ABSTRACT
The Food and Drug Administration (FDA) expanded the emergency use authorization for the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) for children aged 12–15 years on 10 May 2021. To date, less than a year has passed since vaccination against COVID-19 has been used in children and adolescents, and the overall effects and safety of these vaccines are still being assessed. The BNT162b2 vaccine originally had a favorable profile in 12–17-year-old recipients compared with older ages, and no serious adverse events had previously been reported. Despite various adverse events, the benefit of reducing the infection rate or the frequency of severe COVID-19 has been evaluated to outweigh the harm caused by COVID-19 vaccination. Additionally, several cases of sudden development of new-onset or relapsing glomerular diseases, including acute kidney injury (AKI), have been reported in adults following the BNT162b2 SARS-CoV-2 mRNA vaccine. Herein, we present two cases of adolescents who developed AKI following the second administration of the BNT162b2. These are the first pediatric cases of acute tubulointerstitial nephritis temporarily linked to SARS-CoV-2 vaccination.
Introduction
Coronavirus disease 2019 (COVID-19)-associated hospitalization rates increased in March and April 2021. Nearly one-third of them required pediatric intensive care unit admission, and 5% needed invasive mechanical ventilation.Citation1 There is potential for serious disease or death in adolescents, hence vaccination of adolescents is warranted. Accordingly, on 10 May 2021, the Food and Drug Administration (FDA) expanded the emergency use authorization (EUA) for the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) for children aged 12–15 years.Citation2
The BNT162b2 vaccine originally had a favorable profile in 12–17-year-old recipients compared with older ages, and no serious adverse events had previously been reported.Citation3,Citation4 However, various COVID-19 vaccine associated adverse events, including myocarditis in adolescents, have been reported since these vaccinations have become more common worldwide.Citation5,Citation6 Among the solicited systemic adverse events of BNT162b2 vaccination, headache, and fatigue are common, while gastrointestinal symptoms, such as vomiting (1–3%) and diarrhea (4–11%), are uncommon, regardless of age.Citation3,Citation4 A similar distribution of adverse events occurred in Korean adolescents.Citation7
Additionally, several cases of sudden development of new-onset or relapsing glomerular diseases, including acute kidney injury (AKI) have been reported in adults following the BNT162b2 SARS-CoV-2 mRNA vaccine.Citation8 Herein, we present two cases of adolescents who developed AKI following the second administration of the BNT162b2. These are the first pediatric cases of acute tubulointerstitial nephritis temporarily linked to SARS-CoV-2 vaccination.
Case report
Case 1
A previously healthy 17-year-old man presented to our hospital with epigastric pain and poor oral intake for 3 days, and abnormal creatinine (Cr) serum levels following the second dose of the BNT162b2 SARS-CoV-2 vaccine.
The patient received the second dose of COVID-19 vaccine on 8 November 2021. The next day, he had myalgia and a fever of 38℃; therefore, he underwent a polymerase chain reaction test for COVID-19 on 10 November 2021. The result was negative. He denied taking any medication, including herbal teas, except for one dose of acetaminophen for fever that occurred after vaccination, in the past few months. He denied any cardiorespiratory symptoms.
From 11 November 2021, the patient had problems eating because of epigastric pain and nausea, and he visited a nearby hospital emergency room. He was noted to have renal insufficiency, with a serum Cr level of 3 mg/dL determined by a blood test.
On 13 November 2021, the patient was transferred to our hospital because of renal insufficiency and persistent gastrointestinal problems. Upon physical examination, he was acutely ill-looking with a blood pressure of 150/85 mmHg, heart rate of 75 beats/min, respiratory rate of 20 breaths/min, and body temperature of 36.9℃ with a peripheral oxygen saturation of 100% at room air. His weight was 95 kg (>99th percentile), height 178 cm (75–90th percentile) and BMI 30.0 kg/m2 (>99th percentile). Epigastric tenderness and costovertebral angle tenderness were positive on both sides.
shows the initial laboratory findings on day 1. Additional blood tests were performed. The erythrocyte sedimentation rate (ESR) was 43 mm/h. Complement 3 (C3) and 4 (C4) serum levels were 136 mg/dL and 31 mg/dL, respectively, both within the normal range. Results for antinuclear antibody (ANA) and antineutrophil cytoplasmic antibody (ANCA) tests were negative, and immunoglobulin G (IgG), A (IgA), and M (IgM) levels were 846, 166, and 78 mg/dL, respectively. D-dimer level was 2.19 mg/L FEU (normal range, ≤0.5 mg/L FEU). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were 1.09 INR (normal range, 0.88–1.20 INR) and 29 s (normal range, 20.0–36.0 s), respectively
Table 1. Initial laboratory results.
Urine microscopic findings were negative, and the spot urine protein:Cr ratio (PCR) was 0.10 g/g·Cr. Urine N-acetyl-β-D-glucosaminidase (NAG):Cr was 2.34 IU/g·Cr (normal range, ≤5.61 IU/g·Cr) and β2-microglobulin (β2MG) level was 0.29 mg/L (normal range, ≤0.30 mg/L) (). Chest and abdominal radiography revealed no specific findings. Kidney ultrasonography on hospital day 3 revealed normal-sized kidneys with normal echogenicity and normal range of renal arterial resistive index values.
Electrocardiography showed marked sinus arrhythmia with normal findings. Gastroduodenoscopy revealed no mucosal abnormality with a small amount of bile juice in the stomach and no ulcerative lesions in the duodenum.
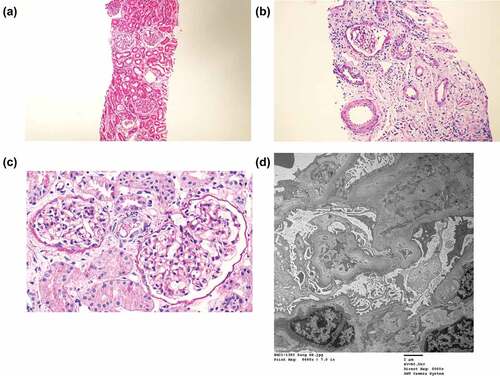
A renal biopsy was performed on day 3 of hospitalization. The glomerulus appeared slightly larger and segmentally hypercellular, involving mesangial cells. Interstitial infiltrates were mainly mononuclear. Focal and moderate interstitial fibrosis and tubular atrophy were noted in approximately 20% of the renal cortices. His blood vessels are unremarkable. Immunofluorescence was negative for IgG, IgM, IgA, C3, C1q, C4, fibrinogen, and kappa and lambda light chains. Electron microscopy revealed a non-significant focal epithelial foot process effacement with no electron-dense deposits. The glomerular basement membrane showed focal wrinkling with partly irregular inner contours (). These findings were consistent with those of acute tubulointerstitial nephritis. Renal insufficiency gradually improved with supportive care, oral intake increased, and he was discharged after 1 week.
Figure 1. Kidney biopsy findings in case 1.
Case 2
A previously healthy 12-year-old man presented to our hospital with 3-week persistent anorexia, nausea, vomiting, and weight loss of 7 kg, followed by renal insufficiency with a Cr level of 2.28 mg/dL.
The patient received a second dose of COVID-19 vaccine on 24 December 2021. The next day, he began to experience nausea, vomiting with gastric juice approximately four times per day, and poor oral intake. He visited a nearby clinic because of persistent anorexia and vomiting. A blood test was performed, and an abnormal Cr level of 2.28 mg/dL was noted on 11 January 2022.
On 17 January 2022, the patient was admitted to our hospital because of renal insufficiency and dehydration with a weight loss of 7 kg. He denied taking any medication, including herbal teas, except for 3 days of medication with trimebutine and domperidone for vomiting that occurred after vaccination, in the past few months. He denied any cardiorespiratory symptoms. There was no family history of chronic kidney disease or hypertension. Upon physical examination, he was acutely ill-looking and dehydrated with a blood pressure of 139/87 mmHg, heart rate of 127 beats/min, respiratory rate of 20 breaths/min, and body temperature of 36.7℃ with a peripheral oxygen saturation of 97% at room air. His weight was 56.3 kg (50–75th percentile), height 148.9 cm (10–25th percentile) and BMI 25.4 kg/m2 (90-95th percentile). Epigastric tenderness was positive, without costovertebral angle tenderness.
shows the initial laboratory findings. Additional blood tests were performed. Venous blood gas analysis showed metabolic acidosis (pH 7.261 and HCO3− 16.3 mmol/L) with an anion gap of 17.7. C3 and C4 serum levels were 120 mg/dL and 13 mg/dL, respectively, both within normal range. ANA, c-ANCA and p-ANCA were all negative, and the IgG, IgA, and IgM levels were 2,834, 557, and 250 mg/dL, respectively. PT and aPTT were 1.29 INR and 31s, respectively. D-dimer level was 0.91 mg/L FEU and lactate dehydrogenase (LDH) was 157 IU/L, respectively. Cardiac enzymes, including creatine kinase MB isoenzyme and troponin-I, were within the normal range, except for the pro-brain natriuretic peptide level of 237.6 pg/ml (normal range, 0–125 pg/ml).
Urine microscopic findings noted pyuria with white blood cells of 10–19/high power field (HPF) and casts of >21/low power field (LPF). The spot urine PCR was 1.95 g/g·Cr, and spot urine calcium:Cr ratio was 0.19. Proteinuria during the 24-h urine collection was 861 mg/day. Urine NAG: Cr was 32.61 IU/g·Cr, and β2MG level was 2.85 mg/L ().
Chest and abdominal radiography revealed no specific findings. Kidney ultrasonography on hospital day 2 revealed normal-sized kidneys with slightly increased echogenicity and a normal range of renal arterial resistive index values. Electrocardiography revealed sinus arrhythmia with a nonspecific ST abnormality. Gastroduodenoscopy revealed no mucosal abnormality in the stomach or duodenum.
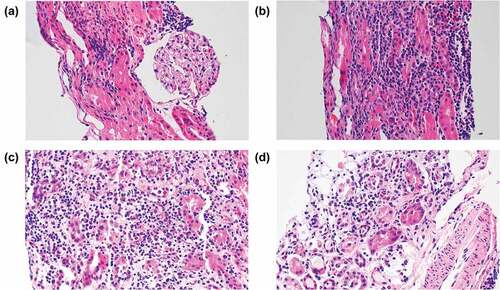
A renal biopsy was performed on day 5 of hospitalization. Immunofluorescence was negative for IgG, IgM, IgA, C3, C1q, C4, fibrinogen, and kappa and lambda light chains. The glomeruli were normal in size and cellularity. Tubules revealed severe necrosis, tubulorrhexis, and loss, with heavy infiltration of neutrophils, eosinophils, and mononuclear cells in the interstitium. Blood vessels were unremarkable. Ultrastructurally, the glomerular basement membrane thickness was normal with relatively smooth contours. No electron-dense deposits were observed. Epithelial cell foot processes showed slight focal effacement ().
Figure 2. Kidney biopsy findings in case 2.
After receiving oral steroid treatment on day 10 of hospitalization, the patient showed remarkable improvement in renal insufficiency ().
Discussion
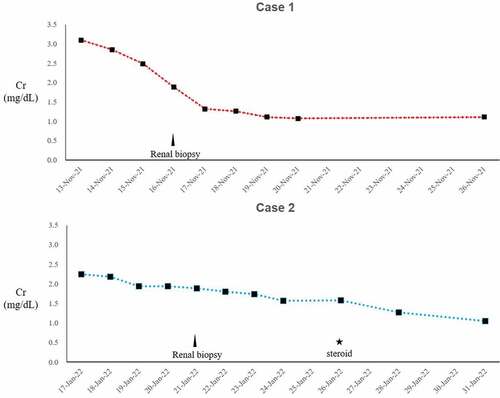
In the two cases described, Cr elevation was confirmed, starting with nonspecific digestive symptoms including vomiting, after the second dose of vaccine against COVID-19. In the first case, Cr elevation recovered rapidly, but in the second case, the Cr did not return to normal, and tubulopathy was continuously noted by urinalysis (). The common feature of both cases was that they were both male, had high BMIs, and their initial symptom presentation appeared after the second dose of BNT162b2 mRNA vaccine.
Despite various adverse events, the benefit of reducing the infection rate or the frequency of severe COVID-19 has been evaluated to outweigh the harm caused by COVID-19 vaccination. In adults, the population vaccinated against COVID-19 is large and sufficient information about reported adverse reactions has been provided. Associations between kidney disease and adult receipt of BNT162b2 vaccination are varied. These include minimal change in disease with AKI,Citation9 and IgA nephropathy.Citation10
A case with acute interstitial nephritis in a 45-year-old female patient was recently reported.Citation11 Similar to our cases, symptoms developed after inoculation with the second dose of the BNT162b2 SARS-CoV-2 vaccine. The patient was treated with corticosteroids and hemodialysis, which led to progressive recovery of renal function. Mira et al. suggested that patient might develop an acute reaction to the BNT162b2 based on immunophenotyping results, which could be useful in diagnosing hypersensitivity to the vaccine and polyethylene glycol excipient.Citation11 However, we have not been able to prove drug hypersensitivity in our cases. Based on that study, we concluded that the acute interstitial nephritis in our cases was related to BNT162b2 vaccination because of the coincidence of symptom onset and similar pathological findings.
Since December 2020, the Pfizer-BioNTech COVID-19 vaccine has been available under EUA for vaccination of individuals aged ≥16 years, and the authorization was expanded to include those 12–15 years of age in May 2021. It was the first COVID-19 vaccine to be approved by the FDA on 23 August 2021. To date, less than a year has passed since vaccination against COVID-19 has been used in children and adolescents, the overall effects and safety of these vaccines are still being assessed. Information regarding adverse reactions is therefore limited. However, the new development of nephrotic syndrome after the first dose of BNT162b2 in 15-year-old Japanese boy has been reported.Citation12 In addition, two pediatric patients with IgA nephropathy presented with macroscopic hematuria within 24 h following the second dose of the Pfizer-BioNTech COVID-19 vaccine. In both cases, new-onset gross hematuria was self-resolved; renal function was recovered spontaneously in one patient, while the other recovered only after receiving methylprednisolone pulses.Citation13
Evidence for drug-induced interstitial nephritis caused by the COVID-19 vaccine is limited. However, other adverse events, including interstitial nephritis caused by influenza vaccination, have also been reported. Moreover, the COVID-19 pandemic may impede patients’ access to general medical care and delay diagnosis of undiagnosed underlying diseases. It is also possible that our cases had a previously undiagnosed kidney disease rather than new-onset glomerular disease resulting from COVID-19 vaccination. Monitoring of renal function is therefore necessary after inoculation with a COVID-19 vaccine, and further research and discussion is necessary. Future reports of similar cases will facilitate the discussion regarding possible major adverse events associated with SARS-CoV-2 vaccines.
Abbreviations
| AKI | = | Acute kidney injury |
| ANA | = | Anti-nuclear antibodies |
| ANCA | = | Antineutrophil cytoplasmic antibodies |
| β2MG | = | β2-microglobulin |
| C3 | = | Complement 3 |
| C4 | = | Complement 4 |
| COVID-19 | = | Coronavirus disease 2019 |
| Cr | = | Creatinine |
| ESR | = | Erythrocyte sedimentation rate |
| EUA | = | Emergency use authorization |
| FDA | = | The Food and Drug Administration |
| HPF | = | High power field |
| IgG | = | Immunoglobulin G |
| LDH | = | Lactate dehydrogenase |
| LPF | = | Low power field |
| NAG | = | N-acetyl-β-D-glucosaminidase |
| PCR | = | Protein-to-creatinine ratio |
| PT | = | Prothrombin time |
| aPTT | = | Activated partial thromboplastin time |
Ethics approval and consent to participate
Ethics committee approval was waived.
Acknowledgements
We really appreciate the patients for allowing us to share their medical information.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Havers FP, Whitaker M, Self JL, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Meek J, Yousey-Hindes K, Anderson EJ, et al. Hospitalization of adolescents aged 12–17 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1, 2020–April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(23):851–6. doi:10.15585/mmwr.mm7023e1.
- U.S. Food and Drug Administration. FDA authorizes Pfizer-BioNtech COVID-19 vaccine for emergency use in children 5 through 11 years of age. 2021 [accessed 2022 January 28]. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–50. doi:10.1056/NEJMoa2107456.
- Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SA, Buddhe S, Misra N, Ramachandran P, Gaur L, et al. COVID-19 vaccination–associated myocarditis in adolescents. Pediatrics. 2021;148(5):e2021053427. doi:10.1542/peds.2021-053427.
- Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, Caron R, Fuss C, Corbin KJE, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNtech COVID-19 vaccination. Pediatrics. 2021;148(3):e2021052478. doi:10.1542/peds.2021-052478.
- Korea Centers for Disease Control and Prevention (KCDC). Status of monitoring adverse events after vaccination against COVID-19 for adolescents (12-17 years old). Public Health Wkly Rep. 2021;14:3542–48.
- Bomback AS, Kudose S, D’-Agati VD. De Novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78(4):477–80. doi:10.1053/j.ajkd.2021.06.004.
- Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, Levin-Iaina N, Fytlovich S, Yagil Y. Minimal change disease following the Pfizer-BioNtech COVID-19 vaccine. Am J Kidney Dis. 2021;78(1):142–45. doi:10.1053/j.ajkd.2021.03.010.
- Tan HZ, Tan RY, Choo JCJ, Lim CC, Tan CS, Loh AHL, Tien CSY, Tan PH, Woo KT. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100(2):469–71. doi:10.1016/j.kint.2021.05.009.
- Mira FS, Carvalho JC, de Almeida PA, Pimenta AC, Coutinho IA, Figueiredo C, Rodrigues L, Sousa V, Ferreira E, Pinto H, et al. A case of acute interstitial nephritis after two doses of the BNT162b2 SARS-CoV-2 vaccine. Int J Nephrol Renovasc Dis. 2021;14:421–26. doi:10.2147/IJNRD.S345898.
- Nakazawa E, Uchimura T, Hirai Y, Togashi H, Oyama Y, Inaba A, Shiga K, Ito S. New-Onset pediatric nephrotic syndrome following Pfizer-BioNtech SARS-CoV-2 vaccination: a case report and literature review. CEN Case Rep. 2021:1–5. Online ahead of print. doi:10.1007/s13730-021-00656-0.
- Hanna C, Herrera Hernandez LP, Bu L, Kizilbash S, Najera L, Rheault MN, Czyzyk J, Kouri AM. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100(3):705–06. doi:10.1016/j.kint.2021.06.032.