ABSTRACT
Hepatitis B Virus (HBV) infection is a major health issue among Asian Americans. The prevalence of chronic Hepatitis B infection in New York City is estimated to be 2.7% compared with .3% in the overall United States. The efficacy and long-term immunity of HBV vaccination in the Korean American pediatric population in Queens, NY, are not well explored. This study aimed to 1) determine the age-specific prevalence of anti-HBs seropositivity in the Korean American pediatric population and 2) assess biologic/demographic factors influencing immunologic response to HBV vaccine. We performed a retrospective chart review of patients registered to a pediatric health clinic located in Queens, NY, from October 2014 to October 2020. Out of 604 medical records of patients aged ≤18 years who received a completed series of HBV vaccines during infancy, we analyzed 91 medical records where HBV serology test (HBsAg and anti-HBs) results were available. Three out of 91 subjects were born to HBsAg-positive mothers. Eight out of 91 subjects were born in South Korea. Overall, 54.9% of subjects were anti-HBs-seropositive. The seropositive rate in the 15 to 18-years-old-age group (14.3%) was significantly lower than that in other age groups: < 1 year (100%) (p = .015), 1–4 years (52.6%) (p = .033), 5–9 years (63.3%) (p = .0034), and 10–14 years (64%) (p = .0063). The mean duration since vaccination in seropositive subjects was 96.5 ± 53.9 months, and that in seronegative subjects was 121.7 ± 64.2 months (p < .047). Gender, BMI, and foreign birth were not significant risk factors affecting the nonseroprotective status. The role of routine screening of anti-HB titers and booster vaccination in this endemic community needs to be further explored.
Introduction
Hepatitis B Virus (HBV) is among the most common causes of acute and chronic liver disease that can lead to liver cirrhosis and hepatocellular carcinoma.Citation1,Citation2 Globally, approximately 257 million people have chronic HB infection, which resulted in 887,000 deaths in 2017.Citation3,Citation4 Global hepatitis B surface antigen (HBsAg) seroprevalence was estimated to be 3.6%, with the highest endemicity in African and Western Pacific regions (8.8% and 5.3%, respectively).Citation5 The prevalence of chronic HB infection in 2016, including undiagnosed infection, in New York City (NYC) was 2.7%Citation6 compared with .3%Citation7 of the overall population in the United States (US). There are considerable racial/ethnic disparities.Citation8 In 2018, Tang et al.Citation9 have shown that of 25,565 adults, 13.4% were currently infected, 52.1% were ever infected, 33.4% were immune from vaccination, and 14.5% were susceptible among Asian Americans in NYC.
Queens, one of the NYC boroughs, known as “Chinese Manhattan,” is a predominantly Asian community. The Korean population is the third most predominant Asian ethnic group in Queens, NY.Citation10 Approximately 70% of persons with chronic HB infection in US were non-US born and acquired the infection in the country from which they emigrated. Distribution of these persons is not even across US. The prevalence estimate in US is likely lower than in a city such as NYC, where about 40% of all residents in 2016 were non-US born.Citation11 A majority of Korean Americans were born in South KoreaCitation10,Citation12where the estimated prevalence of chronic HB infection was approximately 3% in 2016.Citation13
There is no specific treatment for acute hepatitis B. Chronic hepatitis B infection can be treated with medicines, including oral antiviral agents. Treatment has been shown to prevent or delay the progression to cirrhosis, reduce the incidence of hepatocellular carcinoma, and improve survival through long-term viral suppression, but is not curative.Citation14
Vaccination is an easy and cost-effective measure to prevent disease and infection. The remarkable effectiveness of the universal infantile HB vaccination program (UIHBVP) is well documented.Citation15–17 A 3-dose course induces protective antibody concentrations in >95% of healthy infants, children, and adolescents and in >90% of healthy adults.Citation14,Citation18,Citation19 However, Leuridan and Van Damme reported that there is a decrease in the accepted threshold of protection (10 mIU/mL) 10–15 years after a person receives their primary vaccination as infant.Citation20 Tzu-Wei Wu et al.Citation21 have also shown that only about half of adolescent vaccinees were anti-HBs-positive, indicating immunity, and they concluded that a significant proportion of those who received their primary vaccination might have lost their immunological memories against HBsAg.
Seroprevalence of anti-HBs after HB vaccination during infancy in the Korean American pediatric population in Queens, NY, is not well explored. Anti-HBs seropositivity indicates that people either had the disease or were vaccinated and are therefore immune to the disease. Anti-HBs is the easily measurable correlate of vaccine-induced protection using serologic assays. We therefore hypothesized that the efficacy and long-term immunity of HB vaccination can be determined by age-specific seropositivity rate in this intermediate endemic community.
This study aimed to 1) determine the age-specific prevalence of anti-HBs seropositivity through HB vaccination among the Korean American pediatric population living in Queens, NY, by screening for HBsAg and anti-HBs and 2) assess biologic and demographic factors influencing immunologic response to HB vaccine by comparing subjects with anti-HBs and without anti-HBs.
Methods
Study populations and sample design
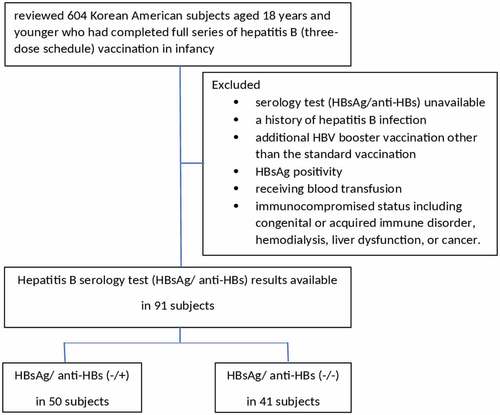
We performed a retrospective chart review of subjects registered in the pediatric health clinic located in Queens, NY, from October 2014 to October 2020. This study was approved by Brookdale University Hospital Medical Center Research and Clinical Projects Committee (RCPC/IRB). The patients’ data were collected via an electronic medical record system operated by MDLand (Electronic Medical Record [EMR] system used to organize and store patient data). We reviewed 604 medical records of Korean Americans aged 18 years and younger who had completed a full series of HB vaccine (three consecutive inoculations) during infancy. HB serology tests in pediatric health care clinic were randomly performed for any reason during the period of study. Among them, HB serology test (HBsAg and anti-HBs) results were available in 91 patients. We grouped them into two, HBsAg/anti-HBs (-/+) and HBsAg/anti-HBs (-/-), and analyzed biologic and demographic data between the groups (). All patients were subjected to review their history including age at the time of serology test, duration since vaccination, birth weight, sex, weight, height, and body mass index (BMI) at the time of the serology test, type of delivery, and socioeconomic status such as health insurance, siblings, and birthplace. Exclusion criteria in the study were as follows: 1) history of HB infection, additional HB booster vaccination other than the standard vaccination; 2) HBsAg positivity, receiving blood transfusion; or 3) immunocompromised status including congenital or acquired immune disorder, hemodialysis, liver dysfunction, or cancer.
Laboratory test
The Hepatitis B serology test was performed in LabCorp (laboratory service that analyzes patient data from bloodwork, urine samples, etc.). Blood sampling occurred at least 1 month after the last dose of serial HB vaccination. Results were expressed qualitatively as positive (or reactive) and negative (or nonreactive) for HBsAg and anti-HBs. Although quantitative results were not analyzed, the laboratory threshold used for a positive anti-HBs test was greater than or equal to 10 mIU/mL.Citation22
Statistical analyses
Categorical data were analyzed using Fisher’s exact test or chi-square test, where applicable. For numerical data, Student's t-test was used to determine differences between the two groups following Levene’s test for equality of variances to determine normality. Non-normal data were analyzed using the Mann-Whitney U test. Significance was set at p < .05, and categorical data are reported as percentages, while continuous data are reported as mean±SD. All analyses were two-tailed and performed using SPSS Software, version 26.0 (SPSS, Inc., Chicago, IL, USA). Graphs were created using Prizm version 7.0 (GraphPad, San Diego CA, USA).
Results
A total of 91 subjects were eligible for our study. All subjects were aged between 0 and 18 years: 33 females and 58 males. Three subjects were born to HBsAg-positive mothers and received hepatitis B immunoglobulin (HBIG) and HB vaccine at the day of birth. The prevalence of chronic HB among Korean American women of childbearing age was estimated to be 49% (3 out of 604) during the period of our study. The overall anti-HB seropositive rate in the subjects was 54.9% (50 of 91), and the anti-HB seronegative rate in the subjects was 45.1% (41 of 91).
The seropositive rates in male and female were 50% (29 of 58) and 63.6% (21 of 33), respectively (); this was not a statistically significant difference (p = .27).
Table 1. Gender-specific seropositive rate after full series of HB vaccination during infancy
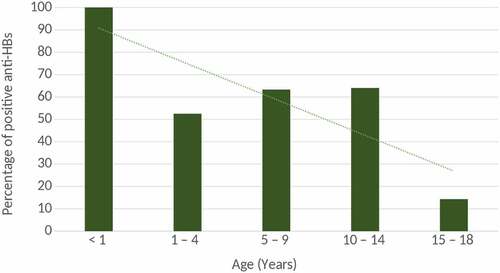
The seropositivity rates were 100% (3 of 3), 52.6% (10 of 19), 63.3% (19 of 30), 64% (16 of 25), and 14.3% (2 of 14) in the <1, 1- to 4-, 5- to 9-, 10- to 14, and 15- to 18-years-old-age groups, respectively (). In all 5 age groups, the seropositive rate in 15- to 18-year-old-age group was significantly lower than other age groups: < 1 year (p = .015), 1 ~ 4 (p = .033), 5 ~ 9 (p = .0034), and 10 ~ 14 (p = .0063). The trend in prevalence of positive anti-HBs between the different age groups is shown in .
Table 2. Age-specific seropositive rate after full series of HB vaccination during infancy
The mean duration since vaccination in 48 seropositive subjects was 96.5 ± 53.9 months, and that in 40 seronegative subjects was 121.7 ± 64.2 months; this was a statistically significant difference (p = .047) (). The mean BMI at the time of serology test in the seropositive subjects was 19.41 ± 3.7 and that in the seronegative subjects was 19.2 ± 4.0 months (p = .82). Eight out of 91 subjects (8.8%) were born and received primary HB vaccines in South Korea. The seropositive rate between US-born and non-US born groups was not significantly different (p > .05).
Table 3. Biologic and Demographic Factors affecting seropositive rate
Discussion
Hepatitis B virus (HBV) is transmitted through percutaneous (i.e., puncture through the skin) or mucosal (i.e., direct contact with mucous membranes) exposure to infectious blood or body fluids. In highly endemic countries, transmission is mainly perinatal, from a carrier mother to her newborn.Citation23 Hur et al.Citation24 illustrated that the HBsAg positivity was 3.5% (n = 5050) and the anti-HB positivity was 75.3% (n = 109,907) among 145,993 women of childbearing age (20–49) during 2001–2018 in South Korea. The national prevalence of new, chronic HB diagnoses among women of childbearing age in US decreased significantly from .83% in 2011 to .19% in 2017.Citation25 In the present study, we observed that 3 out of 91 subjects were born to HBsAg-positive mothers and received hepatitis B immunoglobulin (HBIG) and HB vaccine at the day of birth. They were all born in US. The prevalence of chronic HB among Korean American women of childbearing age in Queens, NY, was estimated to be .49% (3 out of 604) during the period of our study. We assumed that our estimate of .49% was in between .19%Citation25and 3.5%Citation24 because some proportion of childbearing aged Korean American women were born in US and some immigrated from South Korea. This assumption needs to be elucidated in future study.
The overall seroprotective rate was 54.9% in Korean Americans aged 0–18 years, and 45.1% were susceptible (). The percentage of protective antibodies was between 14.3% and 100% in all age groups, with the highest in the <1-year-old group (100%) and the lowest in the 15- to 18-years-old age group (14.3%) (). Although 45.1% of individuals in this study have lost protective levels of antibodies, breakthrough infection, which may be identified by detection of HBsAg and resulting clinical disease, has not been observed. All 3 individuals who were born to HBsAg-positive mothers and received HBIG and 3 doses of HB vaccine during infancy have the protective level of anti-HBs.
Of note, the duration since the completion of serial HB vaccination during infancy, in other words, the age was found to be a significant factor affecting anti-HB seropositivity. Our study indicated that the seropositive rate in 15- to 18-years-old-age group (14.3%) was significantly lower than other age groups: <1 year (p = .015), 1–4 (p = .033), 5 –9 (p = .0034), and 10–14 (p = .0063) (). The mean duration since vaccination in seronegative subjects was significantly longer than that in seropositive subjects (121.7 ± 64.2 months vs 96.5 ± 53.9 months, p < .047) ().
Nonetheless, the decrease in seropositivity is not indicative of a person’s immune response. Protection against disease (i.e. acute hepatitis, prolonged viremia, carriership, and chronic infection) is associated with immune memory.
Currently, the World Health Organization (WHO)Citation26and the European Consensus Group on Hepatitis B ImmunityCitation27state that booster vaccination of healthy individuals after primary vaccination is not recommended. However, this is still being challenged by raising concern about waning immune memory, HBV vaccine breakthrough infection, and nonresponder to the primary course of vaccination. Several studies demonstrated findings, which may indicate waning immune memory among children and adolescents vaccinated with HB vaccines during infancy.Citation28–30 Breakthrough infections occur in 0%–17.7% of the general populationCitation31and in up to 33.3% in children of carrier mothersCitation29after 15 years of follow-up. The main causes of HBV vaccine breakthrough infection include high maternal viral load, intrauterine infection, emergence of S gene mutants, and immunosuppression.Citation32 WHO also stated that additional longer-term studies should be conducted to explore life-long protection conferred by hepatitis B vaccine and the need for booster doses in different subgroups of the population.Citation33
Although a majority of persons vaccinated against hepatitis B successfully respond to vaccination, an estimated 5–15% of persons may not respond due to sex, older age, obesity, smoking, and other chronic illness.Citation34,Citation35 In previous studies, females had a stronger immunogenic response to HB vaccine with higher anti‐HB seropositivity and a reduced chance for HB infection. Ruggieri found sex disparity in infection rates of HBV,Citation36and Tsay also reported that HBsAg was more prevalent in men than in women.Citation37 Females usually develop a stronger innate immune response as opposed to males: estrogens have an immune-stimulating effect, while androgens are immune-suppressing.Citation36 In concordance to those studies, our data showed that females were more likely to be seroprotective (63.6% of female vs 50% of male); however, the role of gender between two groups did not reach statistical significance (p = .27) ().
Another factor that we explored was body mass index (BMI). BMI was important as it identifies whether a patient is at risk of developing weight-related complications, such as poor vaccine-induced immune responses.Citation38,Citation39 Excess lipids can interfere with the patient’s immune response and efficacy of vaccination.Citation40 Although previous studies found that obesity was significantly associated with nonresponse to HB vaccine and that the risk of nonresponsiveness of HB vaccine among obese people with increased BMI, our study did not support those findings (). However, our data should be interpreted with limitation because BMIs were collected at the time of the serology test, not at the time of vaccination.
In addition, we observed that 8 out of 91 subjects were born in South Korea. Our data indicated that birthplace was not a significant risk factor increasing the prevalence of nonseroprotective status. The universal vaccination program with 3 doses of hepatitis B vaccine was globally implemented including in South Korea. The current global coverage is estimated at 85%.Citation14 Therefore, our finding supports the effectiveness of the universal infantile HB vaccination program (UIHBVP).
Limitations for this study were small sample size from a single-center study, limited data about the patient’s history, and data collection method for BMI. Korean American pediatric population in Queens was estimated to be 7,705 approximately.Citation10 The registered 604 Korean American patients in pediatric health clinic represent 7.8% of the overall Korean American pediatric population in Queens, and their residential area spans extensively across Queens region. Our research subjects were randomly selected. It is thought that our data may represent that of the entire Queens region. However, a multicenter study is warranted in the future.
Despite such limitations, our study is valuable and unique in its ethnical as well as geographical focus on the Korean American pediatric population in Queens NY. HBV infection in US is the most common among Asians followed by non-Hispanic blacks. Greater NYC is the second largest region in US where Korean Americans reside. The majority (60%) of them live in Queens. However, studies about chronic HB infection and seroprevalence in Korean Americans were rarely published. Moreover, no studies were aimed at the pediatric population. To our knowledge, this is the first study that revealed the significant lack of seroprotection among Korean American adolescents who reside in the intermediate endemic area of chronic HB infection in US.
In conclusion, we observed a significant decrease in the prevalence of anti-HBs seroprotection in the 15- to 18-years-age group after primary HB vaccination during infancy. It is estimated that the prevalence of chronic HBV infection among Korean Americans is at least 8 times higher than that of the general population in US. Moreover, in Queens, NY, a majority of Korean Americans are composed of immigrants from an endemic country. The US Preventive Services Task Force (USPSTF) recommends with moderate certainty screening for HBV infection in adolescents and adults at risk groups for HBV infection with a prevalence of ≥2%.Citation41 Given that the duration of immune memory has not been fully explored and the question about the necessity of booster doses has not been clearly answered, we suggest routine screening of anti-HBs titers on/after 15 years of age and a booster dose accordingly in this ethnic community. Future studies should determine the potential role of routine screening in anti-HB titers and the effect of booster vaccination in a large-sized sample from a multicenter study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49(5 Suppl):S13–6. doi:10.1002/hep.22881.
- McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–38. doi:10.1016/j.cld.2015.01.001.
- WHO. Global hepatitis report, 2017. Geneva; 2017.
- CDC. Preventing Hepatitis B. 2017 [accessed 2018 Mar 30]. https://www.cdc.gov/globalhealth/immunization/othervpds/preventing_hepatitisb.html .
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. doi:10.1016/s0140-6736(15)61412-x.
- Moore MS, Bocour A, Laraque F, Winters A. A surveillance-based hepatitis C care cascade, New York City, 2017. Public Health Rep. 2018;133(4):497–501. doi:10.1177/0033354918776641.
- Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388–97. doi:10.1002/hep.28109.
- Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, Ruhl C, Unalp-Arida A. Racial/ethnic disparities in the prevalence and awareness of hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;24(11):1052–66. doi:10.1111/jvh.12735.
- Tang AS, Lyu J, Wang S, He Q, Pong P, Harris AM. Disparities in hepatitis B virus infection and immunity among New York City Asian American patients, 1997 to 2017. Am J Public Health. 2018;108(S4):S327–s335. doi:10.2105/ajph.2018.304504.
- Asian Ddfabt CA. Profile of New York City’s Korean Americans. New York, NY: Asian Ddfabt, Center AFCI; Asian American Federation Census Information Center (CIC); 2013. p. 4.
- Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(Rr–8):1–20.
- Hyun CS, Kim S, Kang SY, Jung S, Lee S. Chronic hepatitis B in Korean Americans: decreased prevalence and poor linkage to care. BMC Infect Dis. Aug 15 2016;16(1):415. doi:10.1186/s12879-016-1732-7.
- KCDC. Korea National Health and Nutrition Examination Survey (KNHANES). Korea Centers for Disease Control & Prevention; http://knhanes.cdc.go.kr2016 .
- WHO. Immunization coverage. Fact Sheet. [accessed 2020 Jul]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b2020 .
- Whittle HC, Inskip H, Hall AJ, Mendy M, Downes R, Hoare S. Vaccination against hepatitis B and protection against chronic viral carriage in the Gambia. Lancet. Mar 30 1991;337(8744):747–50. doi:10.1016/0140-6736(91)91367-4.
- Chen HL, Chang MH, Ni YH, et al. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. Jama. 1996;276(11):906–08. doi:10.1001/jama.1996.03540110060032.
- Wang LY, Hu CT, Ho TY, Lin HH. Geographic and ethnic variations of long-term efficacy and immunogenicity of hepatitis B vaccination in Hualien, a HBV hyperendemic area. Vaccine. 2006;24(20):4427–32. doi:10.1016/j.vaccine.2005.12.069.
- Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18(1–2):57–67. doi:10.1016/s0264-410x(99)00179-6.
- Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev Vaccines. 2004;3(2):119–29. doi:10.1586/14760584.3.2.119.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53(1):68–75. doi:10.1093/cid/cir270.
- Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57(1):37–45. doi:10.1002/hep.25988.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;2:489–92. doi:10.1086/314578.
- Navabakhsh B, Mehrabi N, Estakhri A, Mohamadnejad M, Poustchi H. Hepatitis B virus infection during pregnancy: transmission and prevention. Middle East J Dig Dis. 2011;3(2):92–102.
- Hur YJ, Choe SA, Choe YJ, Paek J. Hepatitis B surface antigen and antibody positivity among women of childbearing age after three decades of universal vaccination in South Korea. Int J Infect Dis. 2021;104:551–55. doi:10.1016/j.ijid.2020.11.147.
- Kushner T, Chen Z, Tressler S, Kaufman H, Feinberg J, Terrault NA. Trends in hepatitis B infection and immunity among women of childbearing age in the United States. Clin Infect Dis. Jul 27 2020;71(3):586–92. doi:10.1093/cid/ciz841.
- WHO. Hepatitis B vaccines: WHO position paper, July 2017 - recommendations. Vaccine. 2019;37(2):223–25. doi:10.1016/j.vaccine.2017.07.046.
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355(9203):561–65. doi:10.1016/S0140-6736(99)07239-6.
- Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, Bell BP. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics. 2007;120(2):e373–81. doi:10.1542/peds.2007-0131.
- Lu CY, Ni YH, Chiang BL, Chen P-J, Chang M-H, Chang L-Y, Su I-J, Kuo H-S, Huang L-M, Chen D-S, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. May 15 2008;197(10):1419–26. doi:10.1086/587695.
- Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu T-Y, Huang L-M, Chen C-J, Chen D-S. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology. 2010;51(5):1547–54. doi:10.1002/hep.23543.
- van der Sande MA, Waight P, Mendy M, Rayco‐solon P, Hutt P, Fulford T, Doherty C, McConkey S&, Jeffries D, Hall A&, et al. Long-Term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193(11):1528–35. doi:10.1086/503433.
- Chang MH. Breakthrough HBV infection in vaccinated children in Taiwan: surveillance for HBV mutants. Antivir Ther. 2010;15(3 Pt B):463–69. doi:10.3851/imp1555.
- WHO. Evidence to recommendations table and GRADE table, need for a hepatitis B vaccine booster dose following primary immunization. http://who.int/immunization/policy/position_papers/hepatitis_b/evidence_recommendation_gratable_hepb_duration.pdf2017 .
- Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. Jun 21 2016;6:27251. doi:10.1038/srep27251.
- Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the advisory committee on immunization practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15):455–58. doi:10.15585/mmwr.mm6715a5.
- Ruggieri A, Gagliardi MC, Anticoli S. Sex-Dependent outcome of hepatitis B and C viruses infections: synergy of sex hormones and immune responses? Front Immunol. 2018;9:2302. doi:10.3389/fimmu.2018.02302.
- Tsay PK, Tai DI, Chen YM, Yu C-P, Wan S-Y, Shen Y-J, Lin D-Y. Impact of gender, viral transmission and aging in the prevalence of hepatitis B surface antigen. Chang Gung Med J. 2009;32(2):155–64.
- Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. Jama. 2009;302(17):1896–902. doi:10.1001/jama.2009.1583.
- Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum Vaccines Immunother. 2017;13(5):1014–17. doi:10.1080/21645515.2016.1274475.
- White SJ, Taylor MJ, Hurt RT, Jensen MD, Poland GA. Leptin-Based adjuvants: an innovative approach to improve vaccine response. Vaccine. Mar 25 2013;31(13):1666–72. doi:10.1016/j.vaccine.2013.01.032.
- Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW, Kubik M, et al. Screening for hepatitis B virus infection in adolescents and adults: US preventive services task force recommendation statement. Jama. 2020;324(23):2415–22. doi:10.1001/jama.2020.22980.