ABSTRACT
Although safe, rotavirus vaccines have been associated with increased intussusception risk. In Brazil, after the oral human rotavirus vaccine (OHRV) introduction in the childhood immunization, in 2006, increased intussusception risk was identified after the second OHRV dose, whereas in other countries, higher risk was associated to the first vaccine dose. It was hypothesized that the concomitant use of oral poliovirus vaccine (OPV) in Brazil might explain this difference. In 2012, the inactivated polio vaccine (IPV) was adopted in the first two doses of Brazilian childhood immunization schedule, creating an opportunity to study the subject. Our objective was analyzing the impact of polio vaccines on rotavirus-associated intussusception. We used surveillance data on intussusception in infants living in São Paulo State. Two periods were considered: an OPV-period (March 2006 to June 2012) and an IPV-period (October 2012 to December 2017). The period from June to September 2012 were considered as transition. Self-controlled case series analysis with event-dependent exposure was performed, considering two risk periods (7 and 21 days post-vaccination). We identified 325 intussusception cases in infants reported to the surveillance systems during the study period. The statistical analysis included 221 cases that occurred within 60 days after vaccination. Overall, a higher intussusception risk was observed in the first week after vaccination for both the first (Relative Incidence [RI] = 4.3, 95%CI 2.8–6.5, p < .001) and second vaccine doses (RI = 4.2, 95%CI 2.7–6.4; p < .001). There were no statistically significant differences in intussusception risk according to the rotavirus vaccine dose and the polio vaccine (OPV or IPV) administered concomitantly.
Introduction
Intussusception is a serious condition, in which one intestinal segment invaginates inside another one. It is the main cause of intestinal obstruction in young children, with average annual incidence ranging from 34 cases per 100,000 infants (in African region) to 90 (in Western Pacific region), and the incidence peak occurring in children aged from 29 weeks, in Africa, to 70 weeks, in the Western Pacific region.Citation1 Intussusception cause is usually unknown, but increased intestinal motility during viral infection may play a role.Citation2,Citation3 Rotavirus vaccination have been associated to intussusception since 1999, when the first licensed rotavirus vaccine (RotaShield®) was withdrawn from market due to increased risk of intussusception following vaccination.Citation3 Two of the currently available rotavirus vaccines (human monovalent and human-bovine pentavalent vaccines), although safer, have also been both associated with increased risk of intussusception, particularly within seven days after the first vaccine dose.Citation4
In Brazil, the oral human rotavirus vaccine (OHRV) was introduced in the National Immunization Program (NIP) childhood schedule in March 2006. Rotavirus vaccine coverage increased rapidly (>80% in the second year), reaching the goal (>90% for the second dose) in 2011.Citation5 A previous study, conducted in Sao Paulo state, reported 40% reduction in the annual hospitalizations of under-five children for diarrhea, in the first five years of the vaccination program.Citation6 A case-series and case-control study, conducted in Brazil and Mexico, identified an increased risk of intussusception after the first OHRV dose in Mexico (OR = 5.8 , 95%CI, 2.6–13), but after the second OHRV dose in Brazil (OR = 1.9 , 95%CI, 1.1–3.4).Citation5 During this study period, the oral poliomyelitis vaccine (OPV), administered concomitantly with OHRV, was used in Brazil, whereas the inactivated polio vaccine (IPV) was adopted in Mexico. Lower intestinal OHRV replication has been reported when the vaccine was administered concomitantly with OPV. So, it was hypothesized that the non-increase in the intussusception risk after the first OHRV dose in Brazil could be due to OPV co-administration.Citation6 Up to 2012, the Brazilian childhood immunization schedule included five OPV doses (at 2, 4, 6 and 15 months and 5 years of age). Two mass vaccination campaigns (National Immunization Days), during which OPV was administered to all under-5 children, independently of their vaccination status, were conducted each year. In August 2012, aiming to reduce cases of vaccine-associated paralytic polio (VAPP), Brazil adopted an IPV-OPV sequential schedule, with IPV in the first two doses (at 2 and 4 months of age), establishing an opportunity to evaluate the role of polio vaccines (OPV and IPV) on rotavirus vaccine-associated intussusception.Citation7
The objective of this study was to evaluate the impact of polio vaccines (OPV and IPV) administered concomitantly with rotavirus vaccine on the occurrence of intussusception in infants in Sao Paulo state, at the Southeast of Brazil, based on surveillance data.
Methods
Study design
We conducted a retrospective analysis of confirmed cases of intussusception in children aged from six weeks to 11 months and 29 days, reported to the surveillance system, in Sao Paulo state, Brazil, from March 2006 to December 2017. The OPV to IPV replacement occurred in August 2012, establishing two periods: the OPV period (March 2006 to June 2012) and the IPV period (October 2012 to December 2017). The period from July to September 2012 was defined as transition. A three-month transition period was considered enough since the Ministry of Health coordinates and centralizes vaccines’ purchase and distribution in Brazil. Furthermore, most childhood vaccination is performed at the public Unified Health System (Sistema Unico de Saude, SUS) facilities, which follow standing orders for administering vaccines according to the NIP recommendations. During the study period, both polio and rotavirus vaccines had high coverage in the country. Polio vaccine third dose coverage was lower than 95% in just three years of the time series (94.1%, in 2012; 92.1%, in 2014, and 92.6%, in 2015). Rotavirus vaccine coverage is lower than polio, probably due to the age restriction for its administration. Rotavirus vaccine second dose coverage was 56.2% in the introduction year (2006), increasing to >80% in the second year and achieving >90%, in 2011. There was a reduction (64.9%) in 2012, but it stabilized in >90% in the following years of the time series.Citation7,Citation8
Data sources
Data on intussusception cases were collected from the Surveillance System databases. Adverse events following immunization (AEFI) reporting is mandatory in Brazil since 2005, as part of a national passive surveillance system of vaccine safety (National AEFI Surveillance System), founded in 1984. AEFI may be notified by any health-care worker. Symptoms, signs, vaccines administered, vaccine batch, dates of vaccination and AEFI onset, diagnostic findings, healthcare given, and outcome are registered in the system. All reports are reviewed by the managers physicians and reports of serious AEFI (any of the following: hospitalization, prolongation of hospitalization, life-threatening condition, permanent disability, congenital abnormality, or birth defect and death) are followed-up by the system staff to get more information. In March 2006, concomitantly with rotavirus vaccine introduction, a passive hospital-based sentinel surveillance of intussusception was established in Sao Paulo state. The sentinel hospital staff were trained to identify, investigate, and report intussusception cases. Additionally, from August 2008 to January 2010, a multi-center study of OHRV safety was conducted. This active surveillance study was supported by GAVI, PAHO, and CDC, as part of the rotavirus vaccines’ post-market surveillance.Citation9 In Sao Paulo state, all three surveillance systems were managed by the Immunization Division of the Epidemiological Surveillance Center “Prof Alexandre Vranjac” of the Sao Paulo State Health Department (DI/CVE/CCD/SES-SP) and adopted the same reporting form and the same definitions for intussusception cases (the Brighton Collaborative Group’s definition).Citation10
Data on live births in Sao Paulo state was retrieved from the Unified Health System Department of Informatics (DATASUS), freely available online, and was used to estimate the annual rates of intussusception.
Data collection and study variables
Intussusception cases reported from March 2006 to December 2008 were obtained from a database previously built by EGF, for another study.Citation6 This initial database was adapted by the first author, according to the variables of interest for this study, and was completed with the new intussusception cases reported to the Surveillance Systems from 2009 to 2017.
All reported cases included in this study were classified as level 1 (the highest level of diagnostic certainty) of the Brighton Collaborative Group’s definitions for intussusception and occurred within 30 days of rotavirus vaccination (according to the definitions of vaccine-associated intussusception at the time of data collection for this study).Citation11,Citation12 The authors (CCMR and EGF) had access to all three surveillance systems’ nominal records, and so were able to detect and exclude the record duplicates.
Intussusception cases were described according to demographic and clinical characteristics and history of rotavirus vaccination (age at vaccination, dose, time elapsed from vaccination to intussusception first symptoms). Data on rotavirus vaccination were consistently available for all reported cases, but polio vaccination data was frequently unavailable. OPV or IPV was assumed by the authors according to the period at which the case occurred (OPV period, from March 2006 to June 2012, and IPV period, from October 2012 to December 2017).
Statistical analysis
Demographic data, symptoms, surgical rates, clinical outcomes, and length of hospitalization were described for all reported intussusception cases. The annual reporting rates of intussusception were estimated considering all intussusception cases and the number of live births in Sao Paulo state each year.
Intussusception cases without history of rotavirus vaccination were excluded from the statistical analyses. Cases with a history of rotavirus vaccination were distributed by year of occurrence and age (in weeks). Cases that occurred in the transition period (July to September 2012) were excluded. Cases that occurred after the third vaccine dose were also excluded, since, in Brazil, the pentavalent rotavirus vaccine is only used in the private system, which has low coverage.
To evaluate and compare rotavirus vaccine-associated intussusception rates, according to rotavirus vaccine dose and polio vaccine (OPV or IPV) administered concomitantly, a self-controlled case series (SCCS) analysis was conducted. In this method, each intussusception case acts as its own control for time-invariant confounders. An assumption of the standard SCCS model is that events do not influence subsequent exposures. Considering that the occurrence of intussusception after rotavirus vaccination contraindicates subsequent rotavirus vaccine doses, we decided to use the SCCS model with event-dependent exposure, a variant of SCCS model. The standard SCCS model was also conducted and presented as supplementary material, to allow the models’ comparison.Citation13 Only cases occurring within 60 days after rotavirus vaccination were considered. Two risk periods were analyzed: up to 7 days and up to 21 days after each rotavirus vaccine dose. A control period (31 to 60 days after each rotavirus vaccine dose) was considered to estimate the relative incidence (RI) in each risk period. The natural intussusception incidence rates in infants without rotavirus vaccination, from a previous study,Citation14 was used to control for the variable intussusception risk according to infants age.
The analyses were conducted using R statistical software, version 4.0.0. The SCCS model with event-dependent exposure was performed through the eventdepenexp function of the SCCS library. Asymptotic chi-square test was used to compare intussusception relative incidence in each risk period (7- and 21-day), after each rotavirus vaccine dose, in the historical periods (OPV or IPV).
The study was approved by the Research Ethics Committee of the University of Sao Paulo School of Medicine (CAAE 78883717.70000.0065).
Results
From 2006 to 2017, 325 intussusception cases in children aged from 6 weeks to 11 months and 29 days living in Sao Paulo State were reported to the Surveillance Systems.
Among the 325 confirmed intussusception cases, 296 (91.1%) had a history of rotavirus vaccination. Of these latter, 164 cases occurred within 30 days after vaccination and might be considered associated to the rotavirus vaccine. presents the epidemiological and clinical characteristics of all 325 confirmed intussusception cases, the 296 cases with a history of rotavirus vaccination, and the 164 cases that occurred within 30 days after rotavirus vaccination. Among all 325 cases, vomiting was the most frequently reported symptom, described in 92.3% of 209 records with this information, followed by “strawberry jelly” feces, in 80.8% of 182 records. Surgery was the main diagnostic and treatment method, described in 85.7% of 197 cases with this information. Case-fatality rate was 3.6%, considering 222 cases for which the outcome was reported. Most of the 164 intussusception cases with history of rotavirus vaccine in the previous 30 days were associated with the second vaccine dose (108 cases or 65.9%). Three cases occurred after the third rotavirus vaccine dose. Three infants received the first vaccine dose after the maximum recommended age.
Table 1. Characteristics of confirmed cases of intussusception in infants reported to the surveillance systems. Sao Paulo State, Brazil, 2006 to 2017.
The annual reporting rates of intussusception in infants in the study period is presented in Supplementary Table S1, and the age distribution of cases, in Supplementary Figure S1.
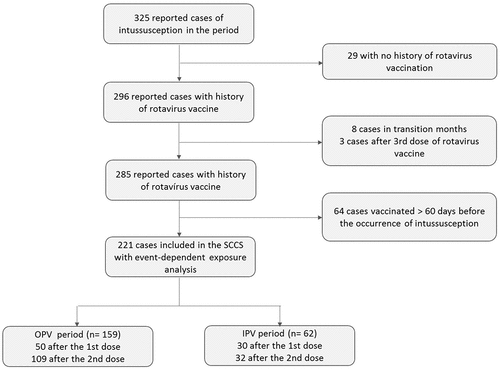
To analyze the role of polio vaccines on intussusception temporally associated to rotavirus vaccine, 11 of the 296 cases with history of rotavirus vaccination were excluded (8 cases that occurred in the transition period and do not have information on polio vaccine, and 3 cases that occurred after the 3rd pentavalent rotavirus vaccine dose). Of the remaining 285 cases with history of rotavirus vaccination, 221 intussusception cases that occurred within the first 60 days after rotavirus vaccination were included in the SCCS analyses (): 159 cases occurred in the OPV period (50 after the 1st rotavirus vaccine dose and 109 after the 2nd dose) and 62 cases were reported in the IPV period (30 cases occurring after the 1st dose and 32 after the 2nd dose).
Figure 1. Flowchart of the analyses of 325 confirmed cases of intussusception in children aged from 6 weeks to 11 months and 29 days of age, reported to surveillance system. Sao Paulo State, Brazil, March 2006 – Dec 2017.
displays the results of the SCCS with event-dependent exposure analyses of intussusception, according to the risk period (7 and 21 days after rotavirus vaccination) relative to the control period (31–60 days after rotavirus vaccination), rotavirus vaccine dose, and study period (OPV-, IPV- and the entire period). At first, each historic period (OPV and IPV) was analyzed independently. In the 7-day risk period, higher relative incidence of intussusception was found for both the first and second rotavirus vaccine dose in both OPV and IPV periods (), and the statistical analyses showed no statistical differences between the OPV and IPV periods (chi-square test p = .606). A similar pattern was observed in the 21-day risk period, however the RIs were lower than in the 7-day risk period (), without statistical differences between the OPV- and the IPV-periods (p = .811). As expected, when the entire study period was analyzed, the relative incidence (RI) of intussusception was increased in both risk periods and for both rotavirus vaccine doses, although higher RI was found for the 7-day risk period as compared to the 21-day risk period ().
Table 2. Relative incidence (RI) of intussusception and respective 95% confidence Iinterval (95%IC), according to risk period (7- and 21-days post-vaccination), rotavirus vaccine dose, and study period (OPV-, IPV- or the entire study period) in the SCCS with event-dependent exposure model. Sao Paulo State, Brazil. March 2006 to December 2017.
Results of the standard SCCS analysis are presented in the Supplementary Table S2. In the 7-day risk period, we observed statistically significant higher RI after the first rotavirus vaccine dose in both OPV (RI = 5.0, 95%CI 2.9–8.5, p < .001), and IPV period (RI = 4.6, 95%CI 2.0–10.4, p < .001), but highest RI after the 2nd dose in both OPV (RI = 8.8, 95%CI 5.5–14.4, p < .001) and IPV periods (RI = 10.9, 95%CI 4.0–29.7, p < .001). The statistical analyses showed no statistical differences between the OPV and IPV periods (chi-square test p = .558). Similar results were observed in the 21-day risk period, statistically significant higher RI after the first dose both OPV (RI = 1.8, 95%CI 1.1–3.0, p = .016), and IPV period (RI = 2.3, 95%CI 1.2–4.6, p < .001), but highest RI after the 2nd dose both in the OPV (RI = 5.1, 95%CI 3.3–7.8, p < .001) and IPV period (RI = 8.9, 95%CI 3.8–20.8, p < .001), without statistical differences between the OPV- and the IPV-periods (p = .75) (Supplementary Table S2).
Discussion
Our study is the first, according to our knowledge, to assess the role of oral and inactivated polio vaccines in the intussusception temporarily associated to the rotavirus vaccine. Both SCCS with event-dependent exposure and standard SCCS models found that both 7- and 21-day risk periods following vaccination were associated with higher risk of intussusception, for both rotavirus vaccine doses, independently of which polio vaccine (OPV or IPV) was administered concomitantly. Higher RI were found in the first week after both vaccine doses, coinciding with the peak of intestinal replication of the rotavirus vaccine virus. As expected, the RIs after the 2nd dose were higher with the standard SCCS model as compared to the SCCS with event-dependent exposure, due to bias introduced in the standard model that does not take into account the contraindication of the 2nd rotavirus vaccine dose for children that had intussusception after the first vaccine dose. “The direction of bias resulting in the standard SCCS model is often predictable. If occurrence of an event decreases the probability of subsequent exposures, then the RI will be biased upwards. This is because exposures will tend to occur prior to events, thus inducing bias in the direction of a positive association”.Citation13 Both the SCCS with event-dependent exposure analysis and the standard SCCs models did not find statistically significant differences in the relative incidence of intussusception according to the polio vaccines (OPV or IPV) used. The higher RI observed within seven days after both rotavirus vaccine doses is different from the previously reported in a case-series with SCCS analyses, in which an increased risk of intussusception was seen in the first week after the first rotavirus vaccine dose in Mexico and after the second dose in Brazil. Active hospital-based surveillance was the source of intussusception cases in this previous study, whereas routine passive surveillance data were used in our study.
Our results (increased RI of intussusception following both doses of rotavirus vaccine) are different from the results of studies conducted in high-income countries, where IPV is used for routine childhood immunization, and where an increased risk of intussusception has been reported within seven days after the first vaccine dose.Citation15 However, an Australian self-controlled case-series study, based on the national hospital databases supplemented by active surveillance, also observed an increased risk of intussusception within the seven-day window after both the first and second vaccine doses of both OHRV and RV5, even though the highest relative incidence of intussusception was found after the first RV5 dose.Citation16
Besides the study carried out in Brazil and Mexico, there is another ecological evidence of a possible role of OPV on rotavirus vaccine-related intussusception. A self-controlled case series study, based on active surveillance data, in seven African countries where rotavirus vaccine and OPV are simultaneously administered, did not find an increased risk of intussusception in the first week after OHRV administration, as compared to the background risk (RI = .25, 95%CI < .001–1.16, after the first OHRV dose and RI = .76 95%CI .16–1.87, after the second dose).Citation17 However, in these countries, the vaccines are administered to younger children (at 6 and 10 weeks of age), which could have also contributed to the low intussusception incidence, since this condition is rare in the first two months of life.Citation17
There is also biological plausibility for the hypothesis of modification of vaccine-related intussusception risk by OPV co-administration. Intestinal replication is critical for immunogenicity and effectiveness of live attenuated oral vaccines. When concurrently administered, these vaccines may interfere with each other.Citation18 OHRV and OPV coadministration do not impair OPV immunogenicity but, decreases the immune response to OHRV, particularly after the first dose, suggesting lower OHRV replication.Citation17,Citation19 This negative effect of OPV on OHRV immune response is overcome after completing the vaccination, with immune response at the end of the schedule similar to administration of OHRV alone.Citation18 Lower rotavirus antigen shedding in feces was demonstrated in infants that received rotavirus vaccine concomitantly with OPV, as compared to infants who received IPV concomitantly, also suggesting OPV interference with rotavirus vaccine replication when both vaccines are co-administered.Citation20 These evidence support the hypothesis of lower risk of vaccine-associated intussusception when the rotavirus vaccine is co-administered with OPV.
So, we expected that the replacement of OPV by IPV in the routine childhood immunization might shift the predominant occurrence of intussusception cases toward to the first dose. The results of our analysis do not allow such conclusion, since we found no significant differences in the relative incidence of intussusception after the first or second rotavirus vaccine dose, in the IPV or OPV-periods, both for the 7-day or 21-day risk periods after vaccination. However, we found an increased relative incidence of intussusception in both 7-day and 21-day period following rotavirus vaccination, for both the first and second doses. The role of OPV vs. IPV in intussusception related to rotavirus vaccine needs further careful evaluation.
Many factors are associated with intussusception, such as infectious agents, genetic predisposition, and intestinal tract abnormalities (intestinal polyps, lipomas), in addition to neuronal intestinal dysplasia, celiac disease, or Crohn’s disease.Citation2,Citation21 Although most intussusception cases did not have an identified cause, the increased intestinal motility during a viral infection has been considered a possible associated factor.Citation22 A retrospective, self-controlled case series study based on data from two North American databases suggested temporal association of rotavirus gastroenteritis with intussusception, with a incidence rate ratios (IRR) of 79.6 (95%CI 38.6–164.4) in the first seven days after gastroenteritis and 25.5 (95%CI 13.2–49.2) for the first 21 days.Citation21 However, it is important to emphasize that the study did not assess other risk factors that could be associated with intussusception.Citation21 The increased intussusception risk within the first week after rotavirus vaccination does not necessarily result in an increased incidence of intussusception, when considering the whole first year of life. This is probably due to a reduction in the intussusception risk in older infants associated to decreased rates of rotavirus infection in vaccinees.Citation23,Citation24
AEFI passive surveillance systems are usually more sensitive to severe cases and to cases occurring in the first months of a new vaccination program implementation, when both the health-care workers and population are more alert to adverse events.Citation25 In this study, the sentinel surveillance of intussusception and the multi-center study on rotavirus vaccine safety, while active (until 2010), contributed to increase the intussusception reporting in the OPV period. The changes in intussusception surveillance over the years contributed to the gradual reduction of the reporting rates. It is also important to mention difficulties faced by health-care workers in emergency rooms with limited resources to perform a correct diagnosis of intussusception, since many services do not have a medical surgeon and complementary exams, such as ultrasonography. Therefore, our surveillance is probably more sensitive to severe cases with late diagnosis.
The Brazilian AEFI Information System has been successful in identifying more reactogenic vaccines or batches, as well as previously unknown AEFIs.Citation25 Unlike the experiences of other countries of the European Community and North America, this system has an exclusive link with the NIP/MoH, without explicit relation to the regulatory agency (ANVISA). Although there are several strategies to increase the AEFI surveillance systems sensitivity, it is important to highlight that passive and active systems have low specificity, since they identify adverse events temporally associated with the vaccine, without necessarily having a causal link.Citation25,Citation26
Our access to data exclusively through surveillance records is a great limit of our work, given the heterogeneity in the pattern of data recording over time, and the important role played by the sentinel surveillance (2006 to 2010), which contributed with many cases. Passive surveillance systems, particularly the AEFI surveillance are more likely to capture intussusception cases that occur proximal to vaccination, given that this is the period when surveillance is indicated. This could bias the results away from the null. This seems more likely to occur when passive surveillance is not supplemented by other surveillance systems, as during the IPV period.
As there was no available Brazilian data on intussusception rates without rotavirus vaccination, we used US rates to adjust age distribution, which is another limitation since the underlying rates could differ by region and population. In addition, as intussusception is a rare event, slight changes in absolute numbers may increase or decrease trend toward the first or second dose without necessarily reflecting actual changes in trends.Citation27 As strengths, we gathered data from the most populous state in Brazil and with a wide distribution of primary, secondary, and tertiary health-care services, as well as emergency rooms, providing good representation of our findings. A similar study with nationwide data, or longer surveillance time after the IPV introduction in childhood immunization may give more solid evidence on the role of polio vaccines in intussusception associated to rotavirus vaccines.
As conclusion, the SCCS model with event-dependent exposure found an increased risk of intussusception temporally associated with rotavirus vaccine in the first 7 and 21 days after both rotavirus vaccine doses, regardless of OPV or IPV concomitant use. The analyzes performed did not show differences in the relative incidence of intussusception, according to rotavirus vaccine dose or polio vaccine administered (OPV or IPV), in the two risk periods considered (7-day and 21-day after vaccination).
Supplemental Material
Download JPEG Image (22.2 KB)Acknowledgement
The authors would like to thank Ana Paula Sayuri Sato, Vivian Iida Avelino da Silva, José Leopoldo Ferreira, Silvia Cristina Martini and Evandro Luis Linhari Rodrigues for their suggestions. We also thank the healthcare workers of the Divisao de Imunizacao, Centro de Vigilancia Epidemiologica Prof. Alexandre Vranjac, Coordenadoria de Controle de Doenças da Secretaria de Estado da Saude de Sao Paulo, Brazil
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2063594.
Additional information
Funding
References
- Clark AD, Hasso-Agopsowicz M, Kraus MW, Stockdale LK, Sanderson CFB, Parashar UD, Tate JE. Update on the global epidemiology of intussusception: a systematic review of incidence rates, age distributions and case-fatality ratios among children aged <5 years, before the introduction of rotavirus vaccination. Int J Epidemiol. 2019;48:1316–7. doi:10.1093/ije/dyz028.
- Yen C, Healy K, Tate JE, Parashar UD, Bines J, Neuzil K, Santosham M, Steele AD. Rotavirus vaccination and intussusception – science, surveillance, and safety: a review of evidence and recommendations for future research priorities in low and middle income countries. Hum Vaccines Immunother [Internet]. 2016;12:2580–89. doi:10.1080/21645515.2016.1197452.
- Rha B, Tate JE, Weintraub E, Haber P, Yen C, Patel M, Cortese MM, DeStefano F, Parashar UD. Intussusception following rotavirus vaccination: an updated review of the available evidence. Expert Rev Vaccines. 2014;13:1339–48. doi:10.1586/14760584.2014.942223.
- Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: an evidence based meta-analysis of cohort and case-control studies. Vaccine. 2017;35:4276–86. doi:10.1016/j.vaccine.2017.05.064.
- Flannery B, Samad S, de Moraes JC, Tate JE, Danovaro-Holliday MC, de Oliveira LH, Rainey JJ. Uptake of oral rotavirus vaccine and timeliness of routine immunization in Brazil’s national immunization program. Vaccine. 2013;31:1523–28. doi:10.1016/j.vaccine.2013.01.004.
- Fernandes EG, Sato HK, Leshem E, Flannery B, de Konstantyner TCR, de SM Veras MA, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations in São Paulo State, Brazil. Vaccine. 2014;32:3402–08.
- Sartori AMC, Vicentine MP, Gryninger LCF, de Soárez PC, Novaes HMD. Polio inactivated vaccine costs into routine childhood immunization in Brazil. Rev Saude Publica. 2015;49:8. doi:10.1590/S0034-8910.2015049005492.
- Domingues CMAS, Maranhão AGK, Teixeira AM, Fantinato FFS, Domingues RAS. The Brazilian national immunization program: 46 years of achievements and challenges. Cad Saude Publica. 2020;36(Suppl 2):e00222919. doi:10.1590/0102-311x00222919.
- Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi:10.1056/NEJMoa1012952.
- São Paulo (Estado) Secretaria da Saúde . Norma Técnica do Programa de Imunização Centro de Vigilância Epidemiológica, (São Paulo: Centro de Vigilância Epidemiológica); 2016.
- Tapiainen T, Bär G, Bonhoeffer J, Heininger U. Evaluation of the Brighton collaboration case definition of acute intussusception during active surveillance. Vaccine. 2006;24:1483–87. doi:10.1016/j.vaccine.2004.11.082.
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde. Manual de Vigilância Epidemiológica de Eventos Adversos Pós-Vacinação Departamento de Vigilância das Doenças Transmissíveis, (Brasília); 2014.
- Farrington P, Whitaker H, Weldeselassie YG. Self-Controlled case series studio a modelling guide with R. 1a ed. Boca Raton: Chapman and Hall/CRC; 2018.
- Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, Parashar UD. Trends in intussusception hospitalizations among US infants, 1993-2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121:e1125–32. doi:10.1542/peds.2007-1590.
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med [Internet]. 2014;370:503–12. doi:10.1056/NEJMoa1303164.
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s national immunization program. Clin Infect Dis an off Publ Infect Dis Soc Am. 2013;57:1427–34. doi:10.1093/cid/cit520.
- Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, Mujuru H, Mpabalwani E, Ngwira B, Cortese MM, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378:1521–28. doi:10.1056/NEJMoa1713909.
- Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30(Suppl 1):A30–5. doi:10.1016/j.vaccine.2011.11.093.
- Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gómara M, Prendergast AJ, Grassly NC. Causes of impaired oral vaccine efficacy in developing countries. Futur Microbiol. 2018;13:97–118. doi:10.2217/fmb-2017-0128.
- Taniuchi M, Platts-Mills JA, Begum S, Uddin MJ, Sobuz SU, Liu J, Kirkpatrick BD, Colgate ER, Carmolli MP, Dickson DM, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine. 2016;34:3068–75. doi:10.1016/j.vaccine.2016.04.080.
- Willame C, Cheuvart B, Aris E, Vetter V, Cohet C. Association between rotavirus gastroenteritis and intussusception: suggested evidence from a retrospective study in claims databases in the United States. Hum Vaccin Immunother. 2021;17:269–77. doi:10.1080/21645515.2020.1770514.
- Koch J, Harder T, von Kries R, Wichmann O. Risk of intussusception after rotavirus vaccination. Dtsch Arztebl Int. 2017;114:255–62. doi:10.3238/arztebl.2017.0255.
- McGeoch LJ, Finn A, Marlow RD. Impact of rotavirus vaccination on intussusception hospital admissions in England. Vaccine. 2020;38:5618–26. doi:10.1016/j.vaccine.2020.06.078.
- Vetter V, Pereira P, Benninghoff B. Rotavirus vaccination and intussusception: a paradigm shift? Hum Vaccin Immunother. 2021;17:278–82. doi:10.1080/21645515.2020.1770035.
- Waldman EA, Luhm KR, Monteiro SAMG, de Freitas FRM. Surveillance of adverse effects following vaccination and safety of immunization programs. Rev Saude Publica. 2011;45:173–84. doi:10.1590/S0034-89102011000100020.
- German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the guidelines working group. MMWR Recomm Reports Morb Mortal Wkly Report Recomm Reports. 2001;50:1–7.
- Fernandes EG, Leshem E, Patel M, Flannery B, Pellini ACG, Veras MA, Sato HK. Hospital-Based surveillance of intussusception among infants. J Pediatr (Rio J). 2016;92:181–87. doi:10.1016/j.jped.2015.06.008.
