ABSTRACT
Since commencement of COVID-19 pandemic, several SARS-CoV-2 variants have emerged amid containment efforts via vaccination. The Delta variant (B.1.617.2), discovered in October 2020, was designated as a VOC by the WHO on May 11, 2021. The enhanced transmissibility of Delta variant has been associated with critical mutations such as D614G, L452R, P681R, and T478K in the S-protein. The increased affinity of the S-protein and ACE2 has been postulated as a key reason for decreased vaccine efficacy. As per evidence, the Delta variant possesses increased transmissibility and decreased vaccine efficacy compared to other VOCs like Alpha and Beta. This has led to concerns regarding the acquisition of novel mutations in the Delta variant and outbreaks in vulnerable communities, including vaccinated people. In this mini-review of Delta variant, we have explained its evolution and characteristics, the impact of spike mutations on infectivity and immune evasion, and measures to combat future outbreaks.
Introduction
Since the COVID-19 pandemic began, many SARS-CoV-2 mutations have been discovered and reported.Citation1–6 These rapid alterations were aided by recombination, selection pressure and point mutations.Citation4,Citation7 SARS-CoV-2 mutations affect the neutralizing activity of vaccine-elicited antibodies and monoclonal antibodies (MAbs), resulting in a mild-to-significant loss of effectiveness.Citation8–11 They may also affect viral transmission, treatment efficacy, and diagnosis. According to the US Department of Health and Human Services, the SARS-CoV-2 variants fall into three broad categories: the variant of interest (VOI), the variant of concern (VOC), and the variant of high consequence.Citation1,Citation12–14
According to recent studies, the Delta variant (B.1.617.2) emerged during India’s second wave of infections with a unique set of mutations (T478K, P681R, and L452R) that make it exceptionally infectious and immune to neutralizing antibodies in previously infected or vaccinated people.Citation2,Citation6,Citation15 Despite the fact that the Delta variant was first found in India in late 2020, it has caused a rise in COVID-19 infection in several other nations.Citation16,Citation17 The Delta variant has progressed effectively, expanding its ancestry into various subgroups or sublineages such as AY.1, AY.2, AY.3, AY.33, AY.34. The AY.1 sub-lineage of delta variant, which contains an extra K417N mutation in its spike protein, has been reported in England.Citation18 The AY.1 variant known as the Delta Plus variant is thought to be the most lethal. It has been postulated that the Delta Plus variant has even more antibody escaping properties because of the K417N mutation. A K417N mutation has been reported in the Beta variant previously.Citation18,Citation19 Furthermore, in comparison to other VOCs, the Delta plus strain has been found to possess a greater risk of transmission and a high affinity to lung epithelial cells.Citation19
However, recent genome sequencing research has confirmed that the Delta variant has spread across at least 32 nations worldwide, the bulk of which are in the United States.Citation18,Citation20
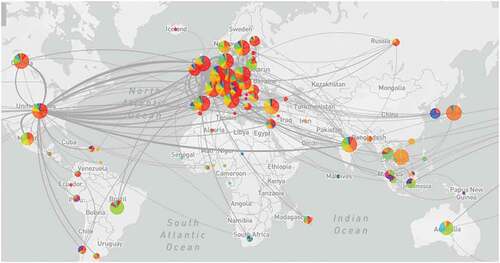
According to a recent comparison analysis, the Delta Plus variant exhibited a much higher number of high-prevalence mutations (20%) than the Delta variant. The Delta Plus version has a higher proportion of signature mutations in Spike (G142D, A222V, and T95I) than the Delta variant. Three spike mutations (K417N, V70F, and W258L) were found only in the Delta Plus version.Citation19,Citation21 A novel mutation in ORF1a (A1146T) was discovered, which was exclusively seen in the Delta Plus variant with a 58% frequency.Citation19 Apart from that other four mutations detected in the ORF1a, i.e., P1604L, A3209V, V3718S, and T3750I,Citation19,Citation21 Delta Plus variant had much more alterations, such as T95I, A222V, G142D, R158G, and K417N than the Delta variant.Citation19 According to structural analysis, mutations alter the sidechain conformation, weakening the antibody interactions.Citation22 Delta Plus originated in India and made its way to the United States through England and Japan before expanding to more than 20 additional nations ().18 Based on the findings, it is evident that the Delta and Delta Plus variants have distinct mutation patterns and that the Delta Plus variant is not just the Delta variant with K417N added. Highly coupled mutations may have developed to maintain the virus’s structural integrity.Citation21,Citation23
Figure 1 A graphical representation of dynamics of the Delta variant (B.1.617.2) first detected in India and emerged to the United States and then to various other countries through the England and Japan (Source: GISAID, https://www.Gisaid.org/hcov19-variants/).
Moreover, when compared with other variants, the Delta Plus variant has been found to withstand MAbs used to treat COVID-19, such as casirivimab and imdevimab and to have increased transmissibility and affinity for the lungs’ mucosal lining 8. On the contrary, neutralization of the Delta Plus variant was discovered in COVID-19 naive or recovered patients inoculated with the BBV152 (Covaxin) vaccine in India.Citation23 Recently another lineage of SARS-CoV-2 named Delta-V has been identified in Vietnam, significantly raising the number of cases daily. Delta-V was formerly assumed to be a hybrid virus because it included mutations in the spike protein (S protein) of the Alpha (B.1.1.7) strain.Citation24 Another Delta variant sublineage, named AY.4.2, has been linked to the gradual rise in COVID-19 cases in the United Kingdom. The N-terminal domain of this lineage is affected by Delta and AY.4 mutations, S: A222V and Y145H. The AY.4.2 variant has been designated as a variant of consideration because it has the potential to exacerbate the situation during ongoing mass vaccination campaigns.Citation25 Thus many, sublineages of the Delta variant have emerged, some of which may have changed biological features and pose a greater risk to human health.Citation15 Although little is known about the Delta variant’s pathogenicity and virulence, it has been suggested that the clinical manifestations may change.Citation26 Moreover, the transmissibility of emerging variants such as the Delta and Omicron varaints may worsen the situation and can lead to more severe disease presentations.Citation14 However, it is still difficult to conclude as there is only a smattering of laboratory data. Despite major advances in vaccine development against COVID-19 and the implementation of rigorous immunization programs worldwide, many scientists believe that the emergence of novel SARS-CoV-2 variants poses a severe threat to the pandemic’s containment efforts.Citation23,Citation27,Citation28 Furthermore, several speculations have been made about the emergence of a super variant named Deltacron, which has been considered a crucial event in the trajectory of the COVID-19 pandemic. Moreover, it was postulated that the Deltacron’s “backbone” is drawn from the Delta strain, while it's spike—the component of the virus that attaches to ACE2 receptors of the host cells—is acquired from the Omicron variant. Therefore, it is essential to understand the mutations and their impact on the various features of the Delta variant (https://www.theguardian.com/world/2022 March2011/what-is-deltacron-covid-variant-uk). Hence, this review article aims to describe the impact of mutations in the Delta variant’s transmissibility and severity. Moreover, the possible impacts on the efficacy of different available vaccines and the repurposed drugs would be explained comprehensively. In addition, we have highlighted the future concerns associated with the Delta variant and possible solutions to contain the possible consequences associated with the Delta variant.
Mutations in the spike protein (S-protein) and their impact on the pathogenesis
The S-protein of SARS-CoV-2 interacts with the host cell receptor, namely ACE2, and the strong link between the S-protein and the ACE2 receptor is the key driving factor in determining SARS-CoV-2 transmissibility. Surprisingly, the Delta variant’s S-protein has shown the most substantial alterations. These alterations are thought to be responsible for the Delta variant being the most transmissible. There is no doubt that the Omicron variant has been widely altered thus far.Citation9,Citation12,Citation26 However, mutations in the Delta variant have been theorized to be more harmful than the Omicron variant. With updated knowledge on mutations and their impact, individually and in combination, on overall viral fitness, researchers will be able to better and more quickly predict the behavior of novel variants of SARS-CoV-2. Additionally, this will be an important consideration when assessing the ramifications of vaccination modifications and booster regimens.Citation17 The S-protein domain spike gene mutations in the Delta variant are D614G, L452R, P681R, and T478K, and these mutations are also recorded in other VOCs and VOIs ().Citation26,Citation29,Citation30 These mutations are highly likely to influence viral infectivity/transmissibility and resistance of the convalescent plasma (CP) or monoclonal antibodies (MAbs).Citation14,Citation31,Citation32
Table 1. Major mutation of Spike protein (S-protein) of the Delta variant and their various impact on viral features such as transmissible nature and capability to evade the immune response and vaccine induced neutralizing antibodies (NABs).
The asparagine-to-glycine substitution at amino acid position 614 in the viral spike protein (D614G) is one of the most critical mutations that has been reported in the current circulating viral strains. Transitions from D614 to G614 happened asynchronously in many parts of the world, including local epidemics where the WT D614 virus was well-established prior to developing the G614 variation.Citation33 This suggests that the D614G change is not the result of an unintentional founder effect or genetic drift. There has been no clinical evidence of a difference in illness severity linked with the D614G alteration because S-protein influences the host range, tissue tropism, and pathogenicity by interacting with the host cell receptors ACE2. However, recent studies have suggested that D614G mutation improved the viral resilience in human hosts, resulting in greater dissemination in the community.Citation34
Furthermore, several analyses, such as the clinical studies, have shown that the D614G mutation significantly increases the replication of the viral particles in the respiratory tract, notably in the upper region of the respiratory tract, which can be associated with the enhanced transmissible potential of the Delta variant. Nevertheless, it is essential to note that the D164G mutation does not lead to the increased severity of the infection. In addition, the enhanced viral replication has been associated with the changes that have occurred in the RBD of the S-protein due to the D614G mutation. In addition, it has been shown through the neutralizing antibody data that the D614G mutation is unlikely to undermine the vaccine effectiveness, but it may influence the potency of therapeutic MAbs in an epitope-dependent way.Citation34 Furthermore, a recent genetic analysis in Qatar showed that the D614G mutation was more dominating and prevalent (approximately 80%) among 2634 SARS-CoV-2 genome sequences.Citation35 In addition, the SARS-CoV-2 variant with D614G mutation has been recorded to have modifications in the viral viability, which improve the dissemination of the Delta variant relative to the WT SARS-CoV-2.Citation33 However, the severity of the infection induced by the variant with D164G mutation may not be affected by this type of mutation.Citation33,Citation36
Another vital mutation in the Delta variant is at position 452, and the L452R mutation substitutes a leucine with an arginine. The L452 residue does not directly contact the ACE2 receptor. Because it is found in the spike protein’s hydrophobic plaques, mutations create structural alterations that increase its interaction with the ACE-2 receptor.Citation37,Citation38 It also aids viral replication by boosting spike persistence, viral transmissibility, and viral fusion.Citation39 Additionally, a pseudovirus analysis demonstrated that the L452R mutation is linked to significant enhancement in the binding of the S-protein to ACE-2 receptors.Citation40 The capacity of a pseudovirus harboring the L452R mutation to infect 293T cells improves by 7 to 23 times, and the ability to infect HAO-ACE2 cells rises about 6 to 15 folds relative to the D614G mutation only.Citation40 Moreover, L452R mutation can cause the vaccine-induced serum neutralizing antibody titer against the pseudovirus to decrease by 3–6 timesCitation39,Citation41 and the pseudovirus can evade several permitted MAbs based therapeutics.Citation41,Citation42
Another notable mutation in the Delta variant is the substitution of proline for arginine at position 681. The P681R mutation promotes the furin-mediated cleavage of the S-protein, which leads to the enhanced fusion of the viral particle with the host cell.Citation28 Compared to the strains lacking this mutation, this would allow for better viral fusion and integration into the host cell.Citation29,Citation30,Citation43 In vitro assays showed that P681R mutation does not increase the infectiousness of the Delta variant. However, the viral strain with P681R mutation exhibits higher pathogenicity in infected hamsters than the WT SARS-CoV-2 strain.Citation43
Recent studies have shown a partial reduction in neutralizing antibodies (NAbs) due to the P681R mutation in the Delta variant. The presence of both D614G and P681R mutation in the viral strain showed moderate (approximately 1.5 times) resilience to some important MAbs against the RBD region of the S-protein, according to a recent pseudovirus neutralization assay.Citation43 In addition, a significant reduction has been observed in neutralizing antibody (NAb) titer of serum elicited by the BNT162b2 vaccination against the D614G/P681R virus.Citation43 It’s worth noting that this neutralization assay implies that the existence of many mutations in the virus strain might have negative repercussions.Citation30
Another substitution (T478K) of the uncharged threonine (T) to a charged amino acid, lysine (K), is a crucial mutation that increases the electrostatic potential of surface S-protein of viral variant. This leads to a more positive value in the region of the S-protein, which directly interacts with the host cell receptor (ACE2). Additionally, the larger side chain of lysine is predicted to increase the steric hindrance of the delta variant, which can be a plausible reason for the significant increment in the interaction of S-protein with the ACE2 receptor.Citation44
Another recent report suggests that the location of T478K increases the interaction of the S-protein with human ACE2 receptors, which leads to enhanced infectiousness of the Delta variant.Citation45,Citation46 Moreover, it has been postulated that this mutation has led to the enhanced infectivity of the Mexican variant (B.1.1.222) of SARS-CoV-2.Citation45 According to the recent information by the global GISAID database, the presence of T478K mutation in the variant may enhance the adaptability of the variant in European countries.Citation44 Moreover, an in vitro analysis has suggested that the presence of this mutation can lead to immune evasion and reduce the sensitivity toward the NAbs ().Citation47
Figure 2. A schematic representation of the mutations in the Spike protein (S-protein) of Delta B.1.617.2 variant. The combination of mutations induces a conformational changes in the S-protein which leads to the substantial increase in the affinity of S-protein to the ACE2 receptors. The increased affinity between the S-protein and ACE2 receptors is a key factor in increased transmission and severity of the Delta variant.
Impact on the transmissibility and severity
A recent study has investigated the dissemination of the Delta variant of SARS-CoV-2 in China.Citation48 One hundred sixty-seven patients infected with the Delta variant were tracked back to the index case, and the viral load was recorded on a daily basis. Sequential polymerase chain reaction tests were used to retrieve the viral load in the patients. According to the investigators, the initial positive test of patients infected with the Delta variant had a viral load 1000 times greater than patients with 19A/19B strain infection, which led to the pandemic in early 2020.Citation48 This suggests that the Delta variant (B.1.617.2) possesses an enhanced capability of viral replication. In addition, the Delta variant is significantly more infectious than the parental strain of SARS-CoV-2.Citation34
R0 (R-naught) is the primary reproduction number, also known as the basic reproduction ratio or rate, which is an epidemiological metric used to measure the transmissibility of infectious agents.Citation49 The contagiousness of an infectious disease is usually measured by this epidemiological term known as R0. R0 is calculated using the patient’s duration of infectivity after infection, the mechanism of transmission, and the contact rate.Citation49 R0 of the initial COVID-19 strain discovered in Wuhan was 2.4–2.6, according to reports.Citation49,Citation50 The R0 for the Alpha (B.1.617.2) strain was 4–5, whereas the R0 for the Delta (B.1.617.2) strain was 5–8. This means that someone infected with the Delta variant of SARS-CoV-2 can infect up to eight other persons. It also suggests that the Delta variant can be twice as transmissible (or perhaps more) as the parental strain of SARS-CoV-2.Citation51
In addition, it has been ascertained that the Delta variant of SARS-CoV-2 (B.1.617.2) is more infectious than smallpox, which has an R0 of 3.5–4.6.Citation52 Furthermore, it has been reported that the vaccination can provide a lesser amount of protection against asymptomatic community transmission of the Delta variant compared to the other variants reported so far.Citation53,Citation54 Moreover, scientists believe that the higher dissemination or the circulation of the Delta variant in the community can lead to high rates of mutations. This, in turn, may enforce the emergence of novel variants of SARS-CoV-2. It has been suggested that because of the emergence of novel variants of SARS-CoV-2, the vaccine manufacturers such as Moderna and Pfizer may have to produce booster doses for their vaccines.Citation30
Immune escape or the resistance to convalescent plasma (CP)
Earlier the E484 mutation was observed in the Beta and Gamma variants of SARS-CoV-2, which was linked to their ability to elude the immunological response, notably the neutralizing effectiveness of CP. However, it is yet unclear how far these variants can evade the neutralizing powers of CP in recovered and vaccinated individuals. Intriguingly, double mutations in the Delta variant’s S-protein, such as E484Q and L452R, have been associated with immunological evasion, thus causing global havoc. The presence of E484 residue as a repulsive residue has been considered as a critical mutation as it is present at the RBD-ACE2 interface,Citation55 which might influence the attachment capabilities of RBD of S-protein with the ACE2.
The E484 and L452 mutations are linked with the resistance capabilities of the Delta variant against the neutralizing antibodies (NAbs) in the sera of convalescent people.Citation56 Hence, the variants including B.1.617, B.1.617.1, and B.1.617.3, possessing the E484 and L452 mutations, were initially considered to be strongly resistant to the body’s immune response.
A two-fold resistance against the NAbs of CP has been observed in the variants such as B.1.617 and B.1.617.1. However, B.1.617.2 (Delta variant) is approximately six to eight times more resistant toward the CP of naturally infected and vaccinated individuals.Citation57–59 Moreover, a significant reduction was reported in the neutralization potentialities of the sera from COVID-19 convalescents against the Delta variant compared to the Alpha and Beta variants.Citation12 In addition, a recent study has revealed that the sera derived from persons immunized with one dose of Pfizer or AstraZeneca have inadequate neutralizing capacities against the Delta variant.Citation12
Influence on the vaccine efficacy (VE)
VOCs, particularly the Delta variant, have a direct influence on COVID-19 vaccines and immunotherapeutics because they can impair the neutralizing activity of vaccine-elicited antibodies and monoclonal antibodies (MAb), leading to a mild-to-significant loss of effectiveness.Citation60–62 BNT162b2 is a well-known mRNA-based vaccine developed by Pfizer and is widely used in the vaccination programs of several countries. Recent comparison studies have raised questions regarding the vaccine’s efficacy, although the two booster doses of this vaccine offer a comparable degree of protection against the Delta strain.Citation12,Citation29,Citation63 Both BNT162b2 and ChAdOx1 nCoV-9 vaccines have been recorded to have reduced effectiveness in the individuals infected with the Delta variant compared to the other VOCs. It is essential to consider that these results were obtained with the individuals given a single dose of the vaccine.
Further analysis with two doses of the vaccine have shown the apparent effectiveness of the major vaccines against the Delta variant. The efficacy of two doses of the BNT162b2 vaccine was 93.7% in people with the Alpha variant and 88% in people with the Delta variant. The efficacy of two doses of the ChAdOx1 nCoV-19 vaccine was 74.5% in people with the Alpha variant and 67.0% in people with the Delta variant. After receiving the two vaccine doses, minor changes in vaccine efficacy were seen between the Delta and Alpha variants. After the first dosage, absolute variations in the vaccine efficacy were more noticeable. This finding would encourage attempts to increase vaccination uptake among vulnerable groups by administering two doses.Citation64
Recently, it has been claimed that the Delta variant has caused a significant increase in vaccine breakthrough infections featuring elevated viral load and transmissibility and has impaired the establishment of effective vaccines.Citation65 In this context, recent research has found a Delta variant outbreak in an ADSC (adult day service center) with a high vaccination coverage rate characterized by a high infection rate, high transmissibility, and mild clinical severity. The outbreak spread to unprotected or partly vaccinated household members, highlighting the need to immunize the high-risk populations’ close contacts.Citation66 In addition, it has also been postulated that the vaccination lowers the chance of infection with the Delta variant and speeds up viral clearance.Citation67 However, it is essential to consider that the fully vaccinated persons with breakthrough infections recorded a high viral load similar to unprotected people and can efficiently spread illness in the household, even among the fully vaccinated people.Citation67 These disparities may possibly be resolved in the future with more immunological studies to find the exact reasons.
In a similar way, numerous recent discoveries in India have found breakthrough infections caused by the Delta variant in people who had been completely vaccinated. However, in the vaccinated group, the proportion of patients who have developed severe illness and died was lower. Both vaccinated and unvaccinated people are at risk of contracting the Delta variant. On the other hand, vaccination appears to stop the disease from progressing. Thus the non-pharmaceutical therapies must also continue to curb the spread of the disease. In order to prevent the pandemic from spreading further, the rate and scope of vaccination must be boosted.Citation68
Effectiveness of various available vaccines against Delta variant
In phase III studies, BNT162b2, mRNA-1273, and Sputnik V exhibited significant effectiveness (>90%) in avoiding symptomatic instances after two doses. COVID-19 symptomatic illnesses and severe diseases with Alpha, Beta, Gamma, or Delta variants were prevented by mRNA vaccines, AZD1222, and CoronaVac. Complete vaccination with mRNA vaccines with AZD1222 appears to either successfully prevent SARS-CoV-2 infection even against parental strain, Alpha, and Beta strains, however with lower efficiency against the Delta variant, according to real-life observational data. BNT162b2 and AZD1222 both showed a decrease in infection protection after six months.Citation69
Effectiveness of various vaccines against infection caused by Delta variant
Fiolet et al., (2022) recently summarized the effectiveness of various available vaccines to protect against SARS-CoV-2 infection by Delta variant in fully vaccinated individuals.Citation69 BNT162b2 recorded 42–79% protection,Citation70,Citation71 mRBA-1273 vaccine recorded 76–84%.Citation72,Citation73 On the other hand, mRNA-vaccines were reported for 64% protection,Citation74 and mRNA-vaccines/Janssen reported 47–79%.Citation75,Citation76 AZD1222 was reported to provide 60–67% protection,Citation70,Citation71 and mRNA/AZD-1222 were shown to provide only 49% protection against the Delta variant.Citation77
Efficacy of vaccines to reduce COVID-19 hospitalization and death
Ad26.COV1.S vaccine is reported to provide 60 to 85% protection against hospitalization in patients infected Delta variant of SARS-CoV-2.Citation78,Citation79 On the other hand, mRNA-vaccines such as mRNA1273 provided 95% protection to reduce hospitalization and death in patients infected with Delta variant.Citation78–80
Effects on other notable drugs
Recently, the Delta variant has been recorded to possess increased resistance against various effective monoclonal antibodies (MAbs) such as Bamlanivimab. The resistance against MAbs has been associated with changes in the RBD of the S-protein.Citation42 Additionally, the alterations in the N-terminal domain (NTD) of S-protein are significant in the development of resistance against several important therapeutic regimens. However, the neutralizing capacity of three additional therapeutically authorized MAbs, including Etesivimab, Basirivimab, and Imdevimab, against the Delta variant was retained. Furthermore, combining numerous neutralizing MAbs that can recognize and bind to distinct and non overlapping epitopes of the S-protein might reduce the loss of individual antibody-mediated neutralization capacities.Citation81,Citation82
Clinical differences among patients infected with Wild type and Delta strain of SARS-CoV-2
According to a recent study, the infection caused by the Delta variant was associated with a lower incidence of symptoms than wild-type infection. Coughing and fever are still the most common symptoms, although gastrointestinal problems have become far less common.Citation83 Moreover, these findings were in agreement with previously published findings.Citation45 Patients infected with the Delta variant of SARS-CoV-2 have also reported sore throats, which were not prevalent in patients infected with wild-type SARS-CoV-2. But the Sore throat symptoms have been reported in a significant proportion of patients infected with a wild-type strain of SARS-CoV-2. In terms of hematological data, patients infected with Delta variant (those who were not vaccinated) reported to have lesser leukocytosis and neutrophilia as compared to the patients infected with a parental strain of SARS-CoV-2. In addition, the lower levels of C-reactive protein (CRP), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), lactic dehydrogenase (LDH), procalcitonin (PCT) and D-dimer have been reported.Citation83 Importantly, varied outcomes were observed in the cases of monocytosis and thrombocytopenia, as well as in the levels of ALP, creatinine, CPK, thrombin time, and fibrinogen, among other parameters.Citation83
However, there is still a lot of work to be done on these clinical symptoms. It is interesting to consider that the partial vaccination did not significantly reduce the severity of Delta variant infection; full vaccination did so by alleviating coagulation dysfunction and mitigating the viremic impact on major organs (as evidenced by lower levels of adenosine deaminase (ADA), blood urea nitrogen (BUN), creatinine, and liver deaminase (LDH).Citation83 It was concluded from these findings that COVID-19 immunization is associated with a considerable reduction in hospitalization and illness progression, despite its decreased efficiency against the Delta variant.Citation68,Citation84
Future concerns/threats
According to current statistics, the Omicron variant is undeniably the most common among the population of numerous countries, including England. However, the Delta variant still exists in several communities and may emerge as a plausible threat in the future via acquiring more mutations in the S-protein or by infecting people who fall on the vulnerability list. In this context, it has recently been suggested that pregnant women are among the most susceptible populations during the Delta variant outbreak, with higher hospitalization, pneumonia, respiratory assistance, and admission to critical care units. Viral infection due to the Delta variant is more likely to occur in pregnant women with gestational diabetes mellitus (GDM). GDM patients are nine and three times more likely to be infected with viral infection compared to pregnant women with diabetes and cardiovascular diseases, and hypertension, respectively. Moreover, it has also been found that pregnant women with GDM are more susceptible to infection of Delta variant than other VOCs and wild-type strains of SARS-CoV-2.Citation85
Furthermore, it has recently been proposed that a single viral strain can only cause SARS-CoV-2 infection, but it is essential to consider that the two strains of SARS-CoV-2- may infect the host at the same time, but this is an exceedingly unusual occurrence.Citation13 In addition, according to some recent information and media coverage, a newly identified Delmicron double variant has been postulated. However, further investigations are required before any conclusions can be drawn. This so-called new super-variant might be created if both variants, including Delta and Omicron variants, infect individuals at the very same time, and people with weakened immune systems can harbor both variants of SARS-CoV-2. The Delmicron variant is considered to have been created by combining the most lethal Delta and most modified Omicron variants of SARS-CoV-2, which is now being blamed for a new outbreak of COVID-19 cases in North America, Europe, and potentially India and other nations.Citation13 However, if such assumptions are proved, they will necessitate more research and certification by the WHO. Because genomic sequencing is used to validate the mutations, which is done on only a tiny fraction of COVID-19 cases, the actual number of Omicron and Delta cases is expected to be much greater than the number of verified instances published thus far.Citation13,Citation86,Citation87 Hence, there is an urgent need to halt the circulations of the variants among the vast population to stop the emergence of any potential viral strain.
Preventive measures
Several reports have clearly shown that the Delta variant is significantly infectious compared to smallpox, with an R0 value of 3.5–4.6. Hence, people must comply with physical distancing and other precautionary measures to take better control of the infection caused by the Delta variant.Citation52
However, we cannot overlook that BNT162b2, mRNA-1273, ChAdOx1, and other commonly used vaccines in European countries and the United States have reported a certain amount of protectiveness against the Delta variant in the people administered with two doses of the vaccine. It is essential to consider that after two doses, the protection rates against diverse SARS-CoV-2 variants, including the Delta variant, were much higher than the single dose of the vaccine. Hence, it is vital to raise the number of completely vaccinated persons to successfully prevent the spread of SARS-CoV-2 internationally.Citation29 Rather than spontaneous infection, vaccination is the only way to establish significant herd immunity against SARS-CoV-2. As a result, global efforts should focus on developing a vaccination program that uses a highly effective vaccine to achieve the largest possible coverage,Citation27 which could serve as an efficient measure to reduce any plausible consequences caused by the Delta variant outbreak.
Moreover, vulnerable people like pregnant women should prioritize self-care and get vaccinated against SARS-CoV-2 as soon as possible. Pregnant women should be prioritized in a rapid immunization campaign.Citation85,Citation88 Until data from the trail bases studies, including the pregnant women, is available, pregnant women and their obstetricians should be informed of the latest facts, including the benefits and risks of the COVID-19 vaccinations.Citation89 Though pregnant women with COVID-19 have a comparable risk of problems than non-pregnant women, intensive care is required in middle and low-income nations. Pregnant women should follow all precautionary measures, with vaccination being a major priority.Citation89,Citation90
Moreover, it is essential to consider that even with the development of effective COVID-19 vaccines, considerable obstacles such as vaccine hesitancy and inequalities in vaccine distribution are still prevalent among several countries.Citation9 All nations should actively evaluate and solve these issues to inhibit the spread of SARS-CoV-2, particularly the Delta variant, and the development of additional SARS-CoV-2 variants.Citation91 Furthermore, the genomic surveillance and evolutionary dynamics studies should be done more quickly, and contact tracing of variants should be done more often as well. This will help a lot to improve our knowledge of VOCs, particularly the Delta variant, so that scientists can update the COVID-19 vaccines and make second-generation vaccines. In addition, the use of booster doses and the development of effective treatment regimens can lessen the risks of the infection with the Delta variant, which possesses a significant advantage of enhanced dissemination potentials. Scientists should keep monitoring and evaluating the Delta variant along with the Omicron variant around the world.Citation13,Citation87,Citation90,Citation92
Genomic surveillance makes use of next-generation sequencing techniques, provides whole-genome data accessible, and improves phylogenetic approaches. These technologies provide new ways to detect phenotypically or antigenically distinct variations.Citation93,Citation94 Genomic monitoring is critical in the fight against COVID-19, and it must be deployed globally in a thorough and coordinated manner. It can give the relevant data needed to develop a more tailored public health plan that targets local priorities through stakeholder engagement and mitigation initiatives while waiting for herd immunity to be achieved through vaccination. Increased international collaboration offers unique prospects for achieving quick genomic monitoring and leveraging the expertise of high-income nations, particularly the United Kingdom, and deploying these to low- and middle-income countries around the globe. Genomic surveillance will allow for better early detection of SARS-CoV-2 mutations and many other new viral strains , as well as the development of effective ways to attenuate and limit epidemics.Citation93
Conclusions and future directions
Since the beginning of the COVID-19 pandemic, which was caused by SARS-CoV-2, the causative agent has undergone several mutations due to recombination, selection pressure, and point mutations. Till now, several variants of concern (VOCs) such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) have been discovered and reported in various parts of the world. These variants may have the potential to significantly influence the dissemination capabilities of the virus, along with reducing the efficacy of presently available vaccines, therapeutic regimens, and diagnostic procedures. The emergence of VOCs has led to challenges in controlling the pandemic amid serious worldwide efforts. However, due to the increased transmissibility and virulence of the Delta variant (B.1.617.2), it has been considered as a major concern and threat to humankind. Recent findings have concluded that the current immunization approaches and the available vaccines may not prevent the Delta variant infection and morbidity. However, they may reduce the severity of the infection hence reducing the mortality rates according to real-world data from various nations. The Delta variant’s enhanced infectivity appears to be the result of a combination of critical changes/mutations that enable the spike protein (S-protein) to interact with the host receptor (ACE-2) more efficiently. The increased affinity of the S-protein toward the ACE-2 receptors leads to lesser effectiveness of the vaccines and a significantly higher number of viral loads in infected people. Moreover, the Delta variant has been reported to exhibit significantly increased resistance to the neutralization by several anti-NTD and anti-RBD monoclonal antibodies such as Etesivimab, Basirivimab, and Imdevimab. In addition, these MAbs have shown decreased affinity to the S-protein of the Delta variant.
Furthermore, the clinical signs of individuals infected with the Delta variant of SARS-CoV-2 are still being worked out. In individuals infected with the Delta variant and wild type SARS-CoV-2, it is critical to distinguish clinical and biochemical markers. There is no doubt that how clinical symptoms differ among individuals infected with sublineages of the Delta variant, such as the Delta plus variant, is still a conundrum. All of these questions, hopefully, will be answered in the future through well-designed and elaborated clinical trials.
Moreover, sera obtained from convalescent people have been found to be four times less effective against the Delta variant than the Alpha variant (B.1.1.7). Even the plasma from those who had received one dose of either the Pfizer or AstraZeneca vaccines have been reported to exhibit a negligible neutralization of the Delta variant. Thus the decline in vaccine effectiveness against the Delta variant is a serious issue, and immunization against COVID-19 should be increased internationally to deal with the Delta variant’s high frequency. With the considerable decrease in the neutralization of the Delta variant with neutralizing antibodies elicited by various available vaccines, it is questionable how vaccinated individuals and vaccine inequalities across the globe will shift the trajectory of the COVID-19 pandemic.
The most critical method to prevent and control the spread of the Delta variant is to increase vaccination coverage. The mortality rate has not ascended in nations with high vaccination rates, such as the United Kingdom, despite the rising incidence, but the pandemic is more dangerous in countries with poor vaccination rates, such as South Africa, India, Thailand, and Indonesia. Among the United States, the reported COVID-19 cases reported are primarily found in the unvaccinated community. As a result, managing the worldwide pandemic would require an accelerated vaccination pace and coverage, with an emphasis on the vaccine-deficient countries.
Abbreviations
| SARS-CoV-2 | = | Severe acute respiratory syndrome coronavirus 2 |
| WHO | = | World Health Organisation |
| VOC | = | variant of concern |
| S-protein | = | spike protein |
| ACE-2 | = | angiotensin-converting enzyme-2 |
| VE | = | vaccine efficacy |
| VOI | = | variant of interest |
| VOC | = | variant of concern |
| Mab | = | monoclonal antibody |
| ORF1a | = | open reading frame 1a |
| NAb | = | neutralizing antibody |
| R0 | = | R naught |
| CP | = | convalescent plasma |
| ADSC | = | adult day service center |
| NTD | = | N-terminal domain |
| RBD | = | receptor binding domain |
| GDM | = | gestational diabetes mellitus |
Authors’ contributions
MD did the ideation, conceptualization, data curation, writing original draft, reviewing and editing. PR executed the conceptualization, writing original draft, reviewing and editing. AS, NT, TKR, OPC did the reviewing and editing. All authors critically reviewed and approved the final version of the manuscript.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Acknowledgements
All the authors acknowledge and thank their respective Universities and Institutes.
Disclosure statement
All authors report no conflicts of interest relevant to this article.
Additional information
Funding
References
- Choudhary OP, Priyanka, Ali RK, Maulud SQ, Dhawan M, Mohammed TA. Will the next spillover pandemic be deadlier than the COVID-19?: a wake-up call. Int J Surg [Internet]. 2022;97:106208.
- Rahman FI, Ether SA, Islam MR. The “Delta Plus” COVID-19 variant has evolved to become the next potential variant of concern: mutation history and measures of prevention. J Basic Clin Physiol Pharmacol. 2022;33(1):109–12. doi:10.1515/jbcpp-2021-0251.
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–24. doi:10.1038/s41579-021-00573-0.
- Souza PFN, Mesquita FP, Amaral JL, Landim PGC, Lima KRP, Costa MB, Farias IR, Belém MO, Pinto YO, Moreira HHT, et al. The spike glycoprotein of SARS-CoV-2: a review of how mutations of spike glycoproteins have driven the emergence of variants with high transmissibility and immune escape. Int J Biol Macromol. 2022;208:105–25. doi:10.1016/j.ijbiomac.2022.03.058.
- Bhattacharya M, Sharma AR, Dhama K, Agoramoorthy G, Chakraborty C. Slowing down as we age: aging of the cardiac pacemaker’s neural control. Geroscience. 2022;44(1):1–19. doi:10.1007/s11357-022-00532-4.
- Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2022;94(4):1641–49. doi:10.1002/jmv.27526.
- Hosch S, Mpina M, Nyakurungu E, Borico NS, Obama TMA, Ovona MC, Wagner P, Rubin SE, Vickos U, Milang DVN, et al. Genomic surveillance enables the identification of co-infections with multiple SARS-CoV-2 lineages in Equatorial Guinea. Front Public Health. 2021;9:818401. doi:10.3389/fpubh.2021.818401.
- Roy B, Roy H. The Delta Plus variant of COVID-19: will it be the worst nightmare in the SARS-CoV-2 pandemic? J Biomed Sci. 2021;8(1):1–2. doi:10.3126/jbs.v8i1.38449.
- Dhawan M, Priyanka, Choudhary OP. Omicron SARS-CoV-2 variant: reasons of emergence and lessons learnt. Int J Surg. 2022;97:106198. doi:10.1016/2Fj.ijsu.2021.106198.
- Rockett R, Basile K, Maddocks S, Fong W, Agius JE, Johnson-Mackinnon J, Arnott A, Chandra S, Gall M, Draper J, et al. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N Engl J Med. 2022. doi:10.1056/nejmc2120219.
- Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, Liu J, Errico JM, Xie X, Suryadevara N, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717–26. doi:10.1038/s41591-021-01294-w.
- Choudhary OP, Dhawan M, Priyanka. Omicron variant (B.1.1.529) of SARS-CoV-2: threat assessment and plan of action. Int J Surg. 2022;97:106187. doi:10.1016/j.ijsu.2021.106187.
- Mohapatra RK, Tiwari R, Sarangi AK, Sharma SK, Khandia R, Saikumar G, Dhama K. Twin combination of Omicron and Delta variants triggering a tsunami wave of ever high surges in COVID-19 cases: a challenging global threat with a special focus on the Indian subcontinent. J Med Virol. 2022;94(5):1761–65. doi:10.1002/jmv.27585.
- Ye G, Liu B, Li F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat Commun. 2022;13(1):1214. doi:10.1038/s41467-022-28882-9.
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–80. doi:10.1038/s41586-021-03777-9.
- Kirola L. Genetic emergence of B.1.617.2 in COVID-19. New Microbes New Infect. 2021;43:100929. doi:10.1016/j.nmni.2021.100929.
- Saville JW, Mannar D, Zhu X, Srivastava SS, Berezuk AM, Demers J-P, Zhou S, Tuttle KS, Sekirov I, Kim A, et al. Structural and biochemical rationale for enhanced spike protein fitness in delta and kappa SARS-CoV-2 variants. Nat Commun. 2022;13(1):742. doi:10.1038/s41467-022-28324-6.
- Arora P, Kempf A, Nehlmeier I, Graichen L, Sidarovich A, Winkler MS, Schulz S, Jäck H-M, Stankov M, Behrens GMN, et al. Delta variant (B.1.617.2) sublineages do not show increased neutralization resistance. Cell Mol Immunol. 2021;18(11):2557–59. doi:10.1038/s41423-021-00772-y.
- Chavda VP, Apostolopoulos V. Global impact of delta plus variant and vaccination. Expert Rev Vaccines. 2022;1–4. doi:10.1080/14760584.2022.2044800.
- Aleem A, Akbar Samad AB, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). Treasure Island (FL): StatPearls Publishing; 2022.
- Kannan SR, Spratt AN, Cohen AR, Naqvi SH, Chand HS, Quinn TP, Lorson CL, Byrareddy SN, Singh K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J Autoimmun. 2021;124:102715. doi:10.1016/j.jaut.2021.102715.
- Jacob JJ, Vasudevan K, Pragasam AK, Gunasekaran K, Veeraraghavan B, Mutreja A. Evolutionary tracking of SARS-CoV-2 genetic variants highlights an intricate balance of stabilizing and destabilizing mutations. mBio. 2021;12(4):e0118821. doi:10.1128/mbio.01188-21.
- Yadav PD, Sahay RR, Sapkal G, Nyayanit D, Shete AM, Deshpande G, Patil DY, Gupta N, Kumar S, Abraham P, et al. Comparable neutralization of SARS-CoV-2 Delta AY.1 and Delta with individuals sera vaccinated with BBV152. J Travel Med. 2021;28. doi:10.1093/jtm/taab154.
- Vietnam says new Covid variant is hybrid of India and UK strains | Coronavirus | The Guardian; n.d. [Accessed 2021 Nov 15]. https://www.theguardian.com/world/2021/may/29/vietnam-discovers-new-hybrid-covid-variant-state-media-reports.
- SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 26; [Accessed 2022 Jan 27]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1009243/Technical_Briefing_20.pdf.
- Gobeil S-C, Janowska K, McDowell S, Mansouri K, Parks R, Stalls V, Kopp MF, Manne K, Li D, Wiehe K, et al. Effect of natural mutations of SARS-CoV-2 on spike structure, conformation, and antigenicity. Science [Internet] 2021. 1979;373(6555):eabi6226. doi:10.1126/science.abi6226.
- Dhawan M, Priyanka, Sahni A, Choudhary OP. Vaccine inequity and hesitancy: dual factors in the emergence of novel SARS-CoV-2 variants. Ann Med Surg (Lond). 2022;73:103186. doi:10.1016/2Fj.amsu.2021.103186.
- Cascella M, Rajnik M, Aleem A, Dulebohn SC, di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Treasure Island (FL): StatPearls Publishing; 2022 [Accessed 2022 Jan 25].
- Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, Mao Q, Xu M, Liang Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(10):1201–09. doi:10.1080/14760584.2021.1976153.
- Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta variant B.1.617.2 COVID-19. Clin Pract. 2021;11(4):778–84. doi:10.3390/clinpract11040093.
- Dhawan M, Priyanka, Parmar M, Angural S, Choudhary OP. Convalescent plasma therapy against the emerging SARS-CoV-2 variants: delineation of the potentialities and risks. Int J Surg. 2022;97:106204. doi:10.1016/j.ijsu.2021.106204.
- Wrobel AG, Benton DJ, Roustan C, Borg A, Hussain S, Martin SR, Rosenthal PB, Skehel JJ, Gamblin SJ. Evolution of the SARS-CoV-2 spike protein in the human host. Nat Commun. 2022 Mar 4;13(1):1178. doi:10.1038/s41467-022-28768-w.
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–27. doi:10.1016/j.cell.2020.06.043.
- Shi AC, Xie X. Making sense of spike D614G in SARS-CoV-2 transmission. Sci China Life Sci. 2021;64(7):1062–67. doi:10.1007/s11427-020-1893-9.
- Benslimane FM, Al Khatib HA, Al-Jamal O, Albatesh D, Boughattas S, Ahmed AA, Bensaad M, Younuskunju S, Mohamoud YA, Al Badr M, et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. Front Cell Infect Microbiol. 2021;11:2235–988. doi:10.3389/fcimb.2021.768883.
- Sabir DK. Analysis of SARS-COV2 spike protein variants among Iraqi isolates. Gene Rep. 2021;26:101420. doi:10.1016/j.genrep.2021.101420.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–20. doi:10.1038/s41586-020-2180-5.
- Pascarella S, Ciccozzi M, Zella D, Bianchi M, Benedetti F, Benvenuto D, Broccolo F, Cauda R, Caruso A, Angeletti S, et al. SARS-CoV-2 B.1.617 Indian variants: are electrostatic potential changes responsible for a higher transmission rate? J Med Virol. 2021;93(12):6551–56. doi:10.1002/jmv.27210.
- Motozono C, Toyoda M, Zahradnik J, Ikeda T, Saito A, Tan TS, Ngare I, Nasser H, Kimura I, Uriu K, et al. An emerging SARS-CoV-2 variant evading cellular immunity and increasing viral infectivity. BioRxiv. 2021b. doi:10.1101/2021.04.02.438288.
- Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, Sotomayor-González A, Glasner DR, Reyes KR, Gliwa AS, et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184(13):3426–37. doi:10.1016/j.cell.2021.04.025.
- McCallum M, Bassi J, De Marco A, Chen A, Walls AC, Di Iulio J, Tortorici MA, Navarro MJ, Silacci-Fregni C, Saliba C, et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021;373(6555):648–54. doi:10.1126/science.abi7994.
- Starr TN, Greaney AJ, Dingens AS, Bloom JD. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep Med. 2021;2(4):100255. doi:10.1016/j.xcrm.2021.100255.
- Saito A, Irie T, Suzuki R, Maemura T, Nasser H, Uriu K, Kosugi Y, Shirakawa K, Sadamasu K, Kimura I, et al. SARS-CoV-2 spike P681R mutation, a hallmark of the Delta variant, enhances viral fusogenicity and pathogenicity. BioRxiv. 2021. doi:10.1101/2021.06.17.448820.
- Di Giacomo S, Mercatelli D, Rakhimov A, Giorgi FM. Preliminary report on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike mutation T478K. J Med Virol. 2021;93(9):5638–43. doi:10.1002/jmv.27062.
- Wang R, Chen J, Gao K, Wei GW. Vaccine-Escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021;113(4):2158–70. doi:10.1016/j.ygeno.2021.05.006.
- Jhun H, Park HY, Hisham Y, Song CS, Kim S. SARS-CoV-2 Delta (B.1.617.2) variant: a unique T478K mutation in Receptor Binding Motif (RBM) of Spike Gene. Immune Netw. 2021:21. doi:10.4110/in.2021.21.e32.
- Muecksch F, Weisblum Y, Barnes CO, Schmidt F, Schaefer-Babajew D, Wang Z, Lorenzi JC C, Flyak AI, DeLaitsch AT, Huey-Tubman KE, et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54(8):1853–68. doi:10.1016/j.immuni.2021.07.008.
- Li B, Deng A, Li K, Hu Y, Li Z, Xiong Q, Liu Z, Guo Q, Zou L, Zhang H, et al. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant. MedRxiv. 2021. doi:10.1101/2021.07.07.21260122.
- Achaiah NC, Subbarajasetty SB, Shetty RM. R0 and Re of COVID-19: can we predict when the pandemic outbreak will be contained? Indian J Crit Care Med. 2020;24(11):1125–27. doi:10.5005/jp-journals-10071-23649.
- Liu Y, Tang JW, Lam TTY. Transmission dynamics of the COVID-19 epidemic in England. Int J Infect Dis. 2021;104:132–38. doi:10.1016/j.ijid.2020.12.055.
- What we know about the SARS-CoV-2 Delta variant [Accessed 2021 Nov 25]. https://newsroom.unsw.edu.au/news/health/what-we-know-about-sars-cov-2-delta-variant.
- Hendaus MA, Jomha FA. Delta variant of COVID-19: a simple explanation. Qatar Med J. 2021;2021:49. doi:10.5339/qmj.2021.49.
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–62. doi:10.15585/mmwr.mm7031e2.
- Torgovnick J. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(25):385. doi:10.1056/NEJMc2113090.
- Yang W, Shaman J. COVID-19 pandemic dynamics in India, the SARS-CoV-2 Delta variant, and implications for vaccination. medRxiv [Preprint]. 2021. doi:10.1101/2021.06.21.21259268.
- Khan A, Zia T, Suleman M, Khan T, Ali SS, Abbasi AA, Mohammad A, Wei DQ. Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: an insight from structural data. J Cell Physiol. 2021;236(10):7045–57. doi:10.1002/jcp.30367.
- Yadav P, Sapkal GN, Abraham P, Ella R, Deshpande G, Patil DY, Nyayanit D, Gupta N, Sahay RR, Shete AM, et al. Neutralization of variant under investigation B. 1.617 with sera of BBV152 vaccinees. BioRxiv. 2021. doi:10.1101/2021.04.23.441101.
- Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, Flowers MW, Wrammert J, Hussaini L, Ciric CR, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385(7):664–66. doi:10.1056/NEJMc2107799.
- Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466–68. doi:10.1056/NEJMc2102017.
- Sharun K, Tiwari R, Dhama K, Emran TB, Rabaan AA, Al Mutair A. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccin Immunother. 2021;17(10):3491–94. doi:10.1080/21645515.2021.1923350.
- Wahid M, Jawed A, Mandal RK, Dailah HG, Janahi EM, Dhama K, Somvanshi P, Haque S. Variants of SARS-CoV-2, their effects on infection, transmission and neutralization by vaccine-induced antibodies. Eur Rev Med Pharmacol Sci. 2021;25(18):5857–64. doi:10.26355/eurrev_202109_26805.
- Biswas B, Chattopadhyay S, Hazra S, Hansda AK, Goswami R. COVID-19 pandemic: the delta variant, T-cell responses, and the efficacy of developing vaccines. Inflamm Res. 2022;15:1–20. doi:10.1007/s00011-022-01555-5.
- Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, Nutalai R, Zhou D, Mentzer AJ, Zhao Y, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–36. doi:10.1016/j.cell.2021.06.020.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–94. doi:10.1056/NEJMoa2108891.
- Zhang M, Liang Y, Yu D, Du B, Cheng W, Li L, Yu Z, Luo S, Zhang Y, Wang H, et al. A systematic review of vaccine breakthrough infections by SARS-CoV-2 Delta variant. Int J Biol Sci. 2022;18(2):889–900. doi:10.7150/ijbs.68973.
- Yi S, Kim JM, Choe YJ, Hong S, Choi S, Ahn SB, Kim M, Park YJ. SARS-CoV-2 Delta variant breakthrough infection and onward secondary transmission in household. J Korean Med Sci. 2022;37(1):37. doi:10.3346/jkms.2022.37.e12.
- Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone MA, Koycheva A, Derqui-Fernandez N, Barnett JL, Whitfield MG, Varro R, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021;22(2):183–95. doi:10.1016/S1473-3099(21)00648-4.
- Thangaraj JWV, Yadav P, Kumar CG, Shete A, Nyayanit DA, Rani DS, Kumar A, Kumar MS, Sabarinathan R, Saravana Kumar V, et al. Predominance of delta variant among the COVID-19 vaccinated and unvaccinated individuals, India, May 2021. J Infect. 2022;84(1):94–118. doi:10.1016/j.jinf.2021.08.006.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–21. doi:10.1016/j.cmi.2021.10.005.
- Sheikh A, McMenamin J, Taylor B, Robertson C; Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021 Jun 26;397(10293):2461–62. doi:10.1016/S0140-6736(21)01358-1.
- Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, House T, Hay J, Bell JI, Newton JN, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127–35. doi:10.1038/s41591-021-01548-7.
- Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Khatib HAA, et al. Bnt162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat Med. doi:10.1038/s41591-021-01583-4.
- Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O’-Horo JC, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021. 2021.08.06.212617072021. doi:10.1101/2021.08.06.21261707.
- Seppälä E, Veneti L, Starrfelt J, Danielsen AS, Bragstad K, Hungnes O, Taxt AM, Watle SV, Meijerink H. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26(35):2100793. doi:10.2807/1560-7917.ES.2021.26.35.2100793.
- Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, Backenson B, Hoefer D, Morne J, Bauer U, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status - New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1150–55. doi:10.15585/mmwr.mm7034e1.
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021 Oct 16;398(10309):1407–16. doi:10.1016/S0140-6736(21)02183-8.
- Elliott P, Haw D, Wang H, Eales O, Walters CE, Ainslie KEC, Atchison C, Fronterre C, Diggle PJ, Page AJ, et al. Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant. Science. 2021 Dec 17;374(6574):eabl9551. doi:10.1126/science.abl9551.
- Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, Naleway AL, Natarajan K, Thompson MG; VISION Network. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - Nine States, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291–93. Erratum in: MMWR Morb Mortal Wkly Rep. 2021 Dec 10;70(49):1717. doi:10.15585/mmwr.mm7037e2.
- Polinski JM, Weckstein AR, Batech M, Kabelac C, Kamath T, Harvey R, Jain S, Rassen JA, Khan N, Schneeweiss S. Durability of the single-dose Ad26.COV2.S vaccine in the prevention of COVID-19 infections and hospitalizations in the US before and during the Delta variant surge. JAMA Netw Open. 2022 Mar 1;5(3):e222959. doi:10.1001/jamanetworkopen.2022.2959.
- Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Takhar HS, Tubert JE, et al. Real-World effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health Am. 2022;6:100134. doi:10.1016/j.lana.2021.100134.
- Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–18. doi:10.1126/science.abd0831.
- Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses: (Trends Immunol 41, 355-359; 2020). Trends Immunol. 2020;41(5):355–59. doi:10.1016/j.it.2020.04.008.
- Hu Z, Huang X, Zhang J, Fu S, Ding D, Tao Z. Differences in clinical characteristics between Delta variant and Wild-type SARS-CoV-2 infected patients. Front Med. 2022;8:792135. doi:10.3389/fmed.2021.792135.
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43–55. doi:10.1016/S1473-3099(21)00460-6.
- Mamun MMA, Khan MR. COVID-19 Delta variant-of-concern: a real concern for pregnant women with gestational diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:778911. doi:10.3389/fendo.2021.778911.
- Yang Y, Zhang Y, Qu Y, Zhang C, Liu XW, Zhao M, Mu Y, Li W. Key residues of the receptor binding domain in the spike protein of SARS-CoV-2 mediating the interactions with ACE2: a molecular dynamics study. Nanoscale. 2021;13(20):9364–70. doi:10.1039/d1nr01672e.
- Wang X, Powell CA. How to translate the knowledge of COVID-19 into the prevention of Omicron variants. ClinTransl Med. 2021;1:e22. doi:10.1002/ctm2.680.
- Priyanka, Choudhary OP. A personal experience of COVID-19 vaccination in pregnancy. Int J Surg. 2021;95:106160. doi:10.1016/j.ijsu.2021.106160.
- Kumar R, Yeni CM, Utami NA, Masand R, Asrani RK, Patel SK, Kumar A, Yatoo MI, Tiwari R, Natesan S, et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: concerns, challenges, management and mitigation strategies–a narrative review. J Infect Public Health. 2021;14(7):863–75. doi:10.1016/j.jiph.2021.04.005.
- Heath PT, Le Doare K, Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020;20(9):1007–08. doi:10.1016/S1473-3099(20)30638-1.
- Choudhary OP, Priyanka, Ahmed JQ, Mohammed TA, Singh I, Rodriguez-Morales AJ. Heterologous prime-boost vaccination against COVID-19: is it safe and reliable? Hum Vaccin Immunother. 2021;13:1–4. doi:10.1080/21645515.2021.2007015.
- Ito K, Piantham C, Nishiura H. Relative Instantaneous Reproduction Number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J Med Virol. 2021. doi:10.1002/jmv.27560.
- Robishaw JD, Alter SM, Solano JJ, Shih RD, DeMets DL, Maki DG, Hennekens CH. Genomic surveillance to combat COVID-19: challenges and opportunities. Lancet Microbe. 2021;2(9):e481–e484. doi:10.1016/S2666-5247(21)00121-X.
- Chiara M, D’-Erchia AM, Gissi C, Manzari C, Parisi A, Resta N, Zambelli F, Picardi E, Pavesi G, Horner DS, et al. Next generation sequencing of SARS-CoV-2 genomes: challenges, applications and opportunities. Brief Bioinform. 2021 Mar 22;22(2):616–30. doi:10.1093/bib/bbaa297.
- Johnson BA, Xie X, Kalveram B, Lokugamage KG, Muruato A, Zou J, Zhang X, Juelich T, Smith JK, Zhang L, et al. Furin cleavage site is key to SARS-CoV-2 pathogenesis. bioRxiv [Preprint]. 2020 . doi:10.1101/2020.08.26.268854.
- Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, Lambson BE, de Oliveira T, Vermeulen M, van der Berg K, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622–25. doi:10.1038/s41591-021-01285-x.
- Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–61.e2346. doi:10.1016/j.cell.2021.02.037.
- Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H, Zhang Y, Li T, Liu S, Zhang M, et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021 Apr 29;184(9):2362–71.e9. doi:10.1016/j.cell.2021.02.042.
