ABSTRACT
Peptide vaccine are a type of immunotherapy that are synthesized according to the amino acid sequence of known or predicted tumor antigen epitopes. They are safe and well tolerated and have shown exciting results in gynecologic oncology. However, no peptide vaccine has yet been licensed in this field. This review examines peptide vaccine clinical trials in gynecology registered on ClinicalTrials.gov through January 1, 2022, analyzes the global progress and current achievements of peptide vaccines in gynecology, and explores the efforts focused on devising new methods to boost immunotherapeutic outcomes, including the use of adjuvants, multi-epitope vaccines, combinations of helper T cell epitopes, personalized peptide vaccines, synthetic long peptides, new peptide delivery, and combination therapy.
Introduction
Compared to traditional surgery, radiotherapy, and chemotherapy, immunotherapyCitation1,Citation2 is a promising new frontier for the treatment of cancer. Immunotherapies include a broad range of therapeutics, including cell therapy, such as chimeric antigen receptor T-cell immunotherapy and T-cell receptor engineered T cells; immune checkpoint inhibitor therapy (CPI) such as cytotoxic T lymphocyte associated antigen 4, programmed cell death protein-1 (PD-1), and programmed death-ligand 1 (PD-L1) antibodies; and cancer vaccines,Citation3 such as peptide vaccines and dendritic cell (DC) vaccines.
Peptide vaccinesCitation4–6 aim to provoke an immune response against tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs) and are created using purified, recombinant or synthetically engineered epitopes to elicit a host immune response with T cell activation. DC vaccines can introduce tumor antigens, such as peptides, proteins, or tumor lysates, to DCs in vitro, which are the most powerful antigen-presenting cells (APCs). Then DCs be injected into patients, and process the antigen and express antigen information on the cell surfaceCitation7 thus initiating the immune response. We will also discuss peptide pulsed-DC vaccines in this review.
A number of peptide vaccine clinical trials are underway for the secondary or tertiary treatment of cancers (for treatment after cancer detection). Peptide vaccines have shown durable clinical responses in a wide variety of tumor types and offer compelling advantages such as high specificity, few side effects, stable chemical properties, and lack of carcinogenic potential.Citation8–10 Because most tumor drugs are macromolecules, and penetrating solid tumors with macromolecules can be difficult, another advantage of peptide vaccines is their structural characterization. Nevertheless, peptide vaccines still have limited therapeutic activity in inducing tumor regression and no vaccine has been licensed for treatment. However, several new developments may change this.
There is a lack of comprehensive data on peptide vaccines in gynecologic oncology, especially regarding the progress of new peptide vaccine clinical trials and recent achievements in this specialized field. Therefore, we analyzed registered clinical trials from Clinical Trials.gov to highlight the current state of peptide vaccines in gynecologic oncology, aiming to provide useful information and fresh ideas for academic organizations, policy makers and pharmaceutical enterprises.
Materials and methods
We searched the ClinicalTrials.gov website using the keywords “peptide” AND “vaccine” in the “Other terms” field, restricting the study start time to before 1 January 2022.
The inclusion criteria for the trials were as follows: (1) a clinical trial must be intended for a peptide vaccine. Peptide-pulsed DC vaccines were also included; (2) trials involving gynecology were deemed eligible; and (3) trials were started before 1 January 2022. The exclusion criteria for the trials were as follows: (1) ambiguous conditions defined as gynecological disease, and (2) no evidence to confirm that the vaccine is a peptide. A peptide vaccine that prevents an infection that progresses to cancer is considered an infectious disease vaccine and not a cancer vaccine, and such vaccines were not included.
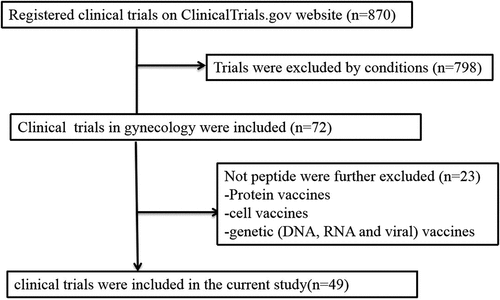
We screened 870 clinical trials, excluded 798 trials based on the condition and 23 trials based on the type of vaccine, and finally included 49 clinical trials. The data-retrieval process is illustrated in .
The following information was collected for analysis: trial identifier, trial title, conditions, start date, trial phases, trial status, location, adjuvant, epitopes, drug target, peptide length, peptide delivery, and combination therapy. Numbers and percentages were used for categorical variables according to descriptive analyses. Microsoft Office Excel 2007 was used for data processing and analysis.
This study was exempt from institutional review board review because it only involved publicly available data without personal information or human subjects.
Results
Basic characteristics
Clinical trials examining peptide vaccines in gynecology were mainly conducted in ovarian cancer (OC) (10, 20.4%), cervical cancer (5, 10.2%), endometrial cancer (1, 2.0%), and multiple cancers (33, 67.3%), which included not only gynecological cancers, such as fallopian tube cancer, vulva, and vaginal cancer, but also other cancers. Most clinical trials of peptide vaccines were concentrated in the United States (36, 73.5%) and Europe (10, 20.4%).
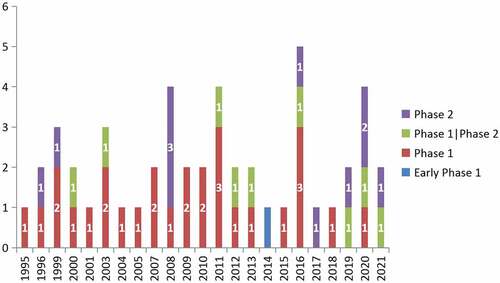
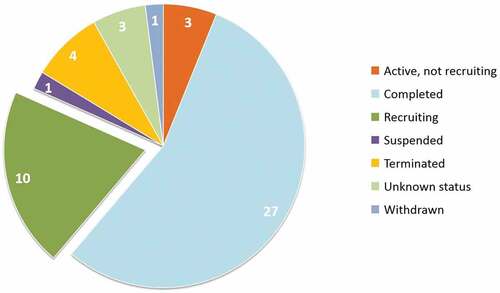
Twenty-eight (57.1%), 9 (18.4%), and 11 (22.4%) trials were phase 1, phase 1/2, and phase 2 trials, respectively. Only 1 trial (2.0%) was in early phase 1.More than half of the peptide vaccine clinical trials (27, 55.1%) were complete; 10 (20.4%) trials were still in the recruiting stage. Four trials had been terminated, including those with vaccines targeting p53 (NCT00001827), EGFRvIII (NCT00023634), MAGE-A1+HER2+FBP (NCT00373217), and FRα (NCT02978222). One trial was withdrawn, which was testing a vaccine targeting p53 (NCT00001827).
and illustrate the numbers of registered clinical trials grouped by study phase and status.
Adjuvants
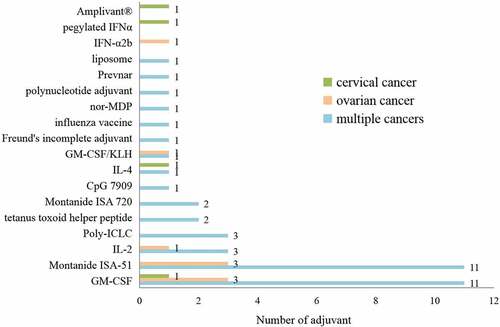
Adjuvants are extensively used to enhance the strength and longevity of immune responses,Citation11,Citation12 significantly influenced the kinetics, magnitude, and quality of responses elicited by vaccination. Therefore, many clinical trials are exploring new adjuvants for peptide vaccines to improve their efficacy. Thirty-five (71.4%) clinical trials in gynecological oncology used adjuvants, and 17 (34.7%) used more than one adjuvant. Six (12.2%) trials using adjuvants were for the treatment of OC, 3 (6.1%) were for the treatment of cervical cancer, and 26 (53.0%) were for the treatment of multiple cancers.
The most common adjuvants were GM-CSF, which stimulates APC maturation, and Montanide ISA-51. There were also trials examining toll-like receptor (TLR) agonists as adjuvants, including poly-ICLC, a synthetic, stabilized, double-stranded RNA viral mimic capable of activating multiple innate immune receptors, and the TLR-2 ligand Amplivant® (NCT02821494). Cytokines, including interleukins and interferons, such as IL-2, IL-4, IFN-α2b, and pegylated IFNα, were also used in trials of peptide vaccines to boost immunity.
As some toxins produced by pathogens,Citation8 such as tetanus toxoid or staphylococcus aureus enterotoxin B, can increase infection and tissue damage by inducing effects such as hemorrhaging or inflammation, two clinical trials (NCT00091273 and NCT00373217) added a tetanus toxoid helper peptide to boost the immune response, and one trial (NCT00478452) added influenza vaccine and Prevnar pneumococcal vaccine to augment T helper (Th) cell immunity.
For example, vaccination with NY-ESO-1 overlapping peptides (OLP) (NCT00616941) for treatment of multiple cancers, including ovarian cancer and fallopian tube cancer. NY-ESO-1 OLP alone were unable to detect NY-ESO-1 specific antibody and CD8+ T cells in patients. But these antibody and CD8+ T cells were found in 6 of 13 (46%) and 8 of 13 (62%) patients, respectively, after vaccination with OLP+Montanide, and in 10 of 11 (91%) and 10 of 11 (91%) patients after vaccination with OLP+Montanide+Poly-ICLC.Citation13 Emulsification of peptides in Montanide was required for the expansion of high-avidity NY-ESO-1 specific CD4+ T-cell precursors, and Poly-ICLC further accelerated and enhanced immune responses by significantly enhancing CD4+ Th1 responses.Citation14
On the other side, as traditional adjuvants and carriers are associated with poor efficacy, peptide vaccine designs with built-in adjuvants have been proposed, including DPX-0907 (NCT01095848), DPX-Survivac (NCT01416038), PGV001(NCT02721043), NeoVax(NCT04024878), PDS0101 (NCT04287868 and NCT04580771), OSE2101 (NCT04713514), and ELI-002 (NCT04853017).
ELI-002 is an amphiphile (AMP) KRAS investigational therapeutic vaccine containing AMP mKRAS peptide antigens and ELI-004 is an AMP-modified immune-stimulatory oligonucleotide adjuvant.
shows the studies examining adjuvants in peptide vaccine clinical trials.
Multi-Epitope peptide vaccines
Epitopes are special chemical groups in antigen molecules that determine antigen specificity and are also known as antigenic determinants. Incorporating multiple peptide epitopes into peptide vaccines can increase the chance of activating multiple T cell populations and avoid tumor immune escape by overcoming loss or changes of epitopes during tumor progression.Citation6,Citation15 Multi-epitope vaccines also have unique advantages in inducing cellular immunity.
As CD4+ Th cells play an important role in the stimulation and maintenance of antitumor immunity, adding Th cell epitopes to peptide vaccines is another effective method to improve the immunogenicity of the vaccine and enhance the T cell response. For example, the pan HLA DR-binding epitope (PADRE) can be fused to peptide sequences to deliver robust help signals in vivo. DPX-0907 (NCT01095848), DPX-Survivac (NCT01416038), TPIV200 (NCT02764333 and NCT02978222), and OSE2101 (NCT04713514) are multi-epitope vaccines. DPX-0907 consists of seven tumor-specific HLA-A2-restricted peptides, a universal Th peptide, a polynucleotide adjuvant, a liposome, and Montanide ISA 51 VG, based on a novel non-emulsion depot-forming vaccine platform called DepoVax™. In a phase I clinical study, the DPX-0907 vaccine was shown to be safe and well tolerated. Further, breast and OC patients exhibited an 89% immune response rate to DPX-0907.Citation16
DPX- SurvivacCitation17 contains Survivin-derived peptides and a Th peptide epitope, based on DepoVax™. A trial of DPX-Survivac with intermittent low dose cyclophosphamide for treatment of recurrent, advanced platinum-sensitive and resistant ovarian cancer showed an overall survival rate of 44.9% at 23.8 months of follow up and a median overall survival of 19.9 months.
There are currently two trials in OC examining TPIV200, a multi-epitope FRα vaccine. TPIV200 combined with the PD-L1 inhibitor durvalumab in advanced, platinum-resistant OC is safe, with a related grade 3 toxicity rate of 18.5%. Increased T cell responses to the majority of peptides were observed in all patients at 6 weeks, and the median overall survival was 21 months, with evidence of benefit from post-immunotherapy regimens.Citation18
A trial for OSE2101, an emulsion of a peptide suspension in Montanide ISA 51 adjuvant containing 10 synthetically manufactured peptides, including a p53 peptide, is still in the recruitment stage.
Examples of multi-epitope peptide vaccines and the addition of Th cell epitopes are shown in .
Table 1. Multi-Epitope peptide vaccines and the addition of Th cell epitopes.
Synthetic long peptide (SLP) vaccines
Synthetic long peptides, which are generally more than 20 amino acids in length require processing and thus favor presentation by APCs, such as DCs, allowing for broader T-cell responses by provoking both CD4+ T cells and CD8+ T cells.Citation19 We identified eight (16.3%) registered SLP vaccine clinical trials in gynecological cancers, including those examining the P53-SLP vaccine (NCT00844506 and NCT01639885) in OC, ISA101/ISA101b (NCT02128126) in cervical cancer, and PGV001 (NCT02721043) in multiple cancers.
ISA101/ISA101b is a novel therapeutic SLP vaccine targeting HPV16 E6/E7. Treatment with the ISA101 vaccine and carboplatin/paclitaxel chemotherapy in 77 patients with advanced, recurrent, or metastatic cervical cancer caused tumor regression in 43% of 72 evaluable patients. Patients mounted type 1 T cell responses to the vaccine across all doses, and it provided a much stronger therapeutic immunity than chemotherapy alone.Citation20
Personalized peptide vaccines
A personalized peptide vaccineCitation21 can consist of synthetic peptides or genes encoding neoantigens designed to target specific epitopes. TSAs are only expressed in tumor cells, not in normal cells; thus, using TSAs in vaccines avoids the issue of host immune tolerance and autoimmunity. NeoantigensCitation22–24 are TSAs formed by non-synonymous mutations in the tumor genome. A trial examining NeoVax (NCT04024878), a personalized neoantigen long-peptide vaccine formulated using TLR-3 and the MDA5 agonist poly-ICLC, for the treatment of OC in combination with the PD-1 blocking antibody nivolumab is still in the recruitment stage.
Combination therapy
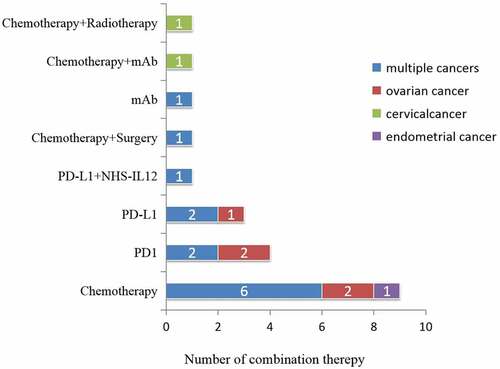
Overall, there were 21 (42.9%) peptide vaccine clinical trials examining combination treatment with other therapies, including chemotherapy, surgery, radiotherapy, and other immunotherapies, such as immune-checkpoint blockade using monoclonal antibodies specific for PD-1 and PD-L1.
Combination therapy was mainly used for multiple cancers (8, 16.3%), OC (3, 6.1%), and cervical cancer (1, 2.0%). Initially, vaccines were combined with chemotherapy, although more recent trials included combination with immune CPI therapies.
The treatment of gynecologic cancers requires appropriate countermeasures. For example, ISA101 can only partially control tumor progression. Systemic delivery of chemotherapy prior to vaccination reduced genital tumor growth at the time of vaccination, and decreased infiltration of tumor-associated macrophages, as a result, significantly increased survival.Citation25
The number of trials examining combination therapies is shown in .
Time trends
The numbers of clinical trials of multi-epitope peptide vaccines, peptide vaccines with Th cell epitopes, personalized peptide vaccines, SLP vaccines, and combination therapies have increased over time. Although the number of trials of peptide-pulsed DC vaccines did not increase between 2011 and 2021, a new attempt was made to identify DC subsets for vaccination. DECENDO (NCT04212377) is a combination of myeloid DCs (myDCs) and plasmacytoid DCs (pDCs) loaded with MUC1 and survivin inhibitors and tumor lysate for immunotherapy in patients with metastatic endometrial cancer. No results available yet.
Details of the clinical trials over time are shown in .
Table 2. Details of the clinical trials over time.
Drug target
The most common targets of peptide vaccines in clinical trials for gynecologic cancers were HPV16 E6/E7 (7, 14.3%), p53 (5, 10.2%), human epidermal growth factor receptor 2 (HER2) (4, 8.1%), New York esophageal squamous cell carcinoma 1 (NY-ESO-1) (4, 8.1%), and FRα (4, 8.1%).
HPV16 E6 and E7 are the main drivers of HPV carcinogenesis, and sustained expression of the viral E6 and E7 oncogenes causes the growth of HPV-positive cancer cells. E6 and E7 are attractive therapeutic targets because their inhibition rapidly induces senescence in HPV-positive cancer cells.Citation26 Examples are ISA101/ISA101b and PDS0101.
PDS0101 consists of HLA-unrestricted HPV16 peptides and the immune-activating cationic lipid R-DOTAP as an adjuvant. R-DOTAP is based on the Versamune® platform, which assembles lipids spontaneously into nanoparticles in a water-based medium and makes them the proper size to mimic an artificial virus. R-DOTAP can act as an immune cell stimulant on immature DCs, thus enhancing immune function. There is a current ongoing clinical trial of PDS0101 (NCT04287868) with two immune modulators for advanced HPV-associated malignancies, which plans to recruit 56 patients. One modulator is Bintrafusp alfa (M7824), a first-in-class bifunctional fusion protein designed as a CPI that blocks PD-L1 and recruits transforming growth factor-β receptor type II to the tumor microenvironment (TME). The other is NHS-interleukin-12 (NHS-IL12), a tumor-targeting immunocytokine that enhances the inflammatory Th1 response by recruiting IL-12 to the TME.Citation2
In the interim data from PDS biotechnology, as of 1 March 2021, the study had enrolled a challenging patient population: 96% had failed both chemotherapy and radiation treatment, and 56% had also failed CPI therapy. Twenty-five patients received triple combination therapy of PDS0101, Bintrafusp alfa, and NHS-IL12, including 3 (12%) patients with vaginal/vulvar cancer and 10 (40%) with cervical cancer, anal cancer, or head and neck cancer. This combination therapy achieved an 83% objective response rate among HPV16-positive CPI-naive patients (5/6) and a 58% tumor reduction rate among HPV16 CPI-refractory patients (7/12). It also showed promising durability in HPV16-positive CPI-naive and CPI-refractory patients. Further, 89% (16/18) of HPV16-positive patients with CPI-naive and CPI-refractory advanced disease were alive at a median 8 months of follow-up. The results in seven HPV16-negative patients suggest a critical role for PDS0101-induced HPV16-specific CD8+ T cells in promoting tumor reduction and survival.
There is also a clinical trial examining PDS0101 for the treatment of locally advanced cervical cancer in combination with chemoradiotherapy (NCT04580771). MD Anderson’s independent study suggests that patients with HPV-specific T cells may have improved clinical outcomes.
The tumor suppressor gene TP53 is the most commonly mutated gene in tumors, with TP53 mutations occurring in 50% of spontaneous cancers in humans.Citation27 The p53 protein encoded by TP53 plays a key role in cell cycle regulation, DNA repair, and apoptosis induction. p53 SLP vaccine (NCT01639885) induced p53-specific T-cell responses in patients with platinum-resistant ovarian cancer ovarian cancer and the combination of gemcitabine and Pegintron stimulated higher frequencies of circulating proliferating CD4+ and CD8+ T-cells but not regulatory T-cells.Citation28
HER2 is a member of the epidermal growth factor receptor family and has tyrosine kinase activity. Carcinogenic activation of HER2 can be caused by overexpression of HER2 protein, gene amplification, or gene mutation and occurs in a variety of malignancies.
NY-ESO-1Citation29 is a cancer-testis antigen with aberrant re-expression in many cancers. It is a good target for cancer immunotherapy because it elicits spontaneous humoral and cellular immune responses and has a restricted expression pattern. An example is NY-ESO-1 overlapping peptides for epithelial ovarian, fallopian tube, or primary peritoneal cancer.
FRα is a folate-binding protein located on the cell membrane. It is overexpressed in many malignant tumors but has limited expression in normal tissues. FRα has a high affinity for non-physiological folic acid and participates in the signal transduction and survival signaling pathway in cancer cells. An example is TPIV200 for ovarian cancer.
Details of drug targets in peptide vaccine clinical trials in gynecological cancers are shown in .
Table 3. Details of drug targets in peptide vaccine clinical trials in gynecological cancers.
Adverse event
The most common adverse events (AEs) associated with this immunotherapeutic paradigm were minor reactions at the injection site, nausea, fatigue, and some AEs were associated with drug combinations or adjuvants. All trials reported either no severe side effects or severe side effects in a small number of participants.
Details of adverse events to peptide vaccines are shown in .
Table 4. Adverse events to peptide vaccines.
Discussion
Thus far, cancer vaccines have had limited clinical success; only one DC vaccine, Provenge (Sipuleucel-T), was licensed for prostatic cancer in 2010, ushering in a new era of cancer immunotherapy. Currently, most peptide vaccines in gynecology are still at an exploratory stage. Many efforts have been made to strengthen immunotherapy, as discussed below.
Adjuvant
Previous studies have shown that the structure of peptide vaccines leads to rapid degradation in vivo, which limits the ability of antigen cross-presentation, and low immunogenicity makes the vaccine unable to induce a persistent and strong T cell immune response. The choice of adjuvant is critical for vaccine efficacy, and there is no consensus on the optimal adjuvant for a therapeutic vaccine. The primary role of adjuvants in peptide vaccines is to ensure sufficient costimulation by APCs that prime T cells, help overcome obstacles, and exert immune-enhancing effects. Additional adjuvants, such as built-in adjuvants, are in development. A promising example is PDS0101 for multiple cancers including cervical cancer.
Multi-Epitope vaccines and SLP vaccines
TAAs often display limited antigenicity, are expressed in both normal tissues and tumor tissues, although at different levels, cause immune tolerance, and can provoke an autoimmune response. Improving the recognition of TAA-specific cytotoxic T lymphocyte (CTL) and helper T lymphocyte (HTL) epitopes is the key to TAA-derived peptide vaccines. The development of multi-epitope, SLP peptide vaccines, as well as adding the Th epitope, are additional solutions to improving the efficacy of cancer vaccines.
Peptide vaccine may confer some advantage over vaccines consisting of larger protein sequences or whole inactivated virus as they are smaller and may elicit a more focused immune response toward critical epitopes.Citation30 However, due to the major histocompatibility complex polymorphism, a vaccine raised against a single-epitope peptide may fail to induce an adequate CTL and antibody response because of the lack of an appropriate HTL for the immune response. Targeting only one epitope should be a matter of concern in heterogeneous antigen expression and the outgrowth of antigen-negative tumor cells; multi-epitope vaccines are a solution to this problem as long as sufficient tumor antigens are identified.
CD4+ T cells, especially Th1 cells, can help prime CD8+ T cells, recruit them to the tumor site, and establish memory, and CD8-independent antitumor functions may be exerted, justifying CD4-inducing approaches in peptide vaccines. The addition of Th cell epitopes to peptide vaccines contributes to this phenomenon.
Therefore, the development of new safe, effective, more widely adapted multi-epitope vaccines containing Th cell epitopes with stronger antigenicity will have broad applicational prospects in immunotherapy. DPX- Survivac and TPIV200 are promising peptide vaccines for OC. Both vaccines have received orphan drug designation status from the U.S. Food and Drug Administration in the OC indication. More clinical trials of DPX- Survivac with CPI or other immunomodulators are ongoing. And although TPIV200 in combination with durvalumab induced robust FRα-specific T cell responses in majority of patients, this study failed to meet its prespecified clinical efficacy endpoint. Even in the setting of a therapy targeting T cell exhaustion, development of vaccine-specific T cell responses was still not sufficient to drive tumor regression.Citation18 More researches are needed.
Short-length peptides, which are approximately 8–11 amino acids in length, are based on MHC class I (MHC-I) molecule presentation and CD8+ T cell recognition. A peptide containing 12–25 amino acids is presented to MHC-II molecules and recognized by CD4+ T cells. Extending short peptides into long peptides can overcome immune tolerance and induce both CD4+ and CD8+ T cell responses.Citation31 Moreover, binding motifs for multiple MHC alleles may be present, which can be further increased by using multiple SLPs.Citation19 Conversely, injection of a short peptide vaccine based on minimal peptide epitopes has the risk of binding exogenously to MHC-expressing cells that do not express costimulatory molecules and, as a result, do not efficiently stimulate T cells. NY-ESO-1 OLP and p53 SLP vaccine are promising peptide vaccine against multiple cancers and OC, respectively.
Personalized peptide vaccines
Personalized peptide vaccines have the potential advantages of maximizing therapeutic specificity, diminishing central immune tolerance, and overcoming immune tolerance. Neoantigens, also known as shared neoantigens, may be prevalent in some patients and tumor types. However, most neoantigens are unique to individual patients.Citation32 With continuous technological progress in genetic and bioinformatic analyses, such as single-cell sequencing and high-throughput sequencing technologies, designing peptide vaccines targeting neoantigens as personalized cancer immunotherapy for individual patients are no longer difficult.Citation33
Although neoantigens are promising targets for immunotherapy, the time required to synthesize personalized peptide vaccines remains a major weakness because of the possibility of missing a crucial period of cancer treatment. Additional factors, such as the influence of neoantigen-specific T cell immunity, complexity, and continuous evolution of the TME, which can attenuate the anticancer immune response of vaccines, must also be considered.
NeoVax is promising against ovarian cancer. Although this clinical trial is still in recruiting stage, another trial of NeoVax for melanoma was completed, and showed a good result by inducing long-term persistence of T cell responses.Citation34
Vaccine delivery
Homing a peptide vaccine to the right location is critical to creating a powerful immune response. Peptide vaccines can be delivered directly to the tumor through various routes, including intravenous, intramuscular, subcutaneous and intradermal.
It is also possible to use DCs, the most potent T cell stimulators in vivo, for delivery to improve the immunogenicity of peptide vaccines. This involves harvesting peripheral blood mononuclear cells, peripheral blood stem cells or autologous DCs, and expanding them in vitro and pulsing them with peptides. The final step is transferring the activated DCs back to the patient to activate the immune response. Most clinical trials use monocyte-derived DCs (moDCs) because of the difficulty in obtaining a sufficient number of more related and available DC subsets for vaccination. However, moDCs do not have all the costimulatory molecular and antigenic cross-presentation mechanisms available for other DC subsets; therefore, they may not be the best source for DC-based immunotherapy. DC subsets have distinct functional characteristics,Citation35,Citation36 and CD1c+ myDC- and pDC-based immunotherapies show encouraging immunological and clinical outcomes.Citation37 There have been studies in this field that have made more attempts, e.g., the DECENDO trial.
Another approach uses nanoparticlesCitation38 as delivery vehicles, including liposomes, amphiphilic compounds, and self-assembled nanoparticles, for example, PDS0101 which delivers the immune nanoparticle liposomal HPV-16 E6/E7 multipeptide. And in animal experiment of ISA101, therapy was further refined by using a optimized nanoparticle-conjugated ISA101, even larger end-stage genital-TC-1 tumors responded, with much higher survival benefit than the traditional ISA101 ‘liquid’ vaccine.Citation25
Delivery systems still have many problems, such as high cost, complex preparation processes, and significant differences in delivery efficiency for different antigens. Sending the vaccine to the right location to mount a robust, antigen-specific immune response is a barrier limiting the clinical application of peptide vaccines. As DC subsets prove more advantageous, the combination of peptides with DC subsets, such as conventional type 1 DCs, is also promising. However, the results in these areas are still limited, and further studies are warranted.
Combination therapy
Peptide vaccines may not be the optimal approach for all cancer treatments; however, they can improve the curative effect of surgery, chemotherapy, radiotherapy, or other immunotherapies.Citation39
Clinical researchers have discovered that certain chemotherapeutic agents used in the right sequence can elicit immunogenic tumor cell death to promote antitumor immunity, anti-angiogenic strategies can enhance T-cell infiltration, and immune checkpoint blockades, which can help rescue exhausted T cells play a prominent synergistic effect by targeting the TME for the treatment of malignant tumors.Citation40
Scientists of this field are trying to make the perfect combination therapy. For example, in addition to combining ISA101 with chemotherapy,Citation20 the combination of ISA101 and nivolumab was also explored. Enrichment in gene sets associated with interferon-γ response and immune infiltration strongly predicted response to therapy. A randomized trial is ongoing to test this strategy and to further explore correlates of immune response with combined ISA101 and nivolumab, versus nivolumab alone.Citation41
Limitations
Several limitations need to be acknowledged. A potential selection bias may exist, and a small amount of data may be missed when manually searching all peptide vaccine clinical trials in gynecology on ClinicalTrials.gov. Further, the database may not contain all clinical trials or lack public data for some trials that explore new ways to boost therapeutic outcomes. Therefore, caution should be taken when interpreting this review. However, these limitations should not affect the overall analysis results.
Conclusions
This review described the progress of peptide vaccine clinical trials in gynecological oncology and analyzed the current achievements. Although much data on peptide vaccine clinical trials have not been published, peptide vaccines were generally safe and well tolerated. From time trends, the potential of peptide vaccines for gynecologic cancer can be seen through the development of new adjuvants, multi-epitope vaccines containing Th cell epitopes, and SPL vaccines. Further studies should also focus on overcoming the complex and time-consuming work of personalized peptide vaccines and finding more efficient ways for vaccine delivery, such as nanoparticles or DC subtypes. Larger obstacles remain that have hampered the clinical use of vaccines, despite their great potential and bright future, and these new developments in peptide vaccines are ongoing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nishio H, Iwata T, Aoki D. Current status of cancer immunotherapy for gynecologic malignancies. Jpn J Clin Oncol. 2021;51(2):167–12. doi:10.1093/jjco/hyaa214.
- Smalley Rumfield C, Pellom ST, Morillon YM II, Schlom J, Jochems C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J Immunother Cancer. 2020;8(1):e000612. doi:10.1136/jitc-2020-000612.
- Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78. doi:10.1038/s41568-021-00346-0.
- Schneble E, Clifton GT, Hale DF, Peoples GE. Peptide-Based cancer vaccine strategies and clinical results. Methods Mol Biol. 2016;1403:797–817.
- Aranda F, Vacchelli E, Eggermont A, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: peptide vaccines in cancer therapy. Oncoimmunology. 2013;2(12):e26621. doi:10.4161/onci.26621.
- Bezu L, Kepp O, Cerrato G, Pol J, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: peptide-based vaccines in anticancer therapy. Oncoimmunology. 2018;7(12):e1511506. doi:10.1080/2162402X.2018.1511506.
- Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med. 2017;214(10):3105–22. doi:10.1084/jem.20170335.
- Malonis RJ, Lai JR, Vergnolle O. Peptide-Based vaccines: current progress and future challenges. Chem Rev. 2020;120(6):3210–29. doi:10.1021/acs.chemrev.9b00472.
- Parmiani G, Russo V, Maccalli C, Parolini D, Rizzo N, Maio M. Peptide-Based vaccines for cancer therapy. Hum Vaccines Immunother. 2014;10(11):3175–78. doi:10.4161/hv.29418.
- Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int Immunol. 2016;28(7):319–28. doi:10.1093/intimm/dxw027.
- Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37(24):3167–78. doi:10.1016/j.vaccine.2019.04.055.
- Firdaus FZ, Skwarczynski M, Toth I. Developments in vaccine adjuvants. Methods Mol Biol. 2022;2412:145–78.
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18(23):6497–508. doi:10.1158/1078-0432.CCR-12-2189.
- Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, Ferran L, Spriggs D, Salazar AM, Gnjatic S, et al. Effect of Montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res. 2013;1(5):340–50. doi:10.1158/2326-6066.CIR-13-0089.
- Pol J, Bloy N, Buqué A, Eggermont A, Cremer I, Sautès-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial Watch: peptide-based anticancer vaccines. Oncoimmunology. 2015;4(4):e974411. doi:10.4161/2162402X.2014.974411.
- Karkada M, Berinstein NL, Mansour M. Therapeutic vaccines and cancer: focus on DPX-0907. Biologics. 2014;8:27–38.
- Berinstein NL, Karkada M, Oza AM, Odunsi K, Villella JA, Nemunaitis JJ, Morse MA, Pejovic T, Bentley J, Buyse M, et al. Survivin-Targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4(8):e1026529. doi:10.1080/2162402X.2015.1026529.
- Zamarin D, Walderich S, Holland A, Zhou Q, Iasonos AE, Torrisi JM, Merghoub T, Chesebrough LF, Mcdonnell AS, Gallagher JM, et al. Safety, immunogenicity, and clinical efficacy of durvalumab in combination with folate receptor alpha vaccine TPIV200 in patients with advanced ovarian cancer: a phase II trial. J Immunother Cancer. 2020;8(1):e000829. doi:10.1136/jitc-2020-000829.
- Calvo Tardón M, Allard M, Dutoit V, Dietrich PY, Walker PR. Peptides as cancer vaccines. Curr Opin Pharmacol. 2019;47:20–26. doi:10.1016/j.coph.2019.01.007.
- Melief CJM, Welters MJP, Vergote I, Kroep JR, Kenter GG, Ottevanger PB, Tjalma WAA, Denys H, van Poelgeest MIE, Nijman HW. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med. 2020;12(535):eaaz8235. doi:10.1126/scitranslmed.aaz8235.
- Aldous AR, Dong JZ. Personalized neoantigen vaccines: a new approach to cancer immunotherapy. Bioorg Med Chem. 2018;26(10):2842–49. doi:10.1016/j.bmc.2017.10.021.
- Maserat E, Nasiri Hooshmand M. Requirements of integrated computational approach for developing personalized cancer vaccines. Hum Vaccines Immunother. 2021;17(12):1–2.
- Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–82. doi:10.1038/nri.2017.131.
- Pan RY, Chung WH, Chu MT, Chen SJ, Chen HC, Zheng L, Hung S-I. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:4325874. doi:10.1155/2018/4325874.
- Domingos-Pereira S, Galliverti G, Hanahan D, Nardelli-Haefliger D. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer. 2019;7:122.
- Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26(2):158–68. doi:10.1016/j.tim.2017.07.007.
- Levine AJ. Targeting the P53 protein for cancer therapies: the translational impact of P53 research. Cancer Res. 2022;82(3):362–64. doi:10.1158/0008-5472.CAN-21-2709.
- Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Nijman HW, van Poelgeest MI, van Erkel AR, Smit VTHBM, Daemen TAHH, van der Hoeven JJM, et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget. 2015;6(31):32228–43. doi:10.18632/oncotarget.4772.
- Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, Decock J. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi:10.3389/fimmu.2018.00947.
- Ghaffari-Nazari H, Tavakkol-Afshari J, Jaafari MR, Tahaghoghi-Hajghorbani S, Masoumi E, Jalali SA, Khodarahmi R, Khodarahmi R. Improving multi-epitope long peptide vaccine potency by using a strategy that enhances CD4+ T help in BALB/c Mice. PloS One. 2015;10(11):e0142563. doi:10.1371/journal.pone.0142563.
- Chen X, Yang J, Wang L, Liu B. Personalized neoantigen vaccination with synthetic long peptides: recent advances and future perspectives. Theranostics. 2020;10(13):6011–23. doi:10.7150/thno.38742.
- Ma M, Liu J, Jin S, Wang L. Development of tumour peptide vaccines: from universalization to personalization. Scand J Immunol. 2020;91(6):e12875. doi:10.1111/sji.12875.
- Sunita SA, Singh Y, Shukla P, Shukla P. Computational tools for modern vaccine development. Hum Vaccin Immunother. 2020;16(3):723–35. doi:10.1080/21645515.2019.1670035.
- Hu Z, Leet DE, Allesøe RL, Oliveira G, Li S, Luoma AM, Liu J, Forman J, Huang T, Iorgulescu JB. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. 2021;27(3):515–25. doi:10.1038/s41591-020-01206-4.
- Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol. 2019;348:123–78.
- Cancel JC, Crozat K, Dalod M, Mattiuz R. Are conventional Type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. 2019;10:9. doi:10.3389/fimmu.2019.00009.
- Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, Teijeira A, Kandalaft LE, Romero P, Coukos G, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7(1):109. doi:10.1186/s40425-019-0580-6.
- Wen R, Umeano AC, Kou Y, Xu J, Farooqi AA. Nanoparticle systems for cancer vaccine. Nanomedicine (London, England). 2019;14(5):627–48. doi:10.2217/nnm-2018-0147.
- Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum Vaccines Immunother. 2017;13(11):2561–74. doi:10.1080/21645515.2017.1364322.
- Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66. doi:10.1158/0008-5472.CAN-18-3962.
- Sousa LG, Rajapakshe K, Rodriguez Canales J, Chin RL, Feng L, Wang Q. ISA101 and nivolumab for HPV-16(+) cancer: updated clinical efficacy and immune correlates of response. J Immunother Cancer. 2022;10.