne F, Boyer O, Vivien D, Le-Mauff B, Latouche JB, Toutirais O. HLA-Class II artificial antigen presenting cells in CD4+ T cell-based immunotherapy. Front Immunol. 2019 [accessed 2019 May 17];10:1081. doi:10.3389/fimmu.2019.01081.
- Goyal K, Goel H, Baranwal P, Tewary A, Dixit A, Pandey AK, Benjamin M, Tanwar P, Dey A, Khan F, et al. Immunological mechanisms of vaccine-induced protection against SARS-CoV-2 in humans. Immuno. 2021;1(4):442–56. doi:10.3390/immuno1040032.
- Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21:475–84.
- Sakurai F, Tachibana M, Mizuguchi H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab Pharmacokinet. 2022;42:100432. doi:10.1016/j.dmpk.2021.100432.
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-Presentation by dendritic cells. Nat Rev Immunol. 2012 [ accessed 2012 Jul 13];12(8):557–69. doi:10.1038/nri3254.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Valdés I, Lazo L, Hermida L, Guillén G, Gil L. Can complementary prime-boost immunization strategies be an alternative and promising vaccine approach against dengue virus? Front Immunol. 2019 [accessed 2019 Aug 27];10:1956. doi:10.3389/fimmu.2019.01956.
- Clements-Mann ML, Weinhold K, Matthews TJ, Graham BS, Gorse GJ, Keefer MC, McElrath MJ, Hsieh RH, Mestecky J, Zolla-Pazner S, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177(5):1230–46. doi:10.1086/515288.
- Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, Larkin BD, Enama ME, Ledgerwood JE, Bailer RT, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One. 2010;5(2):e9015. [accessed 2010 Feb 2]. doi:10.1371/journal.pone.0009015.
- Li M, Yang J, Wang L, Wu Q, Wu Z, Zheng W, Wang L, Lu W, Deng X, Peng C, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. doi:10.1101/2021.08.03.21261544.
- Liu Y, Zeng Q, Deng C, Li M, Li L, Liu D, Liu M, Ruan X, Mei J, Mo R, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discovery. 2022;8(1):10. [accessed 2022 Feb 1]. doi:10.1038/s41421-022-00373-7.
- Shete A, Thakar M, Mehendale SM, Paranjape RS. Is prime boost strategy a promising approach in HIV vaccine development? J AIDS Clin Res. 5:293. doi:10.4172/2155-6113.1000293.
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–63. doi:10.1016/j.immuni.2010.10.008.
- Palgen JL, Feraoun Y, Dzangué-Tchoupou G, Joly C, Martinon F, Le Grand R, Beignon AS. Optimize prime/boost vaccine strategies: trained immunity as a new player in the game. Front Immunol. 2021 [accessed 2021 Mar 8];12:612747. doi:10.3389/fimmu.2021.612747.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-Dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdox1 nCov-19 (AZD1222) vaccine: a pooled analysis of four randomised trials [published correction appears in Lancet. 2021 Mar 6;397(10277):880]. Lancet. 2021;397(10277):881–91. doi:10.1016/S0140-6736(21)00432-3.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia [published correction appears in Lancet. 2021 Feb 20;397(10275):670]. Lancet. 2021;397(10275):671–81. doi:10.1016/S0140-6736(21)00234-8.
- Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials [published online ahead of print, 2021 Dec 7]. Lancet Infect Dis. 2021;S1473-3099(21):00681–2. doi:10.1016/S1473-3099(21)00681-2.
