ABSTRACT
Vaccination remains the most effective and cost-saving measure to protect against hepatitis B, a global health problem. It is crucial to characterize the persistence of the immune response after booster vaccination. This study aimed to quantify the persistence through mathematical modeling. Booster vaccination against hepatitis B was conducted in children 5–15 years in 2009–10 in Zhejiang Province. There were four dosage formulations of hepatitis B vaccines [Shenzhenkangtai Biotechnology Co. Ltd. Dalianhanxin Biotechnology Co. Ltd. NCPC GeneTech Biotechnology Pharmaceutical Co. Ltd. Sinovac Biotech Co. LTD. China]: 5, 10, and 20 μg hepatitis B vaccines or 5 μg hepatitis A and B (HAB) combination vaccine with a 0-1-6-month schedule. These were randomly administered to children negative for all hepatitis B markers, named as the schedule 2 group. Anti-HBs positive subjects were given one dose of booster, named as the schedule 1 group. Anti-HBs antibody was measured 1, 7, 18, 66, and 102 months after the first booster dose. A linear mixed-effects model was proposed to predict long-term persistence. One hundred two months after the booster dose, the mean anti-HBs levels were 33.8 mIU/mL, with 73.7 mIU/mL for the schedule 1 group and 20.2 mIU/mL for the schedule 2 group. The model predicted that 99.5% of subjects would remain seropositive (≥10mIU/mL) at year 20 post booster vaccination, with 100.0% and 98.8% for the schedule 1 group and the schedule 2 group, respectively, whereas at year 30, the seropositivity rates would decrease to 76.8%, with 99.4% for the schedule 1 group and 62.5% for the schedule 2 group. The immunogenicity of the booster vaccination could persist for at least 8 years. Mathematical modeling may predict even longer, up to 30 years of protection.
KEYWORDS:
Introduction
According to the WHO, an estimated 300 million people were living with chronic hepatitis B infection, with 820 000 deaths in 2019. The majority of people died from cirrhosis and hepatocellular carcinoma. In addition, 1.5 million people develop new HBV infections annually.Citation1 Hepatitis B is still a major public health problem.
To date, vaccination is an effective, safe, available and cost-saving preventive measure against hepatitis B. Since the infant vaccination has been recommended, the HBsAg prevalence in the general population has decreased from 4.2% in 1990 to 3.7% in 2005.Citation2 In children aged below 5 years old, the rate was as low as 1.4% in 2016.Citation3 In China, hepatitis B vaccine (HepB) was recommended for infant routine immunization in 1992. However, the family should pay for the vaccine. In 2002, China integrated HepB into the expanded program on immunization (EPI) vaccines, making it free for all infants, and the family only had to pay for the service fee for the vaccination procedure. Since 2005, HepB vaccination has been completely free for all neonates.Citation4 Due to the policy, the prevalence in the general population decreased from 9.8% in 1992 to 7.2% in 2006.Citation5
However, breakthrough of HBV infection would still occur in some of children who had finished the infant immunization. Hence, several scholars favor booster vaccination against HBV infection and found that different booster vaccines had good efficacy.Citation6,Citation7 To date, the persistence of the immune response after the hepatitis B booster vaccine is unknown. As there were few real world studies on the long-term observation of booster immunization efficacy, mathematical modeling could perhaps solve this problem. Hence, this study determined the titers of anti-HBs after 8 years of booster dose of hepatitis B vaccines and then predicted the long-term persistence of anti-HBs protection.
Methods
Study design and subjects
The cohort study was conducted in Changshan, Longquan, Qingyuan, Yuhuan and Xianju counties in Zhejiang Province, China in 2009 and 2010 to evaluate the long-term persistence of antibodies against hepatitis B (anti-HBs). The inclusion and exclusion criteria for participants were similar to those in a previous study.Citation8
The inclusion criteria were as follows: children born between 1 January 1993, and 1 January 1997, who had received full doses of primary vaccination during infancy; never received a hepatitis B booster vaccination before and post booster vaccination study; willing to participate in the study and sign the informed consent form; without acute illness, fever and no allergies or severe reactions to vaccinations; all information regarding the study was provided by the subject’s parents or guardians; having no immune dysfunction; never received any immune suppressive therapy; at no risk of compromised immunity; and did not receive any kind of vaccination within the past 4 weeks. The exclusion criteria were as follows: children with positive HBsAg or anti-HBc; having another booster vaccination before the study and during the follow-up period; and unwillingness to participate in this study.
Initially, the children aged 5 to 15 years old were screened for HBsAg, anti-HBs, and anti-HBc. In order to observe whether the antibody will rise sharply after booster vaccination and whether the persistence will be prolonged, participants who had positive anti-HBs received one dose of hepatitis B vaccine at 0 months, named as the schedule 1 group. Those who were negative for HBsAg, anti-HBs, and anti-HBc received three doses of hepatitis B vaccine at 0-1-6 months, named as the schedule 2 group. Four dosage formulations of hepatitis B vaccines were randomly given to subjects: 5 μg (lot number: 20071223(1-9), Shenzhenkangtai Biotechnology Co., Ltd.), 10 μg (lot number: 20090309(01-06), Dalianhanxin Biotechnology Co., Ltd.), 20 μg (lot number: 200802A21 (01–05), NCPC GeneTech Biotechnology Pharmaceutical Co., Ltd.) and hepatitis A and hepatitis B combined (HAB) vaccine (lot number: 2009040302, dosage: 250 U inactivated HAV-Ag and 5 μg HBsAg; Sinovac Biotech Co., LTD.).
This study was approved by the Institutional Review Board of the Zhejiang Centers for Disease Control and Prevention, and we obtained oral informed consent from each participant’s parents or their guardian. After acquiring consent, 2-ml blood samples were collected from each subject and tested for anti-HBs titers at 1, 7, 18, 66, and 102 months after the first dose booster vaccination.
Lab testing
All serum samples were quantified by chemiluminescence immunoassay (CLIA) and an Architect-i2000 (Abbott Laboratories, USA) was used for the CLIA. The testing at the 66-month follow-up was sent to ADICON Clinical Laboratories Inc. in Hangzhou and the testing at 102 months was sent to KingMed Diagnostics in Hangzhou. The reagent lot number for anti-HBs was 75684M100 (Abbott Laboratories, USA) during the first 5-year follow-up and 91245FN00 (Abbott Laboratories, USA) was used for the last testing.
The geometric mean titer (GMT) of anti-HBs ≥10 mIU/ml was considered positive and was able to provide protection against HBV infection. Because anti-HBs titers <.01 mIU/ml were unable to be detected, we assigned a value of .01 mIU/ml to these subjects when calculating the GMT of anti-HBs. Log transformation was used for calculating the GMT. Anti-HBs >15,000 mIU/mL were assigned a value of 15,000 mIU/mL at 1 month to 66 months after the first dose, while anti-HBs ≥1000 mIU/mL were recorded as 1000 mIU/mL at 102 months after the first dose.
Mathematical modeling for long-term anti-HBs antibody duration
To assess the persistence of HBV vaccine-booster antibody responses, a linear mixed-effects model was designed according to a previous study.Citation9 The anti-HBs antibody titers were log-transformed to ensure the normal distribution of the data. The individual antibody levels of each participant at each visit were used to fit a linear mixed-effects model.
In this model, four components were taken into account, including the fixed-effects parameters, random effects (which account for individual variation), serial correlations and measurement error. Time (sample moment in months), gender, age and vaccine were entered as covariates. The variance of the measurement error was allowed to vary as a function of time.
Data analysis
We established a database using Epidata 3.2 (Epidata; Norway and Denmark), and data were processed using the R4.1.2 open source package lme4 (www.r-project.org). A linear mixed-effects model was used to predict seropositivity rates (anti-HBs cutoff 10 mIU/mL) at 1, 7, 18, 66 and 102 months after the booster vaccination. Symmetrized (antibody levels were log-transformed) and the mean trend of the observed antibody levels was represented as described by Hens et al.Citation10 The Mann-Kendall trend test was used for the trend analysis. The model was fitted using all available data during the follow-up period. The Bayesian information criterion (BIC) was used for model selectionCitation11 and the coefficient of simple determination (R2) was assessed for goodness of fit.Citation10
Results
Recruitment of participants for the modeling
A total of 431 participants were included in the modeling analysis according to the inclusion and exclusion criteria. There were 172 participants in the schedule 1 group and 259 in the schedule 2 group. The mean age was (7.7 ± 2.3) years for the whole population, with (7.2 ± 2.2) years in the schedule 1 group and (7.9 ± 2.4) years in the schedule 2 group. There were totally 196 males with 77 males in the schedule 1 group and 119 in the schedule 2 group (), and there were no differences between the schedule 1 and 2 groups (P > .05). As shows, there were no sex differences at the five time points.
Table 1. The basic information of participants (n,%).
Table 2. Distribution of gender at five follow-up time points (n, %).
Immunogenicity in participants
The total anti-HBs levels were 33.8 mIU/mL (95% CI: 28.1-40.7 mIU/mL) at 102 months after the first dose of booster hepatitis B vaccine, with 73.7 mIU/mL (95% CI: 56.7-95.8 mIU/mL) for the schedule 1 group and 20.2 mIU/mL (95% CI:15.9-25.5 mIU/mL) for the schedule 2 group at 102 months (). Trend analysis showed a decreasing trend in both groups ().
Table 3. Anti-Hbs levels during follow-up period.
Table 4. Mann-Kendall Test Results for anti-HBs levels at five time points.
Modeling results
After BIC selection, the best model formula was log(GMT) ~ schedule * time 2 + (1|id). The effect of the immune response was time-dependent (, ). The use of schedule was a significant fixed effect. Sex, vaccine and age had no significant effects on the model. shows the parameter estimates and standard errors.
Figure 1. Observed individual profiles (gray lines) and population-averaged estimation of Anti-HBs levels (blue and red line).
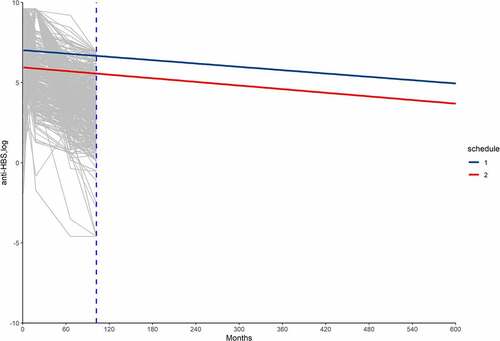
Table 5. Table parameter estimates and standard errors of the model.
Based on the model, the predicted mean anti-HBs levels were 2121.8 mIU/mL, 284.3 mIU/mL and 38.5 mIU/mL at 10, 20 and 30 years after the booster dose of hepatitis B vaccine ().
Observed data were used in the model to predict long-term antibody persistence. Using ≥10mIU/mL as the seropositivity cutoff value, the model predicted that 99.5% of subjects would remain seropositive at 20 years, with 100.0% and 98.8% for the schedule 1 group and schedule 2 group, respectively. At year 30, the seropositivity rates decreased to 76.8%, with 99.4% for the schedule 1 group and 62.5% for the schedule 2 group ().
Table 6. Predicted proportion of participants whose anti-HBs ≥10 mIu/ml.
Discussion
Our results showed that children still had protective antibody levels 8 years after the booster vaccination. This was consistent with other studies that confirmed the good anamnestic response of the booster hepatitis B vaccine.Citation12–16 As most of previous booster studies had short-term follow-up periods, this 8-year cohort study could be a ‘long’-term observation in children. However, longer-term of real-world observations could not be obtained for various reasons. Trend analysis showed a decreasing trend of anti-HBs titers, suggesting that the immune response may wane over time.
Earlier in 1966, a mathematical model was designed to estimate the long-term immunity to diphtheria and tetanus in adults. The study found that the titer at any time following the booster was related to the ratio of the post-to-pre-booster titer, and time was the only factor influencing the predicting function.Citation17 Although individual features such as age and sex could affect the response to vaccination, Honorati MC et al. demonstrated that sex was not a significant fixed effect in the mathematical model, and the effect of the immune response was only time-dependent.Citation18 In our study, not only sex had no association with the model, and age was another nonsignificant factor.
For the prediction model, a random effects model was first generated to predict the long-term immunogenicity of the hepatitis B vaccine. As only individual values were involved in the random effect model, Vellinga A et al. proposed a linear mixed effect model. It included both fixed and random effects. When predicting, the fixed factors should be the significant ones that should be considered according to the clinical practice. For instance, the antiepileptic drugs had a significant fixed linear effect in the mathematical model in Vellinga A’s study.Citation19 In our study, schedule was the significant fixed effect in the model after comparing different models via ANOVA.
Previously published data have determined the relationship of pre-booster anti-HBs titers and long-term efficacy after booster.Citation20 However, Gesemann M and Scheiermann N further identified a stronger correlation of long-term follow-up titers with 1 month post-booster titers than pre-booster titers.Citation21 Hence, anti-HBs titers from 1 month after the booster vaccination were used to predict long-term anti-HBs concentrations. Through BIC analysis, a better choice of linear mixed-effects model was formed to predict the anti-HBs titer persistence. We found that 40 years later, the mean titers were still 25.2 mIU/mL, 99.4% of vaccines in the schedule 1 group still had seropositive anti-HBs and 94.6% of vaccines still had seropositive anti-HBs in the schedule 2 group.
Some limitations should be taken into account in our study. First, an inevitable limitation of this article was that we could not exclude the possible overestimation of the seropositivity or anti-HBs titers. The longer the time to predict was, the more inaccurate the results.Citation22 Second, we found that the majority of subjects were younger children who had better immune responses, hence, the observed predicting results would be higher than a population with an identical number of younger and older individuals. Third, blood samples were not collected every year for various reasons such as financial constraints and children studying in another city. To solve this problem, the units at the follow-up time points were transformed from year to month; at the same time, log-transformation of time was used for analysis. Fourth, the ratio of lost to follow-up was too high, making the sample sizes at 102 months different. Fifthly, the endpoint dilutions were different at 1–66 months and 102 months. Considering the log-transformation of GMT could make little difference on the model, we did not changed 15,000 titers to 1000 titers.
Of course, there were strengths of the study. First, the follow-up period was long enough for the observed data. Second, the sample size was hundreds of fold larger than that in previous studies.Citation17,Citation23
In conclusion, booster vaccination could induce robust immune responses for at least for 8 years. Mathematical modeling predicts that the booster vaccination could persist for at least 20 years. Lifespan is extending with the growth of the aging population. According to the seventh census, there are 264 million elderly people aged 60 and over in the mainland, accounting for 18.7% of the total population.Citation24 Although HBV infection acquired in adulthood leads to chronic hepatitis in less than 5% of cases,Citation1 the treatment is more expensive once someone becomes one of the 5% of cases.Citation25 Therefore, whether adults who have finished primary and childhood booster doses should be screened for anti-HBs 20 or 30 years later, needs further study.
CRediT authorship contribution statement
Yan Qiu: Conceptualization, Data curation, Formal analysis, Visualization, Writing-original draft, Writing-review & editing.
Zi-Kang Wu: Conceptualization, Methodology, Data curation, Formal analysis.
Jie Wu: Methodology, Data curation, Formal analysis, Visualization.
Ying Liu: Data curation, Writing-review & editing.
Wen Ren: Data curation, Writing-review & editing.
Yujing Sun: Writing-review & editing.
Jun Yao: Conceptualization, Methodology, Writing-review & editing.
Lingzhi Shen: Conceptualization, Methodology, Funding acquisition, Supervision, Writing-review & editing.
Jing-Jing Ren: Conceptualization, Funding acquisition, Supervision, Writing-review & editing.
Ethical approval
This study was approved by the Institutional Review Board of the Zhejiang Center for Disease Control and Prevention (No. 2018–45). Oral informed consent was obtained from all participants.
Acknowledgments
We thank the children and parents in this study. We appreciate the staff from the Centres for Diseases Control and Prevention in Long-Quan, Kai-Hua and Yu-Huan Counties of Zhejiang Province.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Hepatitis B. 2021 Jul 27 [accessed 2021 Dec 12]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–6. doi:10.1016/j.vaccine.2011.12.116.
- Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi:10.1016/S2468-1253(18)30056-6.
- Jing W, Liu J, Liu M. Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med. 2020;14:21–29. doi:10.1007/s11684-020-0744-2.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–108. doi:10.1002/hep.27406.
- Kim YJ, Li P, Hong JM, Ryu KH, Nam E, Chang MS. A single center analysis of the positivity of hepatitis B antibody after neonatal vaccination program in Korea. J Korean Med Sci. 2017;32:810–16. doi:10.3346/jkms.2017.32.5.810.
- Alssamei FAA, Al-Sonboli NA, Alkumaim FA, Alsayaad NS, Al-Ahdal MS, Higazi TB, Elagib AA. Assessment of immunization to hepatitis B vaccine among children under five years in rural areas of Taiz, Yemen. Hepat Res Treat. 2017;2017:1–6. doi:10.1155/2017/2131627.
- Qiu Y, Ren JJ, Wu ZK, Shen LZ, Shan H, Dai XW, Li J, Liu Y, Ren W, Yao J, et al. Strategies for hepatitis B booster vaccination among children: an 8-year prospective cohort study. Hum Vaccines Immunother. 2020;16:2822–30. doi:10.1080/21645515.2020.1738169.
- Verbeke G, Molenberghs G. [Lecture notes in statistics] Linear mixed models in practice volume 126. New York: Springer-Verlag; 1997.
- Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis a vaccine-induced antibodies in adults. Vaccine. 2014;32:1507–13. doi:10.1016/j.vaccine.2013.10.088.
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–64. doi:10.1214/aos/1176344136.
- Wang ZZ, Gao YH, Wei L, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu X-E, Zhuang H, et al. Long-Term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccines Immunother. 2017;13:909–15. doi:10.1080/21645515.2016.1250990.
- Zanetti A, Desole MG, Romanò L, D’-Alessandro A, Conversano M, Ferrera G, Panico MG, Tomasi A, Zoppi G, Zuliani M, et al. Safety and immune response to a challenge dose of hepatitis B vaccine in healthy children primed 10 years earlier with hexavalent vaccines in a 3, 5, 11-month schedule: an open-label, controlled, multicentre trial in Italy. Vaccine. 2017;35:4034–40. doi:10.1016/j.vaccine.2017.05.047.
- Avdicova M, Crasta PD, Hardt K, Kovac M. Lasting immune memory against hepatitis B following challenge 10-11 years after primary vaccination with either three doses of hexavalent DTPa HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 a 11-12 months of age. Vaccine. 2015;33:2727–33. doi:10.1016/j.vaccine.2014.06.070.
- Van Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in adolescents 15-16 years of age vaccinated in infancy with three doses of hepatitis B vaccine. Vaccine. 2016;34:2745–49. doi:10.1016/j.vaccine.2016.04.013.
- Norrozi M, Azami A, Sarraf-Neduad A, Chitsaz H, Rahmati N. Persistence of anti-HBs antibody and immune memory to hepatitis B vaccine, 18 years after infantile vaccination in students of Tehran University. Res Med. 2016;40:36–41.
- Gottlieb S, Martin M, McLaughlin FX, Panaro RJ, Levine L, Edsall G. Long-Term immunity to diphtheria and tetanus: a mathematical model. Am J Epidemiol. 1967;85:207–19. doi:10.1093/oxfordjournals.aje.a120684.
- Honorati MC, Palareti A, Dolzani P, Busachi CA, Rizzoli R, Facchini A. A mathematical model predicting anti-hepatitis B virus surface antigen (HBs) decay after vaccination against hepatitis B. Clin Exp Immunol. 1999;116:121–26. doi:10.1046/j.1365-2249.1999.00866.x.
- Vellinga A, Van Damme P, Bruckers L, Weyler JJ, Molenberghs G, Meheus A. Modelling long-term persistence of hepatitis B antibodies after vaccination. J Med Virol. 1999;57:100–03. doi:10.1002/(SICI)1096-9071(199902)57:2<100:AID-JMV2>3.0.CO;2-M.
- Coursaget P, Yvonnet B, Gilks WR, Wang CC, Day NE, Chiron JP, et al. Scheduling of revaccination against hepatitis B virus. Lancet. 1991;337:1180–83.
- Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13:443–47. doi:10.1016/0264-410X(94)00010-K.
- Luo FJ, Dong CM, Shen YG, Huang CJ. Application of exponent curve model to study the hepatitis B DNA recombinant yeast derived vaccine antibody levels [abstract in English]. Chin J Epidemiol. 2004;25:805–07.
- Van Damme P, Leroux-Roels G, Suryakiran P, Folschweiller N, Van Der Meeren O. Persistence of antibodies 20 y after vaccination with a combined hepatitis a and B vaccine. Hum Vaccines Immunother. 2017;13:972–80. doi:10.1080/21645515.2016.1274473.
- National Bureau of Statistics. The seventh national census bulletin (No. 8). 2021 May 11[accessed 2021 Dec 12]. http://www.stats.gov.cn/ztjc/zdtjgz/zgrkpc/dqcrkpc/ggl/202105/t20210519_1817701.html
- Qiu Y, Ren JJ, Yao J. Healthy adult vaccination: an urgent need to prevent hepatitis B in China. Hum Vaccines Immunother. 2016;12:773–78. doi:10.1080/21645515.2015.1086519.
