ABSTRACT
While there are several SARS-CoV-2 vaccines currently available, additional options must be provided that are safe, effective, and affordable for the entire global population. We have developed a novel immune activating platform technology that will fill this need. This recombinant platform protein is produced in insect cells using baculoviral expression technology similar to what is currently used for several other approved vaccines as well as employed by myriad GMP facilities globally. Thus, infrastructure exists for rapid scale up following initial optimizations. Here we report initial results for a SARS-CoV-2 vaccine (OMN008) based on our platform technology. Unadjuvanted OMN008 vaccination resulted in robust antigenicity and neutralization. Additionally, OMN008 vaccination induced a specific CD8 T-cell response. All of these results taken together indicate OMN008 may be an excellent candidate to fill gaps left by the currently available vaccines. Further testing is necessary to fully optimize production; however, overall cost of production should remain low given the simple formulation of this recombinant platform.
Introduction
The threat of the SARS-CoV-2 pandemic is as present now as it was in its onset in late 2019 into 2020. At that time, we were ill equipped to deal with a novel pandemic respiratory virus and while we have made many strides with the development of numerous testing methods,Citation1,Citation2 and vaccines,Citation3 there are still many gaps that need to be filled. Several companies have developed vaccines that have been granted emergency use authorization (EUA) or FDA approval in the United States, i.e. Pfizer-BioNTech, Moderna and Johnson and Johnson.Citation3 While these vaccines have been rigorously tested and deployed to billions of people globally,Citation4 there are still many populations where they are not availableCitation4–6 and there remains a large population of individuals who are hesitant to vaccinate with new mRNA or viral based technologies.Citation7,Citation8
Currently available vaccines are not without risk as there have been reports of myocarditisCitation9,Citation10 and thrombosis.Citation11,Citation12 These issues emphasize the need for additional vaccines that may be developed cost effectively with more common and tested technologies. Several subunit-based vaccines are currently in preclinical or clinical development that address these issues.Citation13–16 In contrast to mRNA-based vaccines, subunit vaccines do not contain nucleic acid. Instead, a subunit vaccine is comprised of a protein subunit of the virus to which the immune response will be directed.Citation17,Citation18 This protein may be soluble or incorporated into lipid nanoparticles and administered with or without adjuvant to assist in stimulating an immune response.Citation17,Citation18 With well-established manufacturing processes, once optimized production scale up would be relatively easy.
We have developed a novel subunit vaccine platform technology, based on our proprietary cancer vaccine platform technology. Previous unpublished results of our cancer vaccine technology indicated a strong induction of cell-based immunity with increased intratumoral T-cells. Given reports that strong t-cell responses correlate with improved overall outcome following infectionCitation19 we modified our cancer vaccine to target SARS-CoV-2.
Results
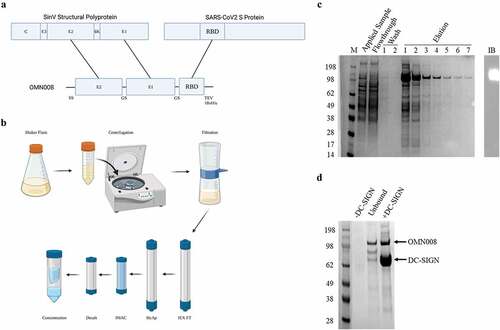
Design and production of VGP platform vaccine for SARS-CoV-2
The platform technology on which this novel vaccine is constructed consists of two domains. The N-terminal domain encodes an immune targeting module derived from Sindbis virus (SinV) glycoproteins (VGP) E1 and E2. These VGPs interacts with the DC specific intracellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) on the surface of dendritic cells.Citation20–23 The SinV VGP was chosen due to reports demonstrating that SinV is capable of inducing durable antitumor responses to model antigens encoded by the virus.Citation21–27 Further, lentivirus pseudotyped with SinV VGPs induced similar responses indicating that the VGPs are sufficient to induce immunity.Citation28 The C-terminus of the platform encodes the receptor binding domain (RBD) of the Sars-CoV-2 Spike protein (). Within the full-length recombinant protein each fragment, i.e. E2, E1, RBD are separated by a flexible GS hinge () to allow for independent folding of each domain. This recombinant protein construct was cloned into a baculoviral transfer vector and transfected into insect cells for virus production. Following amplification, the recombinant baculovirus was used to further infect insect cells at higher MOI for expression. The recombinant protein vaccine (OMN008) was found to be secreted into culture supernatant with a yield of approximately 5 mg protein per liter of cell culture. Following a multistep purification workflow (), an anti-his reactive band of approximately 113 kilodaltons (kDa) was enriched to approximately 80% purity (). Functional release testing was also performed demonstrating that the purified protein does interact with DC-SIGN (). Given yields, purity, and functional confirmation we were able to generate enough OMN008 to support a preclinical efficacy study.
Figure 1. Design and production of OMN008.
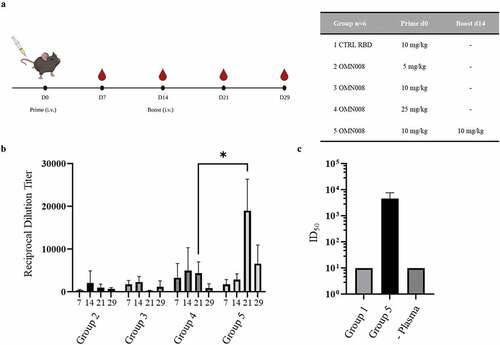
OMN008 vaccination induces neutralizing antibodies to SARS-CoV-2 spike protein
In addition to basic efficacy, we aimed to address questions of dose range and schedule through an initial study (). Briefly, five groups were tested. Group 1 received a single intravenous (IV) injection of SARS-CoV-2 S-RBD (without targeting VGP domain), Groups 2–4 received one IV dose of OMN008 at 5, 10, and 25 mg/kg, respectively. Finally group 5 received 2 IV doses of OMN008 at 10 mg/kg at day 0 and 14. All doses of either control or OMN008 protein were formulated in PBS and were administered without adjuvant. Plasma prepared from weekly blood collection was used for antigenicity and neutralization studies. Immunogenicity was determined using an indirect ELISA. Compared to control (RBD alone), which consistently generated an absorbance similar to plate background, all of the OMN008 vaccinated test groups generated detectable antibody titers. There was a clear dose response observed in groups 2–4 where markedly higher titers were detected in group 4 (25 mg/kg) as compared to the lower dose groups. Additionally, we identified that two doses of OMN008 resulted in significantly higher titers than even the highest single dose group. (). Next, we tested group 5 plasma for the ability to neutralize SARS-CoV-2 Spike protein pseudotyped lentivirus. In this assay, a lentivirus pseudotyped with SARS-CoV-2 S protein encoding a luciferase reporter is used to infect hACE2 expressing cells in the presence or absence of study generated plasma. Group 5 plasma at the 21 day time point was tested here only given that the highest antibody titers were generated in this group at this time point. Control group 1 (RBD alone) plasma at the same time point was used as a negative control. We found that plasma from group 5 effectively neutralized infection with the pseudotyped lentivirus as compared to group 1 plasma which exhibited minimal neutralization which was not affected by plasma dilution ().
Figure 2. OMN008 induces a strong antibody response.
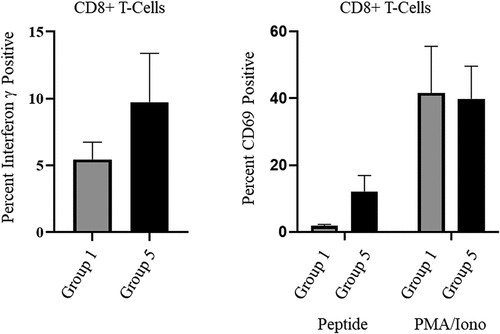
Vaccination with OMN008 induces specific CD8+ T-cell activation
Immune activation from our vaccine platform has been previously demonstrated to induce T-cell expansion within tumors (unpublished results). We aimed to show similar activation here with OMN008. Using peripheral blood mononuclear cells (PBMC) that were collected in parallel with plasma preparations, RBD specific T-cells from groups 5 and 1 at d21 were induced with a SARS-Cov-2 Spike RBD peptide pool. Following activation PBMCs were prepared for flow cytometric analysis. Compared with control group there is an increase in induction of CD69 and secretion of Interferon gamma (IFN-γ) in CD8 positive T-cells vaccinated with OMN008 ().
Figure 3. OMN008 vaccination induces functional CD8 T-cells.
Discussion
Here we have presented a novel subunit vaccine for SARS-CoV-2 based on our proprietary platform technology. This vaccine, OMN008, induces a potent neutralizing antibody response as well as a specific CD8 T-cell response to the viral Spike protein, both of which have been demonstrated to improve overall outcome following SARS-CoV-2 infection.Citation19,Citation29,Citation30 There was no observed subject body weight loss or lethargy at the highest dose level (data not shown) suggesting low overall toxicity, however our results also indicate that lower dosing is likely to be more effective with a multiple dose schedule. We aim to perform additional dosing studies to address the minimal effective dose using the two-dose schedule (day 0 and day 14) as well as addressing toxicity and route of administration in more detail in future preclinical studies. Initially, intravenous administration was chosen for this study given the preliminary success of a cancer therapy based on the same platform technology. Ideally, the route of administration for OMN008 will be intramuscular and we aim to compare this route of administration as well as subcutaneous to the intravenous route in a future study. One would expect to observe increased activity from the IM or SC routes versus IV given the decreased absorption kinetics and thus potential prolonged exposure of vaccine to antigen presenting cells in the tissue or draining lymph nodes. A critical study to demonstrate the utility of OMN008 as a SARS-CoV2 vaccine has yet to be completed at the time of this report. We plan to use a Syrian hamster model to demonstrate the efficacy of OMN008 with respect to protection from infection as well as severe disease. This model has been chosen given the pathological similarities between hamster and human as well as its established role in investigation of therapeutics and vaccines for SARS-Cov-2.
In a time where there is a great need for additional vaccines to prevent SARS-CoV-2 infection and more specifically severe disease and mortality, there are a large number of candidate vaccines in clinical trials that may fill that need. Three notable candidates have similar technology to ours however we believe we have a specific advantage. Candidates from Sanofi, Novavax and Clover have progressed into late stage trials or are awaiting regulatory approval. Sanofi (Vidprevtyn) and Novavax (NVX-COV2373) have developed subunit vaccines produced using the baculoviral expression system in a similar way to OMN008. Clover (SCB-2019) has developed a vaccine using their trimer-tag platform which ensures the mammalian cell expressed protein adopts a homo-trimeric conformation which they suggest will increase immunogenicity. All three of these vaccine candidates are formulated with adjuvant. While expression in a mammalian system may improve the speed from transfection to expression, the added complexities of using a mammalian system would likely increase the overall cost of production. Additionally, our VGP based platform is designed to trimerize as well which would confer similar immunogenicity advantages.
Overall, we believe that this vaccine will fill a need of additional safe and effective vaccines amid the continuing pandemic and has several advantages over these more advanced candidates as well as established mRNA based vaccines. It has recently been reported that while overall safety and efficacy is good, the side effects of mRNA-based vaccines, which can be severe, are due at least in part to triggering of interferon from the mRNA itself.Citation31,Citation32 Given the efficacy observed here in the absence of adjuvant, we expect that OMN008 will have a lower overall toxicity and less side effects than other available vaccines although the latter must also be tested in more thorough pharmacodynamic studies. Production of OMN008 is through established baculoviral expression techniques which have been used to generate several currently approved vaccines including Flublok (Influenza) and Provenge (Sipuleucel-T, Prostate Cancer).Citation33,Citation34 We are currently performing further optimization of expression and purification yield to decrease the overall cost of manufacturing OMN008. While it may not ultimately be as easy to manufacture as mRNA-based vaccines, OMN008 could nonetheless be modified quickly to address new variant challenges as necessary once the manufacturing process has been fully optimized. It has recently been reported that while overall safety and efficacy is good, the side effects of mRNA-based vaccines, which can be severe, are due at least in part to triggering of interferon from the mRNA itself.Citation31,Citation32 Given the efficacy observed here in the absence of adjuvant, we expect that OMN008 will have a lower overall toxicity and less side effects than other available vaccines although the latter must also be tested in more thorough pharmacodynamic studies.Further, we believe that the mechanism through which OMN008 engages the Dendritic cells directly, generating targeted immunity, will drive a more durable immune response that the current mRNA vaccine standards. We aim to test durability of response in subsequent studies.
Acknowledgments
We would like to acknowledge the enormous technical advice and support given by Manon Cox, Ph.D., MBA and Alexander Serganov, Ph.D., Associate Professor, Department of Biochemistry and Molecular Pharmacology, NYU School of Medicine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Guglielmi G. The explosion of new coronavirus tests that could help to end the pandemic. Nature. 2020;583:1–6. doi:10.1038/d41586-020-02140-8.
- Habli Z, Saleh S, Zaraket H, Khraiche ML. COVID-19 in-vitro diagnostics: state-of-the-art and challenges for rapid, scalable, and high-accuracy screening. Front Bioeng Biotechnol. 2021;8.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–36. doi:10.1038/s41577-021-00592-1.
- Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, Giattino C, Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–53. doi:10.1038/s41562-021-01122-8.
- Asundi A, O’-Leary C, Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29(7):1036–39. doi:10.1016/j.chom.2021.06.007.
- Peacocke EF, Heupink LF, Frønsdal K, Dahl EH, Chola L. Global access to COVID-19 vaccines: a scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open. 2021;11(9):49505. doi:10.1136/bmjopen-2021-049505.
- Pan F, Zhao H, Nicholas S, Maitland E, Liu R, Hou Q. Parents’ decisions to vaccinate children against COVID-19: a scoping review. Vaccines. 2021;9(12):1476. doi:10.3390/vaccines9121476.
- Ochieng C, Anand S, Mutwiri G, Szafron M, Alphonsus K. Factors associated with COVID-19 vaccine hesitancy among visible minority groups from a global context: a scoping review. Vaccines. 2021;9(12):1445. doi:10.3390/vaccines9121445.
- Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–22.
- Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–39. doi:10.1056/NEJMoa2110737.
- Ostrowski SR, Søgaard OS, Tolstrup M, Stærke NB, Lundgren J, Østergaard L, Hvas A-M. Inflammation and platelet activation after COVID-19 vaccines - possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front Immunol. 2021;12:779453. doi:10.3389/fimmu.2021.779453.
- Perry RJ, Tamborska A, Singh B, Craven B, Marigold R, Arthur-Farraj P, Yeo JM, Zhang L, Hassan-Smith G, Jones M, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398:1147–56. doi:10.1016/S0140-6736(21)01608-1.
- Hsieh S-M, Liu M-C, Chen Y-H, Lee W-S, Hwang S-J, Cheng S-H, W-C K, Hwang K-P, Wang N-C, Lee Y-L, et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9:1396–406. doi:10.1016/S2213-2600(21)00402-1.
- Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, Li P, Liang P, Han HH, Liang J, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–94. doi:10.1016/S0140-6736(21)00241-5.
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–32. doi:10.1056/NEJMoa2026920.
- Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, Harper WL, Duncanson DM, McArthur MA, Florescu DF, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2021;386(6):531–43. doi:10.1056/NEJMoa2116185.
- Martínez-Flores D, Zepeda-Cervantes J, Cruz-Reséndiz A, Aguirre-Sampieri S, Sampieri A, Vaca L. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.701501.
- Pollet J, Chen W-H, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev. 2021;170:71–82. doi:10.1016/j.addr.2021.01.001.
- Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M, et al. SARS-CoV-2-Specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62. doi:10.1038/s41586-020-2550-z.
- Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for Alphaviruses and distinguish between mosquito cell-and mammalian cell-derived viruses. J Virol. 2003;77:12022–32. doi:10.1128/JVI.77.22.12022-12032.2003.
- Granot T, Yamanashi Y, Meruelo D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol Ther. 2014;22:112–22. doi:10.1038/mt.2013.215.
- Gardner JP, Frolov I, Perri S, Ji Y, MacKichan ML, zur Megede J, Chen M, Belli BA, Driver DA, Sherrill S, et al. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J Virol. 2000;74:11849–57. doi:10.1128/JVI.74.24.11849-11857.2000.
- Tseng J-C, Levin B, Hirano T, Yee H, Pampeno C, Meruelo D. In vivo antitumor activity of Sindbis viral vectors. J Natl Cancer Inst. 2002;94:1790–802. doi:10.1093/jnci/94.23.1790.
- Tseng JC, Levin B, Hurtado A, Yee H, De Castro IP, Jimenez M, Shamamian P, Jin R, Novick RP, Pellicer A, et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotechnol. 2004;22:70–77. doi:10.1038/nbt917.
- Yang L, Yang H, Rideout K, Cho T, Joo K Il, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–34. doi:10.1038/nbt1390.
- Hurtado A, Tseng J-C, Meruelo D. Gene therapy that safely targets and kills tumor cells throughout the body. Rejuvenation Res. 2006;9:36–44. doi:10.1089/rej.2006.9.36.
- Tseng J, Hurtado A, Yee H, Levin B, Boivin C, Benet M, Blank SV, Pellicer A, Meruelo D. Using Sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models using Sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res. 2004;64(18):6684–92. doi:10.1158/0008-5472.CAN-04-1924.
- Morizono K, Ku A, Xie Y, Harui A, Kung SKP, Roth MD, Lee B, Chen ISY. Redirecting lentiviral vectors pseudotyped with Sindbis virus-derived envelope proteins to DC-SIGN by modification of N-linked glycans of envelope proteins. J Virol. 2010;84:6923–34. doi:10.1128/JVI.00435-10.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. doi:10.1038/s41591-021-01377-8.
- Bertoletti A, Le Bert N, Qui M, Tan AT. SARS-CoV-2-Specific T cells in infection and vaccination. Cell Mol Immunol. 2021;18:2307–12. doi:10.1038/s41423-021-00743-3.
- Sprent J, King C. COVID-19 vaccine side effects: the positives about feeling bad. Sci Immunol. 2021;6. doi:10.1126/sciimmunol.abj9256.
- Cagigi A, Loré K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines. 2021;9:61. doi:10.3390/vaccines9010061.
- Richards KA, Moritzky S, Shannon I, Fitzgerald T, Yang H, Branche A, Topham DJ, Treanor JJ, Nayak J, Sant AJ. Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. NPJ Vaccines. 2020;5:77. doi:10.1038/s41541-020-00227-x.
- Cox MMJ. Recombinant protein vaccines produced in insect cells. Vaccine. 2012;30:1759–66. doi:10.1016/j.vaccine.2012.01.016.
