ABSTRACT
Vaccination toward SARS-CoV-2 reduced mortality and ‘boosters’ are being implemented. We offer scientific contribution about IgG production in the COVID-19 experienced population. From January 2021 to March 2021, 183 residents and staff from the Elderly Nursing Home “San Giuseppe Moscati” who had received two doses of the BNT162b2 vaccine were enrolled. The antibody response was assessed by the DiaSorin LIAISON-CLIA S1/S2® IgG solution. Cutoff levels for response (>39 BAU/mL) and neutralizing activity (>208 BAU/mL) were derived from DiaSorin official data. Serology was assessed before and after the first vaccination, and 2 weeks and 6 months after the second vaccination. Anti-S IgG in COVID-19 experienced, baseline IgG producers spiked after the first vaccination to median 5044 BAU/mL and decayed at 6 months to 2467.4 BAU/mL. Anti-S IgG in COVID-19 experienced, baseline IgG non-producers spiked after the second vaccination to median 1701.7 BAU/mL and decayed at 6 months to 904.8 BAU/mL. Anti-S IgG in COVID-19 naïve subjects spiked after the second vaccination to median 546 BAU/mL and decayed at 6 months to 319.8 BAU/mL. The differences between sequential timepoint levels in each group were statistically significant (p < .0001). Serology analysis revealed different kinetics between COVID-19 experienced subjects depending on baseline response, possibly predicting different IgG persistence in blood.
Introduction
In response to the pandemic, 33 COVID-19 vaccines have been approved for use and 10 are in the WHO’s Emergency Use List.Citation1 More than ten billion vaccine doses have been administered, reducing COVID-19 spread and mortality, as well as the risk of escape variants.Citation2,Citation3 Vaccines available in Europe are mainly messenger RNA (mRNA) vaccines, without preservatives or adjuvants. Other vaccines are made using human and primate adenovirus vectors or an inactivated whole-virus SARS-CoV-2 vaccine or only the spike protein, following the influenza vaccine technology.Citation4 Vaccinated people are less prone to acquire infection with the delta variant, the disease is generally milder, contagiousness lasts less and mortality is lower.Citation5 But how long does vaccine-induced immunity last and what is the best timing for a booster dose? Is timing the same for all people? COVID-19 experienced subjects maintain functional natural immunity for a long period of timeCitation6–8and show particularly robust immune responses to vaccines.Citation9 Initial evaluation of the decay after vaccination sorted rates that are similar to immunity after infection, however the threshold achieved for prevention of infection is likely to be crucial.Citation10 The present study aim is to assess the decay in serum anti-spike IgG levels over 6 months from vaccination in a mixed cohort of young and elderly, COVID-19 naïve and COVID-19-experienced subjects.
Materials and methods
We enrolled residents and staff members from the Elderly Nursing Home “San Giuseppe Moscati” (Milan) from January 2021 to March 2021. All subjects received two doses of the BNT162b2 vaccine. Seven hundred thirty-two plasma samples have been analyzed (312 from residents and 420 from staff) in June 2020, after the first wave, in January and February 2021, 14 days after the first and second vaccine shot, and at the end of July 2021 approximately 6 months after the second shot (end of January). Twenty-nine subjects had acquired infection more than 6 months before vaccination and more than 2 before baseline antibody testing (range 41–78 days) and there was a 68% overlap between longer delay and negative result at baseline. The antibody response was assessed by the DiaSorin LIAISON-CLIA S1/S2® IgG solution, and results are reported in the WHO BAU/mL (Binding Antibody Units, 1 BAU/mL = 2.6 × AU/mL). Tests were performed in duplicate for accuracy. The cutoff levels for ‘non responders’ and ‘threshold for neutralization potential’ were derived from DiaSorin official data. The minimal cutoff level for positive antibody production is 39 BAU/mL, while the cutoff level for 100% correlation with 90% Plaque Reduction Neutralization Test (PRNT90) is 208 BAU/mL.Citation11 After signing written informed consent subjects were enrolled in the AntiCROWN longitudinal study of anti-S1/S2 response, approved by the “Comitato Etico Interaziendale Area 1”, n. 2020/ST/158.
The Shapiro–Wilk test was used to test the normal distribution of quantitative variables. When these were normally distributed, the results were expressed as mean values and standard deviation (SD), otherwise we used median values and interquartile range (IQR; 25th −75th percentile). Parametric or non-parametric tests were used to compare quantitative variables (the t-test for independent samples for comparisons between two groups or the Mann–Whitney test). Chi-squared statistics or Fisher’s exact test, as appropriate, were applied to compare qualitative variables. Univariate and multivariate linear regression models for repeated measures were performed to analyze associations between antibody levels (after log transformation) and cohorts (patients and health care workers), sex, age, comorbidities: overweight, anti-flu vaccination side effects after 1st dose and 2nd dose.
A p < .05 was considered to be statistically significant. All tests were two-sided. Data analysis was performed using the STATA statistical package (version 15; Stata Corporation, College Station, 2019, Texas, USA).
Results
The population examined is composed of 78 patients and 105 operators (health workers and administration staff) of a Nursing Home, with huge differences in age, comorbidities and exposure to COVID-19. shows the differences in baseline and demographic data between operators and patients and between COVID-19 naïve and COVID19-experienced subjects. Patients are significantly older than staff and have more comorbidities, particularly cardiovascular, renal, and metabolic diseases (P < .001), while COVID-19 naive are older than COVID-19 experienced (P = .0102), without significant differences in the number of comorbidities nor in immune suppressant, cytotoxic, or antiviral therapies (one subject each). There was no correlation between overall or specific comorbidities and response to vaccination, however larger numbers may be required.
Table 1. Baseline characteristics of the population divided for role and past COVID experience.
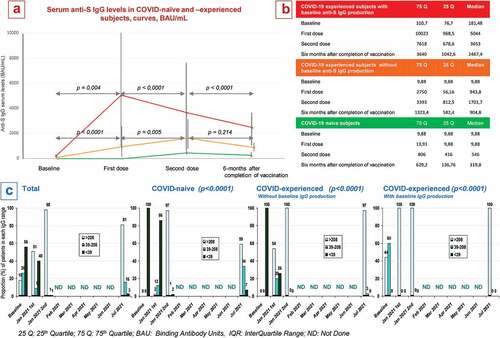
The main comorbidities that have been recorded in the study population are cardiovascular diseases (38.25%), metabolic disorders (17.49%), history of cancer (8.74%), pulmonary diseases (8.2%), autoimmune disease (5.46%), renal disease (5.46%), hepatic diseases (3.83%), cancer (2.73%), diabetes mellitus (1.64%) and immune deficiency (1.09%). shows the decay curves in anti-S IgG serum levels and the proportion of patients at each time point by level ranges (<39 BAU/ml, 39–208 BAU/mL and >208 BAU/mL). As shown in panels A and B, the median IgG loss per month for COVID-19 naïve subjects in the first 6 months of completed vaccination is −36.9 BAU/mL, with an estimated time to decay below 208 BAU/mL equal to 9 months (supposing linear decay from the time of second vaccination). For COVID-19 experienced subjects who produced anti-S1/S2 IgG after infection, the decay rate is −188.8 BAU/mL and the estimated time to below 208 BAU/mL is 18 months. Finally, for COVID-19 experienced subjects who did not produce anti-S1/S2 IgG after infection, the decay rate is −42.6 BAU/mL and the estimated time to be below 208 BAU/mL is 15 months. Interquartile ranges in COVID-19-experienced subjects narrow at 6 months. Differences within time points for each curve are statistically significant, as shown in Panel A. Panel C shows patients who do not produce antibodies (dark bars), patients who produce lower than neutralizing antibody levels (intermediate bars) and patients who produce neutralizing antibody levels (light bars). Trends within subpopulations are statistically significant (P < .0001). We compared COVID-19 naïve trends with both COVID-19 experienced subgroups and the two COVID-19 experienced subgroups both for any antibody production and for neutralizing antibody levels, and all differences were statistically significant (p < .0001). Overall, loss of IgG production at 6 months occurred only in 7% of COVID-19 naive subjects, while decay below the threshold for neutralization occurred in 41% of naive patients and in 3% of COVID-19 experienced subjects who had no IgG production at baseline. COVID-19 experienced subjects who had significant IgG levels at baseline maintained 100% neutralizing levels at 6 months.
Figure 1. a&b: Serum anti-S1/S2 IgG levels in response to 1st and 2nd doses of the BNT162b2 vaccine and 6-months after completion of vaccination, BAU/mL, median value ± IQR. c: proportion of patients by serum level range groups (>208, 39–208 and <39 BAU/mL) at baseline, 1st and 2nd doses and 6-months later.
Discussion
Our study has the advantage of comparing younger vs older, COVID-19-naive vs COVID-19-experienced subjects, and subjects with comorbidity burden with healthier subjects. The main biases are the relatively small population size, the retrospective nature of the analysis, the lack of virus neutralization or plaque reduction assays and the lack of cellular immunity analyses. Another bias, intrinsic to the field, is that IgG testing methods widely differ and there is little in the literature being performed with our test. Several studies have investigated the levels of concordance between different methods, converted in the WHO international standard unit, BAU and against plaque reduction and microneutralization tests in vaccinated non exposed health workers before and after vaccination.Citation12,Citation13 Comparing our study with the only one that we found using our same method (but with the mRNA-123 vaccine) we found more optimistic data in such study, however it should be pointed out that it concerns only younger health care workers. The different decay slope between COVID-19 naive and COVID-19 experienced subjects is confirmed but there is no analysis between baseline IgG producers and non-producers.Citation14 A smaller study by Gimenez et al. on nursing home residents vaccinated with theBNT162b2 vaccine but analyzed with another method that cannot be translated into BAU showed an almost double decay rate compared to ours.Citation15 The literature concerning company-driven follow-up of the main m-RNA vaccines shows retained activity of the mRNA-1273 vaccine, as spike-binding ability, ACE-2 competition and pseudovirus and neutralization activity against wild-type virus and alpha, iota, gamma, and delta variants after 6 months, a longer durability compared to ours.Citation15 Data from the follow-up of the C4591001 Clinical Trial Group original cohortCitation16 reported clinical efficacy declining from 96.2% 2 months after the second dose to 83.7% at month 6, without serologic analysis. Interim results presented on Lancet Infectious Diseases from a phase 2, double-blind, randomized, placebo-controlled trial of CoronaVac show that the best timing for the booster dose, to elicit a robust increase in neutralizing antibodies is 6 rather than 2 months after the second dose.Citation17 Our study revealed interesting findings: the second vaccine dose in the COVID-19 experienced group did not impact on the antibody decline rate, but in the COVID-19 experienced subgroup that had no baseline antibody production it increased the response by approximately 50%. This subgroup showed a three-fold higher antibody production as compared to COVID-19 naïve subjects. Assessing anti-spike IgG levels may distinguish COVID-19 experienced subjects who may benefit from a single-dose vaccination from those who need two doses. The antibody production at 6 months remained well above the threshold of neutralization activity in 59% of COVID-19 naïve subjects and in 99% of COVID-19 experienced subjects. Our prediction of the median duration of IgG protective levels assumes linear decay, while more recently Levin et al. showed that after the first 3 months neutralizing response decays more slowly.Citation18 In general, studies of neutralizing antibody decay after COVID-19 vaccination are relatively small in size, are based on different methods to measure antibody serum levels, and describe divergent kinetics. Kinetics in COVID-19 experienced subjects, however, appears to be peculiar. Our study warns that not all COVID-19 infected subjects are to be considered the same with respect to vaccination, given that among asymptomatic and mild COVID-19 experienced subjects many do not produce anti-S antibodies.Citation19 Also, cellular immunity may play a crucial role in enabling also patients with low-level antibody production to respond in case of exposure to the virus. Indeed, research is moving toward the assessment of plasma cell-mediated immunity, which may sustain antibody production over time although conclusion cannot be driven yet.Citation6
Linked dataset
The dataset containing the source data for this article is available at “Dbase of Decay rate of antiS1-S2 IgG serum levels after 6 months of BNT162b2 vaccine”, Mendeley Data, V1, doi: 10. 17632/rr5r3mht9 r.2
Acknowledgements
We acknowledge Mrs Simona Bocchio for managing the organizational aspects of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- COVID-19 vaccine market dashboard. [accessed 2022 Feb 4]. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
- Hopkins’ J COVID-19 Dashboard. [accessed 2022 Feb 4]. https://coronavirus.jhu.edu/map.html
- Niesen MJM, Anand P, Silvert E, Niesen MJM, Corchado-Garcia J, O’Horo JC, Virk A, Swift MD, Halamka J, Badley AD, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. MedRxiv. 2021. 2021.07.01.21259833. doi:10.1101/2021.07.01.21259833.
- European Medicines Agency. COVID-19 Vaccines [accessed 2022 Mar 12]. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–5. doi:10.1016/S0140-6736(21)00947-8.
- Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, Davenport MP. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi:10.1038/s41577-021-00550-x.
- Capetti AF, Borgonovo F, Mileto D, Gagliardi G, Mariani C, Lupo A, Dedivitiis G, Meraviglia P, Pellicciotta M, Armiento L, et al. One-Year durability of anti-spike IgG to SARS-CoV-2: preliminary data from the anticrown prospective observational study one year durability of COVID-19 anti-spike IgG. J Infect. 2021;83(2):237–79. Epub 2021 May 27. doi:10.1016/j.jinf.2021.05.023.
- Yang Y, Yang M, Peng Y, Liang Y, Wei J, Xing L, Guo L, Li X, Li J, Wang J, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol. 2022;7:423–33. doi:10.1038/s41564-021-01051-2.
- Amedeo FC, As C, Fabio B, Mileto D, Letizia O, Gianfranco D, Angelica L, Maria VC, Lara B, Giacomelli A, et al. Impressive boosting of anti-S1/S2 IgG production in COVID-19-experienced patients after the first shot of the BNT162b2 mRNA COVID-19 vaccine. Clin Infect Dis. 2021:ciab214. doi:10.1093/cid/ciab214.
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. doi:10.1038/s41591-021-01377-8.
- LIAISON® SARS-CoV-2 S1/S2 IgG a quantitative assay with correlation to neutralizing antibodies [accessed 2021 Nov 9]. https://www.diasorin.com/sites/default/files/allegati_prodotti/liaisonr_sars-cov-2_s1s2_igg_m0870004366-d_lr.pdf
- Altawalah H. Antibody responses to natural SARS-CoV-2 infection or after COVID-19 vaccination. Vaccines. 2021;9:910. Giavarina D, Carta M. Improvements and limits of anti SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization Diagnosis, 2021. doi:10.3390/vaccines9080910.
- Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, Papleux E, Vekemans M, Beukinga I, Blairon L. Six-Month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: a third dose is expected. J Infect. 2021;S0163-4453(–3):433. Epub ahead of print. doi:10.1016/j.jinf.2021.08.031.
- Giménez E, Alberola J, Torres I, Albert E, Alcaraz MJ, Botija P, Amat P, Remigia MJ, Beltrán MJ, Rodado C, et al. Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty® COVID-19 vaccine. J Infect. 2021; S0163-4453(21)542–49 DOI: 10.1016/j.jinf.2021.10.026.
- Pegu A, O’-Connell S, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021:eabj4176. doi:10.1126/science.abj4176.
- Thomas SJ, Ed M Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;NEJMoa2110345. Epub ahead of print. doi:10.1056/NEJMoa2110345.
- Pan H, Wu Q, Zeng G Yang J, Jiang D, Deng X, Chu K, Zheng W, Zhu F, Yu H, Yin W Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. Lancet Infect Dis 2021;S1473-3099(21)681–82. doi: 10.1016/S1473-3099(21)00681-2
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, Rubin C. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021 Dec 9;385(24):e84. Epub 2021 Oct 6. doi:10.1056/NEJMoa2114583
- Wu F, Liu M, Wang A. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med 2020;180(10):1356–62. doi: 10.1001/jamainternmed.2020.4616. Erratum in: JAMA Intern Med. 2020 Oct 1;180(10):1405.
