ABSTRACT
This study aimed to evaluate the implementation and impact of rubella-containing vaccine (RCV) by describing the rubella epidemiology and seroepidemiology in Hangzhou. We collected rubella cases of Hangzhou in the Information System for Disease Control and Prevention in China between 2009 and 2020, and performed a descriptive analysis. We applied a multi-stage stratified random sampling method to recruit participants for serological tests of rubella. Enzyme Linked Immunosorbent Assay (ELISA) was used to detect Immunoglobulin G (IgG) antibodies against rubella in serum samples. Univariate and multivariate analyses are used to detect the association between the level of rubella IgG and related factors. The incidence of rubella cases per million population decreased from 15.8 in 2009 to .1 in 2020. The proportion of rubella cases in women of childbearing age was higher than in men. A total of 4,362 subjects were tested serologically for rubella. The percentage of people whose rubella IgG antibody titers were above the minimum protective level (20 IU/ml) was 80.60% (95% CI: 79.4%–81.8%) and the geometric mean concentration (GMC) for rubella IgG was 58.34 IU/ml. The data indicated that Hangzhou had made good progress toward the elimination of rubella, whereas women of childbearing age still had a higher proportion of rubella cases, which might lead to increased risk of subsequent CRS. The positive rate and GMC of rubella IgG were significantly influenced by age and immunization history of RCV. Therefore, we should stress the importance of pushing forward the campaign for supplementary vaccination of rubella in adults.
KEYWORDS:
Introduction
Rubella is a common acute respiratory infectious disease caused by the rubella virus, as well as a mild self-limiting infection. Although rubella is regarded as a benign illness with acute symptoms, such as low temperature, rash, and swollen lymph nodes, arthralgia and arthritis are frequent in adults, particularly women.Citation1 Rubella virus (RV) has a significant potential for teratogenicity.Citation2 If RV infection occurs during the first 4 months of pregnancy, it can be transmitted across the placenta, causing fetal infection and resulting in spontaneous miscarriage, stillbirth, and a spectrum of birth abnormalities known as congenital rubella syndrome (CRS).
To date, rubella is still prevalent in many areas, and CRS with long-term health consequences is still being reported.Citation3 The number of rubella cases fell sharply from 670 894 to 26,006 between 2000 and 2018, but due to the large epidemics in China and Japan, the number of cases increased to more than 30,000 in 2019, and approximately 100 000 CRS cases were estimated to occur annually in 92 low- and middle-income countries now.Citation2 In 2015, the Pan American Health Organization declared that rubella and CRS had been eliminated from the WHO Americas region,Citation4 which demonstrated that rubella vaccination might successfully prevent RV infection and further reduced rubella and CRS. As of July 2020, 84 of the 195 (43%) WHO member states had been verified achieving rubella elimination; 173 member states had initiated rubella vaccination, and the global coverage rates of both MCV2 and rubella vaccines had reached 71%.Citation3 By the end of 2020, WHO updated the target to “achieve and sustain regional measles and rubella elimination goals” by 2030 in all six WHO regions.Citation3
Rubella infection is an important public health issue in China, which influences a population of more than 1.4 billion people, due to the teratogenic effects of the rubella virus on the developing fetus. RCV was introduced nationwide into the Expanded Immunization Program (EPI) in 2008, with a schedule of 1 dose of measles and rubella combined attenuated live vaccine (MR) at 8 months and 1 dose of measles, mumps, and rubella combined attenuated live vaccine (MMR) at 18 months, in response to WHO’s goal of rubella elimination. According to the Chinese Centers for Disease Control and Prevention (CDC), after the 2008 national introduction of rubella vaccination into China's EPI system, the incidence of rubella has progressively fallen from 91.0 per million in 2008 to 1.16 per million in 2017.Citation5 China has made considerable strides in rubella management and is approaching to the goal of rubella elimination.
In 1994, the rubella attenuated live vaccine was first used in Hangzhou.Citation6 Starting in 2008, RCV was introduced into the EPI in Hangzhou. In 2011, Hangzhou implemented a routine vaccination campaign for rubella which contained a vaccine for teenagers in the third grade of junior high school. Rubella epidemiology in developing countries can derive from rubella antibody seroprevalence.Citation1 Since 2009, Hangzhou has been conducting annual serological tests for healthy people. In this study, we aimed to report the epidemiology of rubella infection by age group and identify the challenges in rubella elimination in Hangzhou during the period of 2009–2020.
Materials and methods
Setting
Hangzhou is the capital of Zhejiang province in the East of China. Based on the annual census data from Hangzhou municipal Bureau of Statistics, Hangzhou comprised of 10,360,017 inhabitants in 2020.
Case definition
A rubella surveillance case is an illness with one of the symptoms of fever, rash, cough, catarrhal rhinitis, conjunctivitis, swollen lymph nodes, arthritis or joint pain, or the rubella case suspected by an infectious disease responsible outbreak reporter. There are three types of cases: the clinically confirmed cases, the laboratory testing confirmed cases and the epidemiological linkage confirmed cases.
Clinically confirmed: those who have one of the symptoms of fever, rash with lymphadenopathy, arthritis/arthralgia, or being suspected rubella by the reporter responsible for epidemic of infectious diseases, have no specimens or unqualified specimens, no epidemiological association with laboratory confirmed rubella cases, and have not been clearly diagnosed with other diseases.
Laboratory testing confirmed: a. positive rubella IgM antibody detected in blood samples. b. positive rubella virus nucleic acid test or rubella virus isolated from pathogen specimens. c. The titer of serum rubella IgG antibody in the convalescent stage is ≥4 times higher than that in the acute stage, or the antibody in the acute stage is negative, but the antibody in the convalescent stage is positive.
Epidemiological linkage confirmed: a. There are no specimens or unqualified specimens of the monitored cases, but they are epidemiologically related to laboratory-confirmed rubella cases.
Target population and data resources
Since 2004, all detected cases (2622) in Hangzhou have been required to report in China Information System for Disease Control and Prevention (CISDCP) within 48 h, once the diagnosis of rubella has been made. Data from our study between January 2009 and December 2020 were obtained from CISDCP. Information on the notified cases included case number, date of symptom onset, and demographic characteristics, such as age, gender, birth date, household registration, and residential address. The census data were obtained from the information system of the National Center for Disease Prevention and Control.
Since 2009, we have been conducting yearly rubella serological tests to assess the seroprevalence of the Hangzhou local population. The healthy population of Hangzhou was obtained using a stratified random sampling method. All personal information was recorded, and the gender was also taken into account; males and females were equally likely to be sampled. The participant was chosen to use a multi-stage stratified random sampling process. For the cross-sectional study, a sample size of at least 3,696 people was used, with the formula: n = (Z1-a/2/d)2p(1-p), a = .05, d = .1, p-rubella = 92%,Citation7 p: the rates of rubella IgG seropositivity. Rubella IgG sero-epidemiology was examined among 11 different age groups: <1, 1, 2, 3–4, 5–6, 7–9, 10–14, 15–19, 20–29, 30–39, ≥40 y. Every year, at least 30 samples from each age group were collected.
Experimental approach and results calculation
The SERION ELISA classic rubella virus IgG kit (Serion, Germany) was used to detect the presence of specific rubella IgG antibodies. Values <10 IU/ml were considered seronegative, values 10–20 were considered equivocal, and values >20 IU/ml were considered seropositive. A level of >10 IU/ml for rubella IgG antibodies was an indicator of immunity.Citation8 However, confirmed cases of re-infection also occurred in populations with titers greater than or equal to the cutoff value of 15 IU/ml,Citation9 so we defined the levels of immunity to rubella as: rubella antibody levels of ≤20 IU/ml = “sero-negativity,” and >20IU/ml = ‘sero-positivity’.
Statistical analysis
We calculated the rates and proportions to describe the sociodemographic and confirmed the type of the rubella cases and drew diagrams with Microsoft Excel 2019. Serological analysis of rubella was carried out using SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA). The positive rates of rubella IgG were expressed in frequencies, and Pearson’s chi-square test was used to determine group differences for categorical data. Two independent variables t-test or one-way ANOVA test was used to compare the measurement data for the GMC for rubella IgG levels. Logistic regression and linear regression were used for multivariate analysis. The 95% confidence intervals (CIs) were calculated. A value of P < .05 (2-sided) was considered statistically significant.
Results
Epidemiological profile
Overall assessment
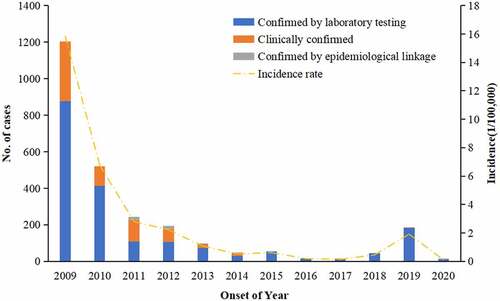
During 2009–2020, 2,622 cases of rubella were reported to the Hangzhou CDC, which included 1925 laboratory-diagnosed cases, 656 clinically diagnosed cases and 41 epidemiological linkage diagnosed cases, and the total annual incidence rate was 2.45/100,000. The annual incidence rate of reported cases decreased by 99.2% from 1202 (15.8/100,000) in 2009 to 10. (1/100,000) in 2020. Overall, the incidence of rubella in Hangzhou showed a downward trend, except in 2019 with a small epidemic peak of 187 (1.9/100,000). The proportion of laboratory confirmed rubella cases was 69.97% during 2009–2013 and increased to 97.84% during 2014–2020 ().
Rubella incidence by age
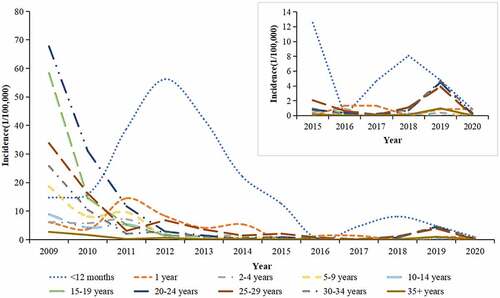
During 2009–2020, generally the incidence rate of rubella declined in all age groups except for the <12 months old group. In 2009, the most affected age group was the 20–24 years old group with an average annual incidence rate of 67.7 per 100,000 population, followed by the 15–19 years old group (58.3/100,000) and the 25–29 years old group (33.8/100,000). From 2009, incidence in all age groups decreased except for the <12 months old group with an epidemic peak in 2012. During 2011–2015, children aged <12 months old group became the age group with highest incidence of rubella (56.2/100,000 in 2012), followed by the 1-year-old group (14.5/100,000 in 2011) and the 2–9 years old group (8.7/100,000 in 2011). From 2011, the average annual incidence rate of other age groups ranged from .0 per million to 5.5 per million ().
Distribution by gender and age
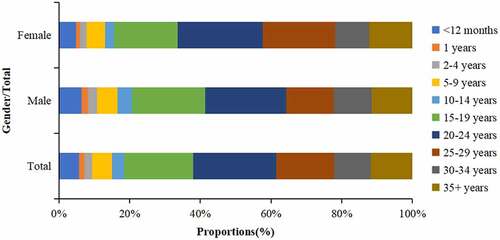
During 2009–2020, among the 2,622 rubella cases, 1470 (56.1%) were male and 5803 (43.9%) were female, with an average annual ratio of 1.3:1. Adolescents and young adults had the largest proportions. The proportion of 15–19, 20–24, 25–29 years old group and 30–34 years old group were 19.49%, 23.49%, 16.48% and 10.34%, respectively. The proportion of rubella cases of young women was higher than that of men in 20–24 years old group and 25–29 years old group ().
Vaccination status of the patients by age group
During 2015–2020, we monitored the age distribution of reported cases and the vaccination status of cases by age. The numbers of RCV 0 dose, 1 dose, 2 dose and unknown cases were 16 cases, 30 cases, 8 cases and 269 cases, accounting for 4.94%, 9.26%, 2.47% and 83.02%, respectively. For <12 months old group and 1-year-old group, the proportion of cases with histories of receiving at least 1 dose of RCV were 80.77% and 75%, and there was 19.23% of patients of <12 months old group having no RCV history. For cases in the 2–5 years old group and 5–9 years old group, the proportion of cases with histories of receiving 2 doses of RCV were 100%. For cases in the 10–19-year-old group and over 20-year-old group, the proportions of having unknown vaccination histories were 85.19% and 94.27%, respectively ().
Table 1. Rubella vaccination status by age group, 2015–2020
Seroepidemiology
Univariate analysis
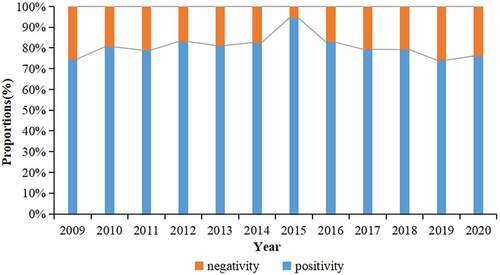
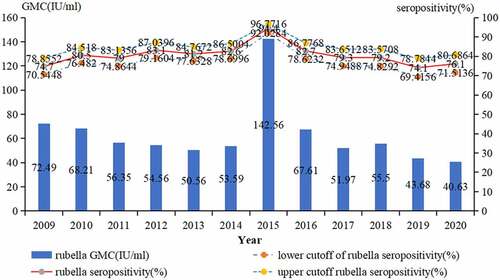
During the period from 2009 to 2020, a total of 4,362 subjects were tested serologically for rubella. 3,515 patients were positive for rubella IgG antibody, the positive rate was 80.6% (95%CI: 79.4–81.8%), and the GMC was 58.34 IU/ml. The proportion of positive rate for rubella IgG antibody remained approximately 80.60% during 2009–2020, excepted in 2015 which was 94.4% ().
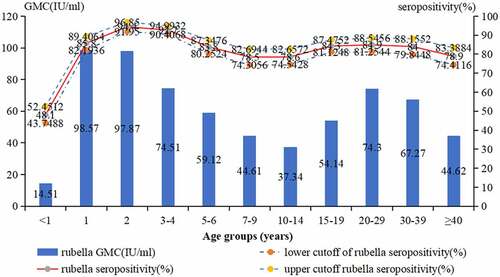
There were no significant differences in the seropositive rates by gender (male: 80.3%, female: 80.9%, 2 = .274,P = .601), but a significant difference in the seropositive rates by age group (2 = 450.978, P < .001). The highest positive rate of rubella IgG antibody was 94.4% (95%CI: 91.9–96.8%) in the 2-year-old group. The lowest positive rate of rubella IgG antibody was 48.1% (95%CI: 43.7–52.4%) in the <1-year-old group. Other age groups’ rates were between 83.8% and 84.9%, except 3–4 years old group’s rate was 92.7% (95%CI: 90.4–94.9%), and 7–14 years old group and ≥40 years old group’s rates lower than 80% (, ).
Table 2. Rubella IgG detection results in population among various groups, 2009–2020
The GMC (IU/ml) of IgG antibodies by gender (male: 55.37 IU/ml, female: 61.47 IU/ml, t = > −2.751, P = .006) and district (urban: 56.01 IU/ml, rural: 59.26 IU/ml, t = > −2.317, P = .021) had significant difference, and there was a significant difference in the GMC among the different age groups (F = 42.878, P < .001). The value of GMC increased from 14.51 IU/ml in <1-year old group to 98.57 IU/ml in 1-year-old group, then decreased to 37.34 IU/ml in 10–14 years old group, and increased again in 15–19 years old group (54.14 IU/ml), and the subsequent age groups remained between 67.27 IU/ml-74.3 IU/ml, except for the ≥40 -year-old group (44.62 IU/ml) (, ). Seropositivity and GMC for rubella IgG had significant difference by year (2 = 71.432,P < .001, F = 14.181, P < .001) ().
Multivariate analysis
There were no significant differences in the seropositive rates by gender (OR = 1.035 , CI 95%: .881–1.217, P = .675) and district (OR = 1.018 , CI 95%: .861–1.207, P = .835); taking the <1-y-old group as a reference, the seropositivity for rubella IgG in all age groups was significantly lower (all P-values <.05) ().
Table 3. Association of seroepidemiology of rubella IgG and related variables via multivariate analysis
The GMC for rubella IgG by gender (OR = 1.024, CI 95%: .995–1.06, P = .104) had no significant difference; higher seropositivity GMC for rubella IgG was found in rural compared with urban (OR = 1.033, 95% CI: 1.005–1.074; P-value < .05). Taking the <1-y-old group as a reference, the GMC for rubella IgG in subjects aged 2–4 y were significantly higher, and those in subjects aged 10–19 y were significantly lower (all P-values < .05) ().
Discussion
Since 2008, all medical agencies were required to report rubella cases via CISDCP in China. During the period of 2009–2020, our results showed that the overall estimated incidence rate of rubella had a downward trend. The age profile of reported cases in Hangzhou had shifted from a predominance of 5–9 years old group to a predominance of 20+ years old adults with no history of immunization, and the number of rubella cases in the 20–29 years old group of females was the majority. The serosurvey results indicated that the positive rate of rubella IgG antibody in healthy people in Hangzhou from 2009 to 2020 was 80.6%.
Since 2009 to 2020, 2622 rubella cases were identified, nearly 45.8% of which were in the 2009 epidemic. There had been a decline in the incidence rate of reported rubella from 15.8/100,000 in 2009 to .1/100,000 in 2020, which showed that the rubella vaccines use in Hangzhou demonstrated good effectiveness, and the incidence of rubella remained a low status between .1/100,000–.6/100,000, a similar downward trend as Guangzhou.Citation10 These results are consistent with several studies showing increased rubella incidence in most regions of China in 2019, which may be related to the cycle of rubella epidemic peak occurring every 7–8 years in China.Citation10, Citation11 The incidence of rubella is usually ranked first in the <12 months old group, similar to that of Poland, where the highest incidence rate was observed among the 0–4 years age group (11.8 per 100,000) in 2017,Citation12 and <12 months age group had an epidemic peak in 2012 in Hangzhou that was similar to the whole countryCitation5 and may be related to the failure of immunization and not timely inoculation.Citation13 Although a national cross-sectional serological survey during 2014 indicated that many adolescents remained susceptible to rubella,Citation14 we have found that the decline of rubella incidence was most evident in the 15–19 years old group in Hangzhou, which was related to the RCV supplementary immunization activities for middle school students in Zhejiang province every year since 2011.
In recent years, the epidemiological characteristics of rubella in Hangzhou have changed, and the age of rubella occurrence has moved toward being older. Although there are a large number of teenage cases, adult cases have an increasing proportion. The age profile of reported cases in China has shifted from a predominance of 5–9 years old group to a predominance of 20+ years old adults with no history of immunization. This is similar to the trend of age composition ratio of rubella incidence in Xi ‘an and Shenyang of China.Citation15, Citation16 Although the rubella vaccination campaign in Hangzhou has reduced the incidence of rubella among people under 20 years old, adults over the age of 20 who lack the corresponding protective antibodies because of no vaccination or natural infection are still susceptible to rubella. This may be one of the important reasons for the significant rise in rubella since 2019. In 2018, Japan had a second large rubella outbreak, and more than 5,000 rubella cases including five CRS cases were confirmed.Citation17 The main reason was that the male birth cohort from 1962 to 1979 was not covered in Japan’s rubella immunization strategy at the beginning of the immunization program (1977–1995).Citation18 Similar to Japan, according to a report released by the National Bureau of Statistics, Chinese women between age of 25 and 35 are at high fertility.Citation5 Both men and women are more susceptible to rubella in this age group, and women have a higher risk of CRS. To reduce the increased risk of CRS in women of childbearing age, Chiba city in Japan has expanded its rubella vaccination target to husbands of pregnant women or women who wanted to become pregnant.Citation17 Italy also offers Measles Mumps Rubella Varicella (MMRV) vaccine to childbearing and pregnant women.Citation19 Therefore, Hangzhou further extends rubella vaccination coverage to women of childbearing age and adult men.
During 2009–2020, the positive rate of Rubella IgG antibody of male in Hangzhou was 80.3%, the one of the female was 80.9%, and GMC of male was 55.37 IU/ml, and the one of the female was 61.47 IU/ml. The results of our investigation failed to meet the requirements that rubella can be effectively eliminated when the population’s immune level is above 85%.Citation20 However, the serosurvey studies indicated that except 1–4 years old group, the IgG positive rate for each age group maintained a relatively low level (<85%), which was different to the results of healthy people in Shandong Province in China, where total IgG positive rate was 91.08% from 2014 to 2015.Citation21 The results of GMC for rubella IgG in different age groups showed that rubella IgG antibody had a slow downward trend in 3–9 years old group after RCV2 compared to 15+ years old group after a third dose of RCV. This trend was similar to the finding that MMR3 provided long-lasting sero-protection against measles, mumps, and rubella.Citation22 One study showed that, in Bangladesh, when rubella seropositivity among women of childbearing age was 81.0% overall, the estimated incidence of CRS was .99 per 1,000 live births.Citation23 In India, a study showed that the seroprevalence of rubella antibodies was 82.3% (95% CI: 80.4–84.0) in 1800 women, and constructed catalytic-models (age-dependent and constant force of infection models) to estimate the incidence and burden of CRS as being 14,520 (95% CI: 9,225–23,100) and 50,028 (95% CI: 48,234–51,543) infants with CRS every year based on age-dependent and constant force of infection models, respectively.Citation24 Our study showed that the acquisition age of rubella infection had already shifted to the ≥25 years age group and the seroprevalence of rubella antibodies was lower than 85% which greatly increased the potential risk of CRS. CRS monitoring is helpful to assess the burden of CRS. However, in Hangzhou, CRS monitoring is not well developed, so the true burden of CRS in Hangzhou is still unknown. The combination of RCV coverage, serological investigation, rubella case surveillance, Rubella virological surveillance and mathematical modeling may provide support for routine CRS surveillance in the future.Citation14, Citation21, Citation25
The strength of the present study was the use of CISDCP for data collection and the Rubella serological research program supported by Hangzhou CDC which was reliable. In addition, our study spanned a long time, which well demonstrated the prevalence trend of rubella in population. Limitations in this study included undetection, because rubella was a milder disease than measles and in the vaccine era some rubella patients had mild or no specific symptoms, so they did not visit doctors; nevertheless, China’s Measles surveillance system (MSS) had good sensitivity by requiring medical facilities to report excluded cases of measles and rubella. For example, in 2015 and 2016, the reported incidence of measles and rubella cases was 3.99/100,000 and 2.90/100,000, respectively;Citation26 the detailed immunization history of all subjects was not collected before 2015 so the impact of immunization history had not been thoroughly analyzed in this study.
In conclusion, based on our findings, all available data indicated that Hangzhou had made good progress toward the elimination of rubella. The current childhood immunization strategy and the RCV supplementary immunization activities for teenagers are contributing to the goal of eliminating rubella, but the women of childbearing age still have a higher proportion of rubella cases, which may lead to increased risk of subsequent CRS. At the same time, the positive rate and the GMC of IgG antibodies were significantly influenced by age and the immunization history of RCV. Therefore, we recommend to strengthen the routine children vaccination schedules and the research on the immune strategy of adult rubella and establish CRS surveillance system to further accelerate the control of rubella and CRS to achieve the rubella elimination goal.
Ethical considerations
Epidemiological study of rubella cases was decided to be exempt from ethical review by the Hangzhou CDC institutional review board. As the rubella cases were collected for the purpose of routine public health surveillance activities, informed consent was waived by the Committee. The data was anonymized before analysis and was not linked to specific identifiers. Rubella serological research program was approved by the ethics committee of the Hangzhou CDC. All participants or legal guardians were given a brief oral description of the objectives of the present study, and we obtained verbal consents from the participants.
Acknowledgements
We wish to thank the staff at the county-level Center for Disease Control and Prevention and at vaccination clinics and hospitals in Hangzhou, for their disease surveillance, control, vaccination service, and seroprevalence surveillance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Plotkin SA, Orenstein WA, Offit PA , et al. Vaccines. 7th ed. Holland: Elsevier; 2017. p. 970–8. ISBN: 9780323357616.
- Plotkin SA. Rubella eradication: not yet accomplished, but entirely feasible. J Infect Dis. 2021;224(12 Suppl 2):S360–S366. doi:10.1093/infdis/jiaa530.
- World Health Organization. Measles and rubella strategic framework 2021–2030. Geneva: World Health Organization; 2020. https://measlesrubellainitiative.org/measles-rubella-strategic-framework-2021-2030/ .
- Kirby T. Rubella is eliminated from the Americas. Lancet Infect Dis. 2015;15(7):768–69. doi:10.1016/S1473-3099(15)00102-4.
- Su Q, Ma C, Wen N, Fan C, Yang H, Wang H, Yin Z, Feng Z, Hao L, Yang W. Epidemiological profile and progress toward rubella elimination in China. 10 years after nationwide introduction of rubella vaccine. Vaccine. 2018;36(16):2079–85. doi:10.1016/j.vaccine.2018.03.013.
- He H, Xie S, Li Q, et al. Serologic and epidemiological effect of rubella-containing vaccine immunization activities among third-year students of middle school in some areas of Zhejiang Province. Chin J Vaccines Immunization. 2016;22(1):20–23.
- Wang Y, Xu W, Wang H, et al. Rubella antibody levels among children before and after implementing the Expanded National Immunization Program in five counties of China. Chin J Vaccines Immunization. 2017;23(2):162–6+161.
- Charlton CL, Lai FY, Dover DC. How to determine protective immunity in the post-vaccine era. Hum Vaccin Immunother. 2016;12(4):903–06. doi:10.1080/21645515.2015.1128600.
- Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol. 1996;106(2):170–74. doi:10.1093/ajcp/106.2.170.
- Ni L, Xu J, Zhang C. Epidemiological characteristic and trend of rubella in Guangzhou, 2005-2019. Morden Prev Med. 2021;48(11): 1938–40+2074.
- Ma J, Luo H, Hao L, et al. Analysis on epidemiological characteristics of rubella in China during 2005-2011. Chin J Vaccines Immunization. 2012;18(6):500–3+540.
- Bogusz J, Paradowska-Stankiewicz I. Rubella in Poland in 2017. Przegl Epidemiol. 2019;73(3):305–10. doi:10.32394/pe.73.35.
- Zhiying Y, Jianyue Z, Xiaoying G. Epidemiology of rubella in Quzhou,Zhejiang, 2004 to 2012. Dis Surveillance. 2013;28:672–75.
- Su Q, Feng Z, Hao L, Ma C, Hagan JE, Grant GB, Wen N, Fan C, Yang H, Rodewald LE, et al. Assessing the burden of congenital rubella syndrome in China and evaluating mitigation strategies: a metapopulation modelling study. Lancet Infect Dis. 2021;21(7):1004–13. doi:10.1016/S1473-3099(20)30475-8.
- Liu J. Epidemiological characteristics and coping strategies of rubella in Xi’An City from 2004-2011. Occup Health. 2012;28(17):2128–9+2132. doi:10.13329/j.cnki.zyyjk.2012.17.036.
- Zhu L, Zhang Z, Dong G. Epidemiological analysis of measles in Shenyang City from 2010 to 2019. J Community Med. 2021;19(3):146–49. doi:10.19790/j.cnki.JCM.2021.03.04.
- Takeshita K, Takeuchi N, Ohkusu M, Ohata M, Suehiro M, Maejima H, Abe H, Ohta F, Ohama Y, Tamai K, et al. Population-Based study of a free rubella-specific antibody testing and immunization campaign in Chiba city in response to the 2018–2019 nationwide rubella outbreak in Japan. Hum Vaccin Immunother. 2021;17(6):1779–84. doi:10.1080/21645515.2020.1847584.
- Oishi K, Satoh H, Tanaka-Taya K. Re-emerging rubella epidemic and public health measures in Japan. Yakugaku Zasshi. 2020;140(7):901–04. doi:10.1248/yakushi.19-00255-3.
- Piffer S, Pedron M, Dal Martello M, Caciagli P, Lanzafame P, Saugo M, Ferro A. Rubella immunity status and the active offer of MMR/MMRV vaccination during pregnancy. Ann Ist Super Sanita. 2021;57(1):26–32. doi:10.4415/ANN_21_01_04.
- Knapp JK, Mariano KM, Pastore R, Grabovac V, Takashima Y, Alexander JP, Reef SE, Hagan JE. Progress toward rubella elimination — Western Pacific Region, 2000–2019. MMWR Morb Mortal Wkly Rep. 2020;69(24):744–50. doi:10.15585/mmwr.mm6924a4.
- Wang C, Zhu Z, Xu Q, Fang X, Liu X, Xiong P, Song L, Xu W, Xu A. Progress towards rubella elimination after implementation of rubella immunization for over 20 years in Shandong province, China. Sci Rep. 2017;7(1):17982. doi:10.1038/s41598-017-18281-2.
- Kaaijk P, Nicolaie MA, van Rooijen D, van Houten MA, van der Klis FR, Buisman A-M, van Binnendijk RS. Dynamics of the antibody response after a third dose of measles-mumps-rubella vaccine indicate a slower decline compared with a second dose. Open Forum Infect Dis. 2020;7(11):ofaa505. doi:10.1093/ofid/ofaa505.
- Chan J, Wu Y, Wood J, Muhit M, Mahmood MK, Karim T, Moushumi F, Jones CA, Snelling T, Khandaker G, et al. Burden of Congenital Rubella Syndrome (CRS) in Bangladesh: systematic review of existing literature and transmission modelling of seroprevalence studies. Infect Disord Drug Targets. 2020;20(3):284–90. doi:10.2174/1871526518666181004092758.
- Shanmugasundaram D, Awasthi S, Dwibedi B, Geetha S, Jain M, Malik S, Patel B, Singh H, Tripathi S, Viswanathan R, et al. Burden of congenital rubella syndrome (CRS) in India based on data from cross-sectional serosurveys, 2017 and 2019–20. PLoS Negl Trop Dis. 2021;15(7):e0009608. doi:10.1371/journal.pntd.0009608.
- Motaze NV, Mthombothi ZE, Adetokunboh O, et al. The impact of rubella vaccine introduction on rubella infection and congenital rubella syndrome: a systematic review of mathematical modelling studies. Vaccines. 2021;9(2):84. doi:10.3390/vaccines9020084.
- Ma C, Su Q, Wen N, et al. Evaluation of the measles surveillance system performance in China, 2015-2016. Chin J Vaccines Immunization. 2018;24(2):141–45.