ABSTRACT
MenACYW-TT (MenQuadfi®) is a quadrivalent meningococcal tetanus toxoid conjugate vaccine licensed in Europe for use in individuals ≥12 months. This study assessed whether serogroup C immune responses with MenACYW-TT were at least non-inferior, or superior, to those of quadrivalent meningococcal ACWY (MCV4-TT; Nimenrix®) and monovalent meningococcal C (MenC-TT; NeisVac-C®) vaccines in toddlers (12–23 months). In this modified, double-blind Phase III study (NCT03890367), 701 toddlers received one dose of MenACYW-TT (n = 230), MCV4-TT (n = 232) or MenC-TT (n = 239). Serum bactericidal assays with human (hSBA) and baby rabbit (rSBA) complement were used to measure anti-meningococcal serogroup C antibodies at baseline and 30 days post-vaccination. A sequential statistical approach was used for primary and secondary objectives. For the primary objectives, superiority of serogroup C was assessed in terms of hSBA seroprotection rates (defined as titers ≥1:8) and GMTs for MenACYW-TT compared to MCV4-TT, and rSBA GMTs compared to MenC-TT. The safety of all vaccines within 30 days post-vaccination was described. When administered as a single dose to meningococcal vaccine-naïve healthy toddlers the superiority of the MenACYW-TT serogroup C immune response versus MCV4-TT was demonstrated for hSBA GMTs (ratio 16.3 [12.7–21.0]) and seroprotection (difference 10.43% [5.68–16.20]); and versus MenC-TT in terms of rSBA GMTs (ratio 1.32 [1.06–1.64]). The safety profiles of a single dose of MenACYW-TT, MCV4-TT and MenC-TT were similar. In meningococcal vaccine-naïve toddlers, MenACYW-TT induced superior immune responses to serogroup C versus MCV4-TT in terms of hSBA seroprotection and GMTs and versus MenC-TT in terms of rSBA GMTs.
Introduction
Invasive meningococcal disease (IMD), which typically presents as meningitis and septicemia, had an incidence in Europe of 0.62 cases per 100,000 people in 2018, with the highest incidence in infants and young children; 8.34 cases per 100,000 children <1-year-old and 2.38 cases per 100,000 1–4-year-olds.Citation1,Citation2 The most common causes of IMD in Europe are meningococcal serogroups B and C, with an increasing number of cases caused by serogroups W and Y in recent years.Citation3 Brazil has similarly seen the majority of its IMD cases caused by serogroups B, C and W and has reported an incidence of 0.50 cases per 100,000 as of 2018,Citation4 while Australia has seen a significant proportion of its cases caused by serogroups B and W, with cases caused by serogroup C comparatively low following the introduction of a meningococcal serogroup C (MenC) conjugate vaccine immunization program.Citation5 MenC vaccine immunization programs were also launched in the UK and the Netherlands in response to the 1999–2001 serogroup C outbreaks,Citation6 successfully reducing overall disease incidence of IMD caused by serogroup C in both countries.Citation7–9 In response to the recent increases in serogroup W in Europe,Citation10,Citation11 quadrivalent meningococcal conjugate vaccines (MCV4) have been progressively introduced, replacing MenC conjugate vaccines in national immunization programs in several countries.Citation12 Among those countries that recommend meningococcal vaccination during childhood, some use a mixture of MenC and MCV4 vaccines according to the age group, while others still offer exclusively MenC, or exclusively MCV4.
MenACYW-TT (MenQuadfi®, Sanofi Pasteur, USA) is a quadrivalent meningococcal tetanus toxoid conjugate vaccine licensed from 12 months of age in Europe and other countries, such as Brazil, Australia and Canada, and licensed for use in individuals from 2 years of age in the US. The development for use in infants from 6 weeks of age is still ongoing.Citation13–21 The immunogenicity and safety of this vaccine has been compared to a licensed MCV4-TT (Nimenrix®, Pfizer, Sandwich, UK) in two studies in toddlers aged 12–23 months in Europe.Citation19,Citation20 In the pivotal phase III study, MenACYW-TT demonstrated non-inferiority for seroprotection (defined as titers ≥1:8) against all four meningococcal serogroups versus MCV4-TT using a human complement serum bactericidal assay (hSBA); for serogroup C, the lower bound of the two-sided 95% CI of the overall difference of the proportion seroprotected was greater than 0.Citation20
The tetanus toxoid conjugate monovalent meningococcal C vaccine, MenC-TT (NeisVac-C®, Pfizer, Sandwich, UK), is used extensively worldwide as part of meningococcal C vaccination programs and its immunogenicity was evaluated during vaccine development using the baby rabbit complement serum bactericidal assay (rSBA). To date, the serogroup C response to the MenACYW-TT vaccine has not been directly compared to MenC-TT.
The objective of this study was to compare the immune response to serogroup C elicited by a single dose of MenACYW-TT to the response elicited by a single dose of MCV4-TT or MenC-TT, and to describe the safety of healthy meningococcal vaccine-naïve toddlers. Using a sequential testing approach, the serogroup C immunogenicity of MenACYW-TT in terms of seroprotection rates and GMTs was tested for non-inferiority to the immunogenicity of MCV4-TT (measured by hSBA) and MenC-TT (measured by rSBA). If seroprotection rates and GMTs were non-inferior, the subsequent aim was to demonstrate statistical superiority of MenACYW-TT versus MCV4-TT for hSBA GMTs and seroprotection rates, and superiority of rSBA GMTs versus MenC-TT. Different SBA assays were utilized to align with assays used in the development of the vaccines. As the objective of the study focussed on serogroup C responses, the responses to the other serogroups were not assessed.
Methods
Study design and participants
This was a Phase III, multi-center, modified double-blind trial conducted to evaluate the immunogenicity of the serogroup C response and safety of a single dose of the MenACYW-TT vaccine versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy meningococcal vaccine-naïve toddlers aged 12–23 months (EudraCT #: 2018–003790–10; NCT03890367). The study was conducted in 29 centers in Denmark, Germany and Finland between 12 September 2019 and 14 October 2020.
Participants had an informed consent form (ICF) signed and dated by their parent(s)/legally acceptable representative(s). Exclusion criteria included participation in another study 4 weeks preceding or during the study; receipt or planned receipt of any vaccine in the 4 weeks preceding or during the study (except for an influenza vaccine administered at least 2 weeks before or after the study vaccination); receipt of immune globulins, blood or blood-derived products in the past 3 months; previous vaccination against meningococcal disease; receipt of immunosuppressive therapy; history of or high risk of meningococcal infection; history of Arthus-like reaction; history of Guillain-Barré syndrome (GBS); receipt of oral or injectable antibiotic therapy within 72 hours prior to the first blood draw.
Participants were randomized in a 1:1:1 ratio using an Interactive Response Technology to receive a single dose of either MenACYW-TT, MCV4-TT or MenC-TT on Day 0, and the randomization lists of participants were stratified by center. Both the participant and the investigator were blinded to study treatment, as were the laboratory personnel. As the vaccines differ in their presentation, the vaccine administrator was unblinded to study group allocation, and was not involved in safety data collection.
Participants received a single dose of either MenACYW-TT (MenQuadfi®, Sanofi Pasteur, Swiftwater, PA, USA), presented in .5 mL of saline solution containing 10 μg of each of meningococcal capsular polysaccharides serogroups A, C, W and Y and approximately 55 μg of tetanus toxoid protein carrier; MCV4-TT (Nimenrix®, Pfizer, Sandwich, UK), provided as a powder and solvent for reconstitution, with each .5 mL dose containing 5 μg of each of meningococcal polysaccharides serogroups A, C, W and Y and approximately 44 μg of tetanus toxoid protein carrier, or MenC-TT (NeisVac-C®, Pfizer, Sandwich, UK), presented in .5 mL of saline solution containing 10 µg of serogroup C polysaccharide and 10–20 µg of tetanus toxoid protein carrier and adsorbed on aluminum hydroxide, hydrated (.5 mg Al3+). All vaccines were administered intramuscularly on Day 0.
The study was conducted in agreement with the Declaration of Helsinki and with the International Conference on Harmonization guidelines for good clinical practice as well as with all local and/or national regulations and directives.
Immunogenicity
Blood samples for immunogenicity assessment of serogroup C response were taken before (Day 0) and 30 days (Day 30 + up to 14 days) after vaccination. Serum bactericidal antibody assays (SBAs) using human complement (hSBA; Global Clinical Immunology, Sanofi Pasteur, Swiftwater, USA) and baby rabbit complement (rSBA; Public Health England, Manchester, United Kingdom) were used to measure functional antibodies against meningococcal group C, both of which were performed by qualified laboratory personnel as described previously.Citation22 The lower limit of quantitation (LLOQ) of both assays was a titer of 1:4. Seroprotection was defined as titers ≥1:8 at Day 30 for both hSBA and rSBA. Comparisons of antibody responses in terms of geometric mean titers (GMTs) at Day 0 and Day 30 measured with both hSBA and rSBA were also performed. An exploratory endpoint of vaccine seroresponse was measured by hSBA defined as a post-vaccination titer ≥1:16 for participants with pre-vaccination titers <1:8, or a post-vaccination titer ≥4-fold greater than the pre-vaccination titer for participants with pre-vaccination titers ≥1:8, and measured by rSBA defined as a post-vaccination titer ≥1:32 for participants with pre-vaccination titers <1:8, or a post-vaccination titer ≥4-fold greater than the pre-vaccination titer for participants with pre-vaccination titers ≥1:8.
Safety
Participants were observed for 30 minutes after vaccination to ensure their safety, during which, any unsolicited systemic adverse event (AE) was recorded. The occurrence of solicited injection site (tenderness, erythema and swelling) and systemic reactions (fever, vomiting, abnormal crying, drowsiness, appetite loss, irritability) were recorded up to 7 days after vaccination in the participant’s diary card provided to the parents/guardians. Parents or guardians were asked to inform the investigators of any potential serious adverse events (SAEs) immediately.
Unsolicited AEs were collected from Day 0 to Day 30, and SAEs including AEs of special interest (AESIs) were collected throughout the trial. Adverse events classified as AESIs were generalized seizures (febrile and non-febrile), Kawasaki disease, GBS and idiopathic thrombocytopenia purpura, as directed by European regulators.
Statistical analyses
For the determination of sample size, a total of 675 participants were initially planned to be enrolled according to a ratio 1:1:1. This sample size was to provide acceptable global powers considering an overall one-sided alpha of 2.5% (i.e., 1.25% adjusted alpha for each of the 2 main non-inferiority objectives tested, then the same level of alpha was used for the subsequent tests as a ranking testing strategy was used), assuming an initial drop-out rate of 10%. As a consequence of the COVID-19 pandemic, enrollment was paused on 17 March 2020 and the study protocol amended. Approximately 30 participants were identified to be excluded from the per protocol analysis set (PPAS) as they were unable to complete their follow-up visit as planned. To maintain the planned study statistical power and the randomization ratio, the protocol was amended to modify the sample size with 30 participants added to replace these participants, so that the study planned to enroll 705 participants in total.
Three analysis sets were used: the hSBA and rSBA full analysis sets (FAS), the hSBA and rSBA per protocol analysis sets (PPAS) and the safety analysis set (SafAS). The FAS was defined as the subset of participants who received one dose of study vaccine and were analyzed by the treatment group to which they were assigned. The PPAS included all participants from the FAS who adhered to the protocol-specific inclusion criteria, did not have relevant deviations from the study protocol and had a valid hSBA or rSBA test result at Day 30. The SafAS was defined as those participants who had received a single dose of the study vaccine and had any safety data available, and were analyzed by the vaccine they actually received.
The testing for non-inferiority and superiority was conducted on the PPAS (hSBA or rSBA) and was done in parallel with a step-by-step approach (). For the primary objectives of the comparison of MenACYW-TT versus MCV4-TT, if the non-inferiority in terms of seroprotection rates measured by hSBA was demonstrated, then non-inferiority in terms of hSBA GMTs was tested; if the latter was demonstrated, then the superiority of the GMTs was tested, and if the latter was demonstrated, then the superiority of the seroprotection rates was tested. For the primary objectives of the comparison of MenACYW-TT versus MenC-TT, if the non-inferiority in terms of rSBA seroprotection rates was demonstrated, then non-inferiority of the rSBA GMTs was tested, if the latter was demonstrated, then the superiority of the GMTs was tested. For the secondary objectives, a similar sequential testing approach was used; for the comparison of MenACYW-TT versus MCV4-TT, if the non-inferiority in terms of rSBA seroprotection rates was demonstrated, then the non-inferiority of the rSBA GMTs was tested; if the latter was demonstrated, then the superiority of the GMTs was tested. For the secondary objectives of the comparison of MenACYW-TT versus MenC-TT, if the non-inferiority in terms of hSBA seroprotection rates was demonstrated, then non-inferiority of the hSBA GMTs was tested; if the latter was demonstrated, then the superiority of the GMTs was tested.
Figure 1. Sequential statistical testing approach for primary objectives (a) and the secondary objectives (b).
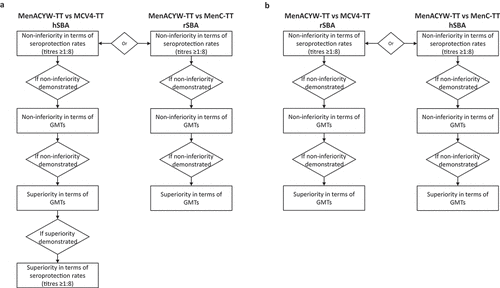
Testing for the primary and secondary objectives was based on the difference in post-vaccination seroprotection rates (computed with their associated 97.5% confidence intervals [CI] and calculated using the Wilson Score method without continuity correction [Newcombe method]) and on the ratio of post-vaccination GMTs (computed first using normal approximation of log-transformed titers and then by taking the anti-logarithms of the lower and upper limits of the provided 97.5% CI). Non-inferiority was demonstrated for seroprotection rates if the lower limit of the two-sided 97.5% CI for the difference was >-10%; superiority was demonstrated if the lower limit was >0%. Non-inferiority was demonstrated for GMTs if the lower limit of the two-sided 97.5% CI for the ratio was >1/1.5; superiority was demonstrated if the lower limit was >1. For safety results, the number and percentages with their respective 95% CIs were calculated using the exact binomial distribution (Clopper–Pearson method).
Analyses were conducted using SAS® Version 9.4 or later (SAS Institute Inc.; Cary, NC).
Results
Study participants
The flow of participants through the study is summarized in Supplementary Figure 1. Out of a total of 707 participants enrolled, 701 received a study vaccine: 230 received MenACYW-TT, 232 received MCV4-TT and 239 received MenC-TT; with a total of 696 participants (228, 229 and 239 in each group, respectively) completing the study. A total of 52.8% of participants were male and 47.2% female for both the hSBA and rSBA FAS groups. The average age of the participants in the MenACYW-TT group was 16.5 months, while the average of participants in both the MCV4-TT and MenC-TT groups was 16.7 months. Baseline demographics are summarized in . The participants were balanced between each of the vaccine groups for both the hSBA and rSBA FASs.
Table 1. Baseline characteristics (FAS)
Primary immunogenicity objectives
For the comparison of MenACYW-TT versus MCV4-TT, following sequential testing, noninferiority and superiority in terms of hSBA serogroup C seroprotection rates were demonstrated. Noninferiority and superiority in terms of hSBA serogroup C GMTs were also demonstrated ().
Table 2. Summary of the primary objective results: serogroup C seroprotection rates (≥1:8) and GMTs following a single dose of MenACYW-TT, MCV4-TT or MenC-TT (PPAS)
For the comparison of MenACYW-TT versus MenC-TT, non-inferiority in terms of rSBA seroprotection rates was demonstrated. Following sequential testing, noninferiority and superiority in terms of rSBA serogroup C GMTs were also demonstrated ().
Secondary immunogenicity objectives
For the comparison of MenACYW-TT versus MCV4-TT, non-inferiority in terms of rSBA serogroup C seroprotection rates was demonstrated. Following sequential testing, noninferiority and superiority in terms of rSBA serogroup C GMTs were also demonstrated ().
Table 3. Summary of the secondary objective results: serogroup C seroprotection rates (≥1:8) and GMTs following a single dose of MenACYW-TT, MCV4-TT or MenC-TT (PPAS)
For the comparison of MenACYW-TT versus MenC-TT, noninferiority in terms of hSBA serogroup C seroprotection rates was demonstrated. Following sequential testing, noninferiority and superiority in terms of hSBA GMTs were also demonstrated ().
Similar conclusions for the primary and secondary objectives were observed in the hSBA and rSBA FAS (data not shown).
Serogroup C vaccine seroresponse at Day 30, as measured by both hSBA and rSBA, was high across all vaccine groups (Supplementary Figure 2).
Safety
Overall, the safety profile was comparable between vaccine groups in terms of solicited and unsolicited adverse reactions () with solicited reactions (solicited injection site reactions and solicited systemic reactions) observed in 80.4% of the MenACYW-TT group, 78.8% of the MCV4-TT group and 74.9% of the MenC-TT group. The proportion of participants who reported at least one unsolicited adverse reaction (AR) was 6.5% (15/230) in the MenACYW-TT group, 4.7% (11/232) in the MCV4-TT group and 5.0% (12/239) in the MenC-TT group. No immediate unsolicited AEs were reported within 30 minutes of vaccination in any group. There was one SAE reported during the study in the MenACYW-TT group (respiratory syncytial virus infection), one in the MCV4-TT group (febrile convulsion [occurring 9 days after vaccination]) and four SAEs reported in two participants in the MenC-TT group (respiratory tract infection, febrile convulsion [occurring 6 days after vaccination and then a second subsequent episode], foreign body in throat and urticaria); none of them were considered vaccine related. No deaths were reported during the study.
Table 4. Safety outcomes (SafAS)
Discussion
This study assessed and demonstrated the superiority of the bactericidal antibody responses to serogroup C following administration of a single dose of MenACYW-TT compared to MCV4-TT in terms of hSBA seroprotection and GMTs in meningococcal vaccine-naïve toddlers 12–23 months of age. This study also demonstrated the superiority of the bactericidal antibody responses to serogroup C in terms of GMTs and the non-inferiority in terms of seroprotection measured by rSBA compared to the licensed MenC-TT. Furthermore, the secondary objectives were also met, with superiority of serogroup C GMTs of MenACYW-TT versus MCV4-TT measured by rSBA and versus MenC-TT measured by hSBA.
MenACYW-TT has been evaluated in toddlers, adolescents and adults (including those ≥56 years) in phase II and III studies.Citation23 These trials have demonstrated that MenACYW-TT elicits a non-inferior immune response to existing licensed meningococcal quadrivalent vaccines against serogroups A, C, W and Y.Citation13–21This current study, conducted in toddlers, focused on serogroup C responses as MenACYW-TT had shown numerically higher titer of bactericidal antibodies against serogroup C in previous studies in toddlers.Citation19,Citation20 However, until now, the superiority of this immune response was not previously tested. Nor had the serogroup C responses of MenACYW-TT been compared to those of MenC-TT, a vaccine widely used as part of serogroup C vaccine programs. To our best knowledge, this phase III study is also the first study which compared serogroup C responses of a quadrivalent ACWY tetanus toxoid conjugate vaccine to MenC-TT in meningococcal vaccine-naïve toddlers. Before this study, the only studies in naïve toddlers of Men C responses were between MCV4-TT and a CRM197 conjugated monovalent serogroup C vaccine.Citation24,Citation25 As the MenC-TT vaccine is known to have increased immunogenicity compared to CRM conjugated vaccines, MenC-TT was proposed as a control for this study.
In Europe, meningococcal C conjugate vaccines were introduced in national immunization programs in several countries in the early 2000s. But the changing epidemiology, with an increase of serogroups W and/or Y, and in particular, the emergence of a hypervirulent meningococcal W:cc11, has led many countries to shift from existing monovalent meningococcal C to quadrivalent ACWY conjugate vaccines in their national immunization, or to start a new program with an ACWY conjugate vaccine. For example, national immunization programs in the UK and the Netherlands introduced MenC vaccines in response to the 1999–2001 outbreak periods caused by serogroup C.Citation6 The introduction of MenC vaccination successfully reduced, through direct and indirect protection, the incidence of IMD caused by serogroup C in both countries. In the UK by 2015/16, an overall reduction in serogroup C cases of 95.6% was achieved,Citation7,Citation8 and a decade after routine use of the MenC conjugate vaccine in the Netherlands, serogroup C disease reduced by 99% in vaccinated age groups and by 93% in unvaccinated age groups.Citation9 Due to increases in IMD caused by serogroup W,Citation11 the UK then replaced the MenC vaccine in adolescents with a MCV4, and in the Netherlands MenC vaccination was switched to a MCV4 for toddlers 14 months of age and adolescents aged 13–14 years.Citation12 Evidence from the UK showed that this change let to a reduction in serogroup W cases.Citation26 Given the success of MenC vaccine programs, it is important that MCV4 vaccines are not inferior to the established monovalent vaccine while also providing protection against other serogroups.
It is equally important to understand the persistence of bactericidal antibodies following priming with a MCV4 vaccine. The immunogenicity of MenACYW-TT 3 years after the vaccination of toddlers (12–23 months) has been shown in a study of a booster dose of MenACYW-TT, conducted in children aged 4–5 years who were primed 3 years earlier with a single dose of MenACYW-TT or MCV4-TT.Citation27 That study showed that meningococcal hSBA and rSBA GMTs decreased over the 3 years following the primary vaccination (either MenACYW-TT or MCV4-TT), but remained higher than pre-vaccination GMTs for all four serogroups, indicating the persistence of the antibody response, with similar or higher titers elicited by MenACYW-TT versus MCV4-TT. Considering the serogroup C results, hSBA titers [95% CI] were higher in the MenACYW-TT-primed vs MCV4-TT-primed group 3 years after the priming dose (106 [73.2, 153] vs 11.7 [7.03, 19.4], respectively), and all participants had hSBA titers ≥1:8 for serogroup C in the MenACYW-TT-primed group compared with 54.5% in the MCV4-TT-primed group. Similar results were observed for GMTs of rSBA against meningococcal serogroup C, with higher levels (non-overlapping 95% CIs) in the MenACYW-TT-primed group than the MCV4-TT-primed group rSBA.Citation27
One of the strengths of this study was the consistency of the antibody titer data between this study and through the rest of clinical development program as a result of the same laboratories being used for the assessment of hSBA (GCI Sanofi Pasteur) and rSBA (Public Health England) across all the MenACYW-TT studies. We also saw a consistency in the results from both the hSBA and rSBA antibody assays in this study. In the phase II and pivotal Phase III studies of MenACYW-TT conducted in toddlers (12–23 months of age) hSBA assays were used for the primary endpoint;Citation19, Citation20 the same assay was used in this study for the comparison to MCV4-TT in order to compare the findings to previous trials. MenC-TT has been studied using the rSBA testing method; therefore, in order to evaluate the non-inferiority of MenACYW-TT compared to MenC-TT, titers were measured by rSBA. The similar trend in responses of seroprotection and GMT levels (hSBA and rSBA) between this study and two previous studies conducted for MenACYW-TT in toddlers indicates the consistency of these assays and results.Citation19,Citation20
The safety profile of reactogenicity and systemic reactions of a single dose of MenACYW-TT was comparable to MCV4-TT and MenC-TT, with reported solicited reactions ranging from 80.4% of the MenACYW-TT group to 74.9% of participants in the MenC-TT group. No immediate unsolicited adverse events were reported in the study, nor vaccine-related serious adverse events or deaths. The similarity of the safety profiles of MenACYW-TT and MCV4-TT is consistent with the safety findings from previous Phase III trials comparing MenACYW-TT to other licensed quadrivalent meningococcal vaccines, which further underlines the safety of this vaccine.Citation14,Citation16–18,Citation20,Citation21,Citation27
A potential limitation of this study is that due to the COVID-19 pandemic study entry was paused, and a number of participants (fewer than 2% in each study group) had protocol deviations due to incomplete follow-up. This loss was mitigated through a protocol amendment, which increased the planned number for enrollment.
In conclusion, this study showed that MenACYW-TT elicited a robust immune response against serogroup C without safety concerns. The safety profile is comparable for all three vaccines. The superior immune response to serogroup C compared with the licensed MCV4-TT vaccine (hSBA seroprotection and GMTs) and licensed MenC-TT vaccine (rSBA GMTs) when administered as a single dose to meningococcal vaccine-naïve toddlers aged 12–23 months supports the potential switch from monovalent MenC to quadrivalent MenACWY vaccination, providing reassurance that MenC protection is maintained, while providing additional benefits of protection against serogroups A, W and Y. The use of quadrivalent vaccines offers the advantage of broadening protection against other serogroups and contributes to addressing the public health need with regard to a changing and unpredictable epidemiology.
Supplemental Material
Download Zip (114.8 KB)Acknowledgements
The authors wish to thank the participants and their parents, the investigators, coordinators and their site staff, the team at Public Health England and GCI laboratories, and the Sanofi Pasteur study team responsible for the design and coordination of the study, particularly to Bertrand Lassalvy and Mae Ann Verdan. Editorial assistance with the preparation of the manuscript was provided by Chandara Olgunsoy MSc and Nicola Truss PhD, inScience Communications, Springer Healthcare Ltd, London, UK. Funding for this assistance was provided by Sanofi Pasteur. The authors would like to thank Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
Disclosure statement
MK serves as NCI and PI in clinical studies and Consultant for GSK, Pfizer, Baxter, Novartis, Astra Zeneca, MedImmune, Sanofi Pasteur, MSD, Jansen, Takeda, BioNTech and others. He perceived activities as official duties and did not personally receive any fees from companies. There is also no target agreement with his employer in this regard.
MR serves as NCI and/or PI SI in clinical studies for GSK, Pfizer, AstraZeneca, MedImmune, Sanofi Pasteur, MSD, Jansen and others. He has not personally received any fees from companies. The institution was reimbursed for study costs.
NBS serves as PI and SI in clinical studies with Pfizer, Gilead and Sanofi Pasteur. She has not personally received any fees from companies. The institution was reimbursed for study costs.
IBG, YT, SB, HA and PO are employees of Sanofi Pasteur and may hold shares and/or stock options in the company.
Data availability statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2052657
Additional information
Funding
References
- European Centre for Disease Prevention and Control. Disease data from ECDC surveillance Atlas for meningococcal disease. European Centre for Disease Prevention and Control. 2018. https://www.ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas
- Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–8. doi:10.1016/j.jinf.2020.05.079.
- European Centre for Disease Prevention and Control. Factsheet about meningococcal disease. 2019. https://www.ecdc.europa.eu/en/meningococcal-disease/factsheet
- Aparecido Nunes A, De Jesus Lopes De Abreu A, Cintra O, Actc M, Barbosa Coelho E, Nogueira Castro De Barros E. Meningococcal disease epidemiology in Brazil (2005-2018) and impact of MenC vaccination. Vaccine. 2021;39:605–16. doi:10.1016/j.vaccine.2020.11.067.
- Australian Government-Department of Health. Invasive meningococcal disease National Surveillance Report-1 January to 30 June 2019. Invasive Meningococcal Disease National Surveillance Report: Australian Government-Department of Health; 2019. https://www1.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/IMD-surveil-report-Jan-June-2019.pdf
- Knol MJ, Hahne SJM, Lucidarme J, Campbell H, de Melker HE, Gray SJ, Borrow R, Ladhani SN, Ramsay ME, van der Ende A, et al. Temporal associations between national outbreaks of meningococcal serogroup W and C disease in the Netherlands and England: an observational cohort study. Lancet Public Health. 2017;2:e473–e82. doi:10.1016/S2468-2667(17)30157-3.
- Poellabauer EM, Pavlova BG, Fritsch S, Singer J, Neubauer C, Doralt J, Valenta-Singer B, Ehrlich HJ. Single priming dose of meningococcal group C conjugate vaccine (NeisVac-C®) in infants. Vaccine. 2013;31:3611–16. doi:10.1016/j.vaccine.2013.04.070.
- Findlow H, Campbell H, Lucidarme J, Andrews N, Linley E, Ladhani S, Borrow R. Serogroup C Neisseria meningitidis disease epidemiology, seroprevalence, vaccine effectiveness and waning immunity, England, 1998/99 to 2015/16. Euro Surveill. 2019;24. doi:10.2807/1560-7917.ES.2019.24.1.1700818.
- Bijlsma MW, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin Infect Dis. 2014;59:1216–21. doi:10.1093/cid/ciu601.
- Knol MJ, Ruijs WL, Antonise-Kamp L, de Melker HE, van der Ende A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Eurosurveillance. 2018;23:18–00158. doi:10.2807/1560-7917.ES.2018.23.16.18-00158.
- Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15:470–80. doi:10.1080/21645515.2018.1532248.
- Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8:307–33. doi:10.1007/s40121-019-0254-1.
- Kirstein J, Pina M, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adults 56 years of age and older: a Phase II randomized study. Hum Vaccin Immunother. 2020;16:1299–305. doi:10.1080/21645515.2020.1733868.
- Esteves-Jaramillo A, Koehler T, Jeanfreau R, Neveu D, Jordanov E, Singh Dhingra M. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in >/=56-year-olds: a Phase III randomized study. Vaccine. 2020;38:4405–11. doi:10.1016/j.vaccine.2020.04.067.
- Chang LJ, Hedrick J, Christensen S, Pan J, Jordanov E, Dhingra MS. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine. 2020;38:3560–69. doi:10.1016/j.vaccine.2020.03.017.
- Anez G, Hedrick J, Simon MW, Christensen S, Jeanfreau R, Yau E, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Hum Vaccin Immunother. 2020;16:1292–98. doi:10.1080/21645515.2020.1733867.
- Baccarini CI, Simon MW, Brandon D, Christensen S, Jordanov E, Dhingra MS. Safety and Immunogenicity of a quadrivalent meningococcal conjugate vaccine in healthy meningococcal-naive children 2-9 years of age: a Phase III, randomized study. Pediatr Infect Dis J. 2020;39:955–60. doi:10.1097/INF.0000000000002832.
- Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Vaccine. 2020;38:5194–201. doi:10.1016/j.vaccine.2020.06.013.
- Vesikari T, Borrow R, Forsten A, Findlow H, Dhingra MS, Jordanov E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a Phase II randomized study. Hum Vaccin Immunother. 2020;16:1306–12. doi:10.1080/21645515.2020.1733869.
- van der Vliet D, Vesikari T, Sandner B, Martinon-Torres F, Muzsay G, Forsten A, van der Vliet D, Adelt T, Diaz Gonzalez C, Simko R, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine-primed toddlers: a phase III randomised study. Epidemiol Infect. 2021;149:e50. doi:10.1017/S0950268821000261.
- Dhingra MS, Namazova-Baranova L, Arredondo-Garcia JL, Kim KH, Limkittikul K, Jantarabenjakul W, Perminova O, Kobashi IAR, Bae C-W, Ojeda J, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine administered concomitantly with other paediatric vaccines in toddlers: a phase III randomised study. Epidemiol Infect. 2021;149:e90. doi:10.1017/S0950268821000698.
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al.; The Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–67. doi:10.1128/cdli.4.2.156-167.1997.
- Martinón-Torres F, Bertrand-Gerentes I, Oster P. A novel vaccine to prevent meningococcal disease beyond the first year of life: an early review of MenACYW-TT. Expert Rev Vaccines. 2021;20:1123–46. doi:10.1080/14760584.2021.1964962.
- Serra L, Knuf M, Martinon-Torres F, Yi K, Findlow J. Review of clinical studies comparing meningococcal serogroup C immune responses induced by MenACWY-TT and monovalent serogroup C vaccines. Hum Vaccin Immunother. 2021;17:2205–15. doi:10.1080/21645515.2020.1855952.
- Findlow J, Knuf M. Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence. Future Microbiol. 2019;14:563–80. doi:10.2217/fmb-2018-0343.
- Campbell H, Edelstein M, Andrews N, Borrow R, Ramsay M, Ladhani S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015-2016. Emerg Infect Dis. 2017;23:1184–87. doi:10.3201/eid2307.170236.
- Piazza FM, Virta M, Paassilta M, Ukkonen B, Ahonen A, Esteves-Jaramillo A, Forsten A, Seppa I, Ding J, Neveu D, et al. Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: a Phase III, open-label, multi-center study. Hum Vaccin Immunother. 2021;1–10. doi:10.1080/21645515.2021.1902701
